The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Characterization of Studied Coatings
2.2. Topography and Mechanical Properties of Studied Coatings
2.3. Analysis of Studied Coatings Biointegration Properties
2.3.1. Cell Culture
2.3.2. Proliferation of L929 Fibroblasts and MG-63 Osteoblasts Detected by MTT Assay
2.3.3. Cell Morphology
2.3.4. Statistical Analysis in the MTT Assay
3. Results
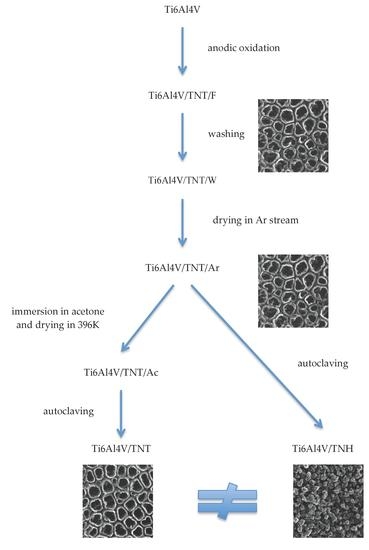
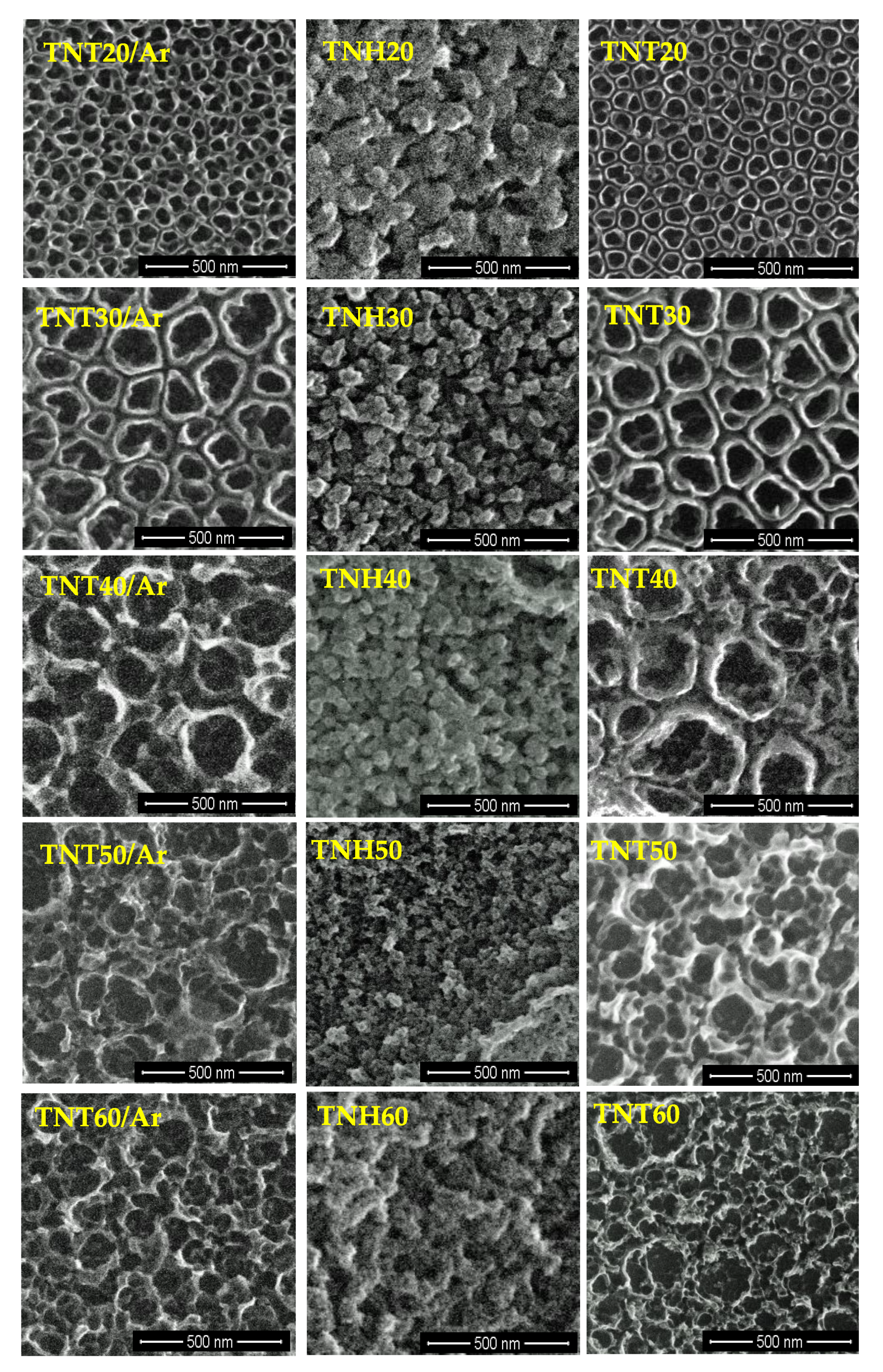
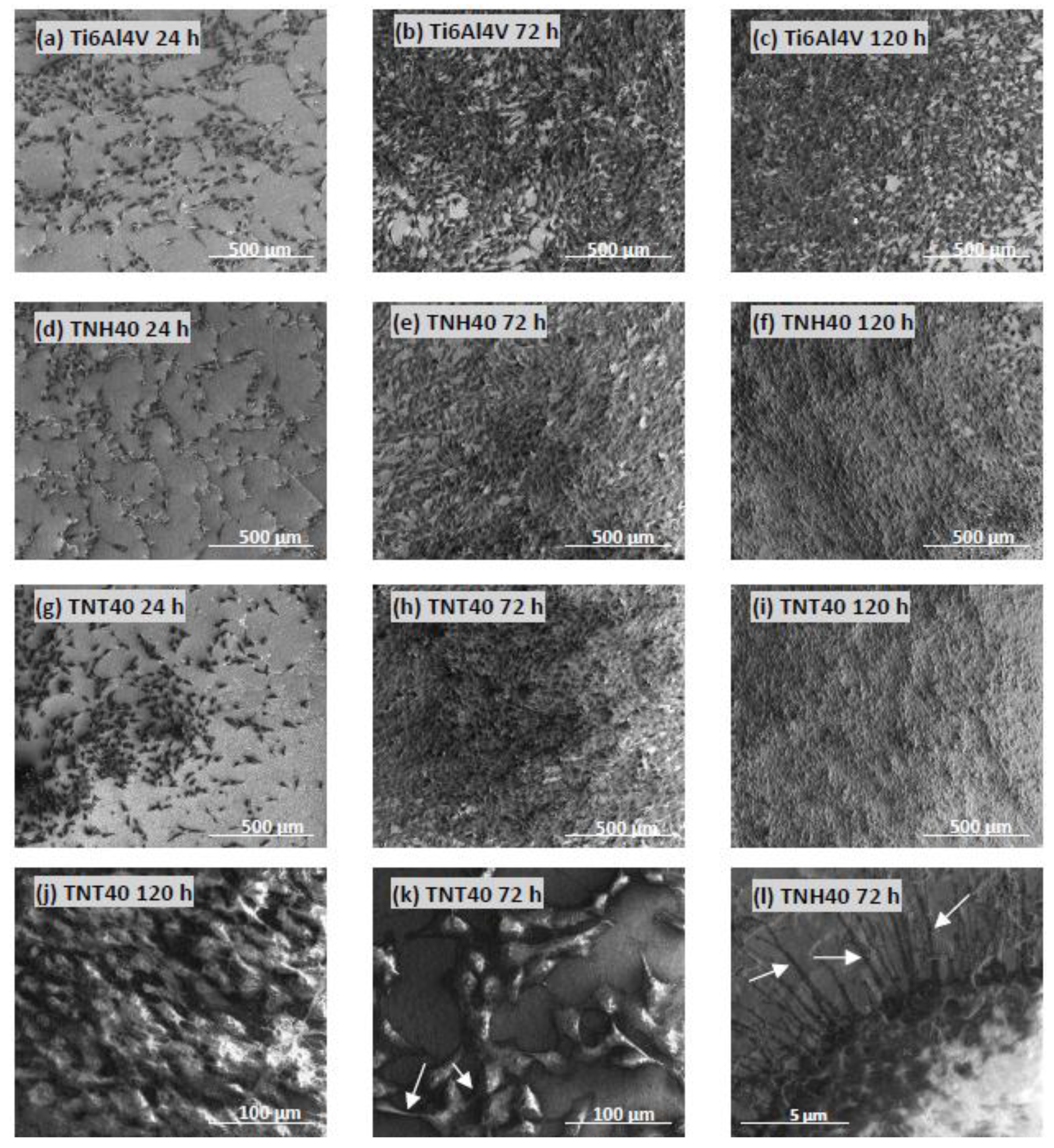
3.1. Synthesis of Nanotubular Coatings and Analysis of Their Surface Morphology on Different Steps of Experimental Procedure
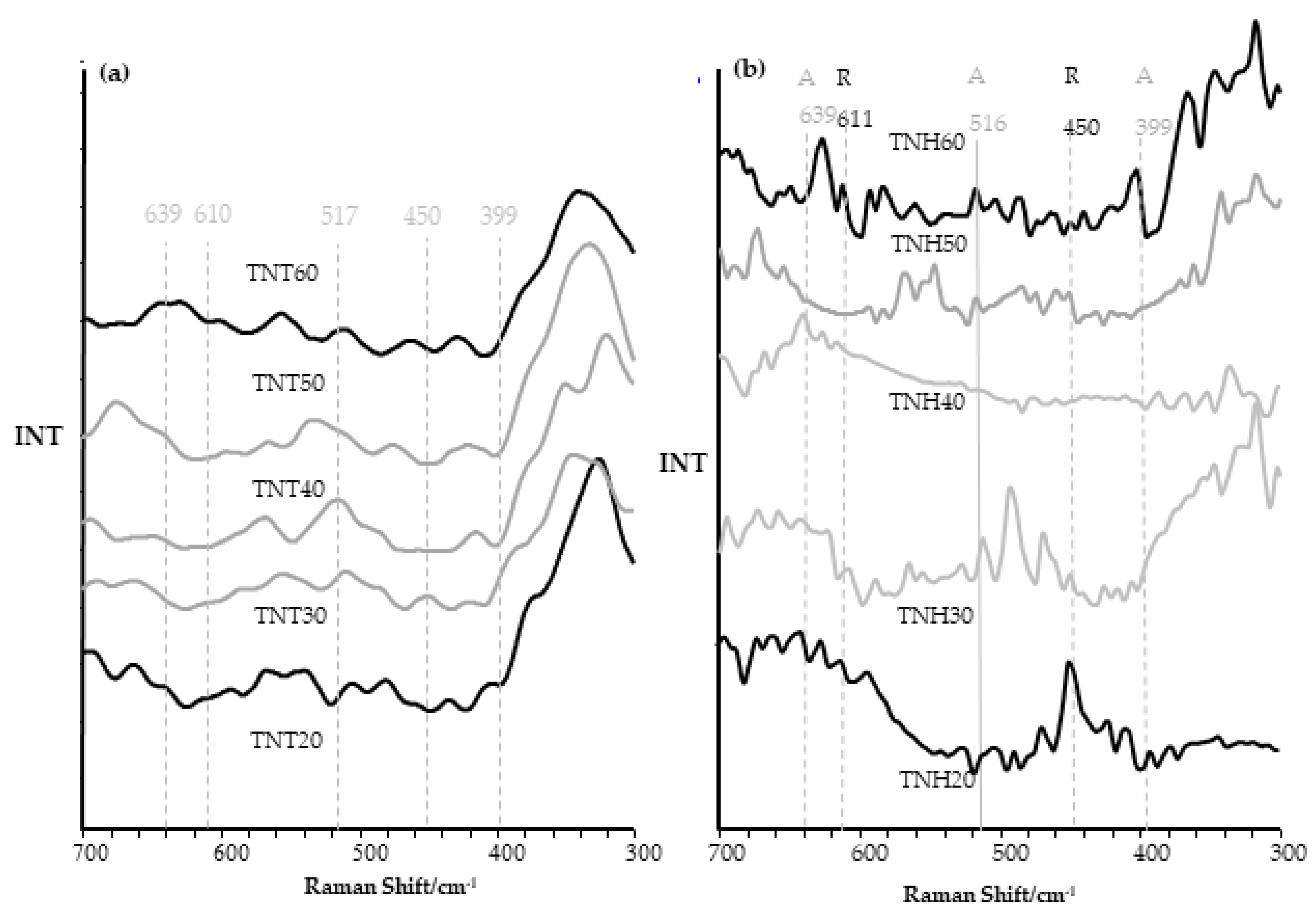
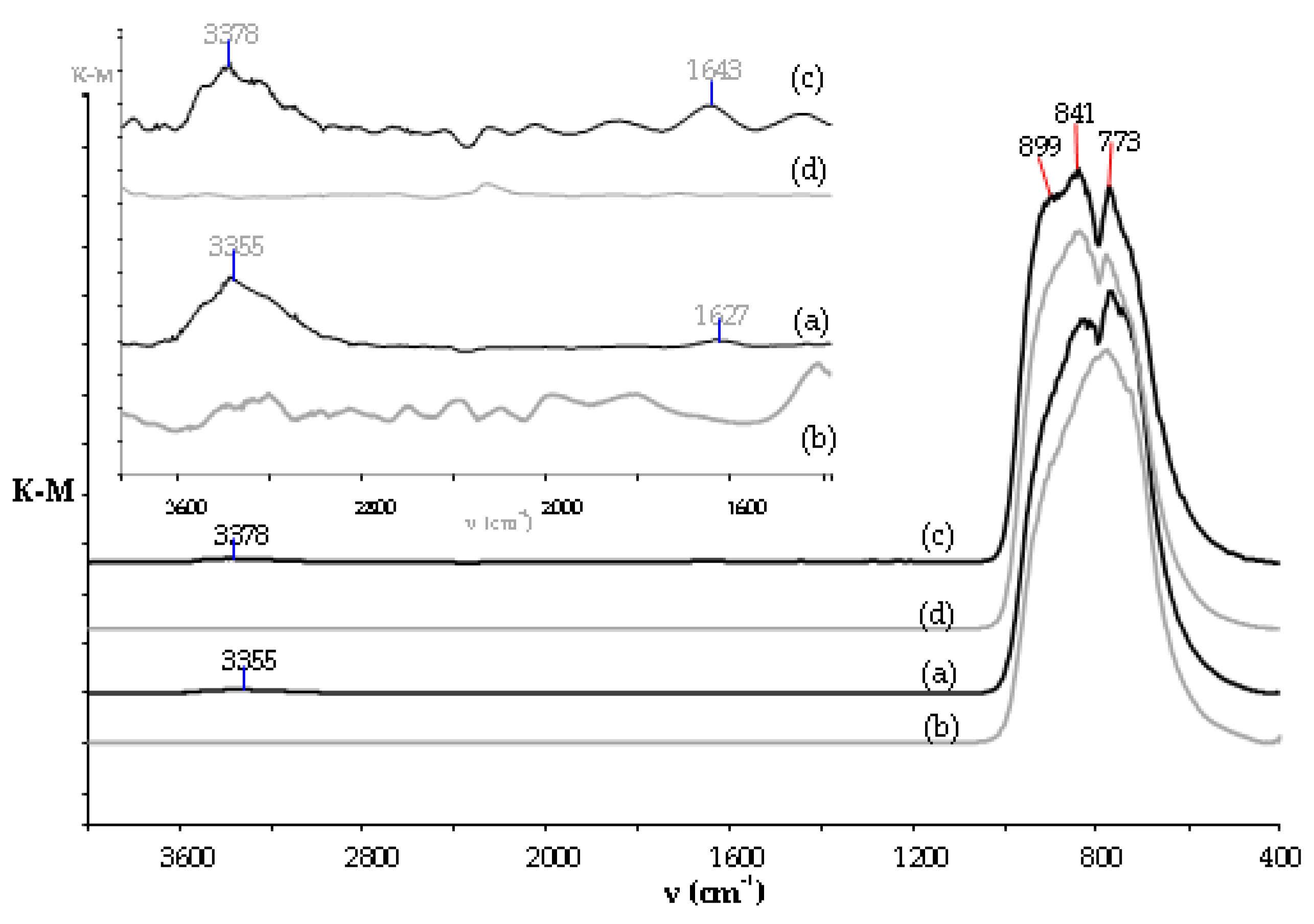
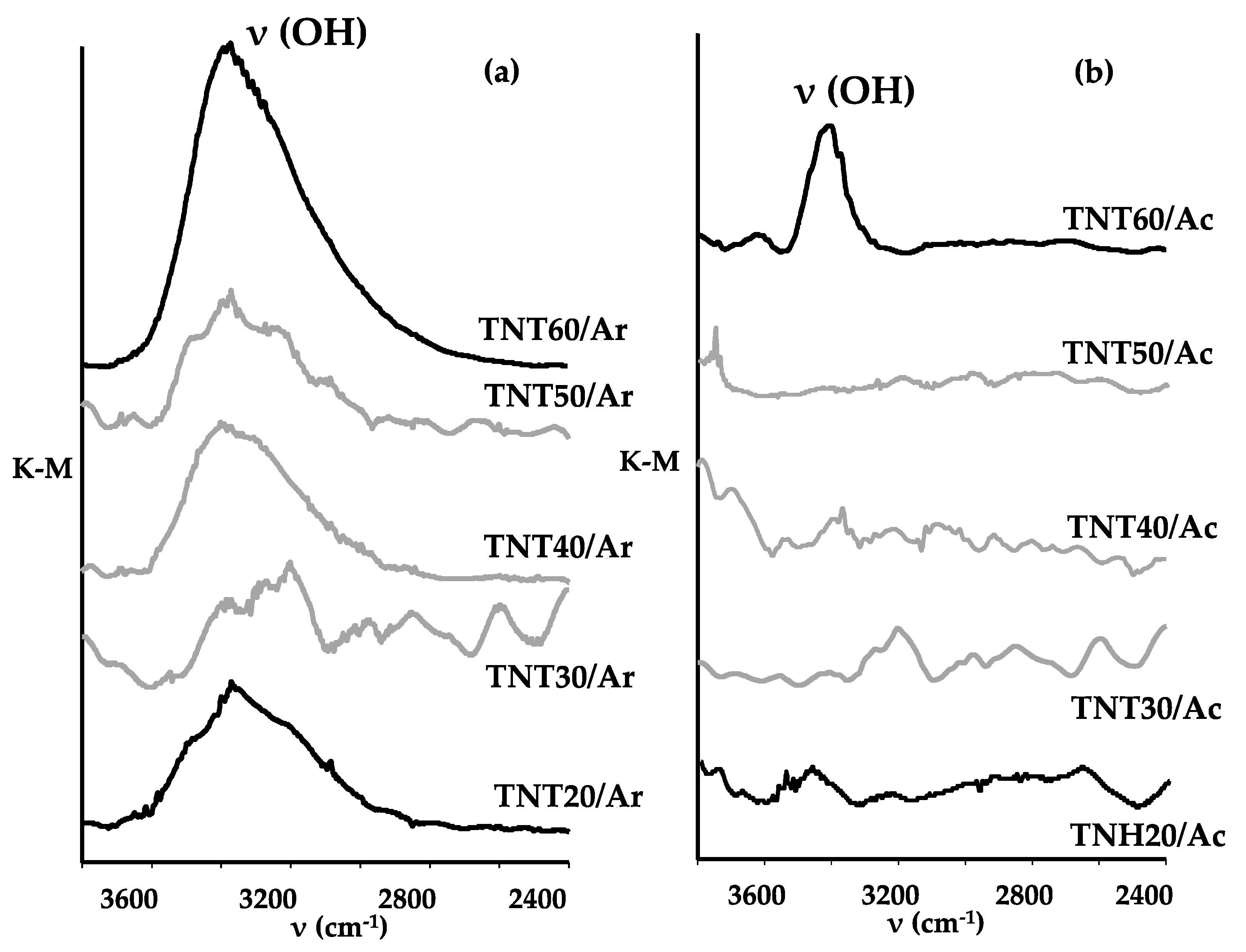
3.2. Structural Studies on TNH20-60 and TNT20-60 Coatings and Their Wettability Analysis
3.3. Topography and Mechanical Properties of Ti6Al4V/TNH20-60 and Ti6Al4V/TNT20-60 Samples
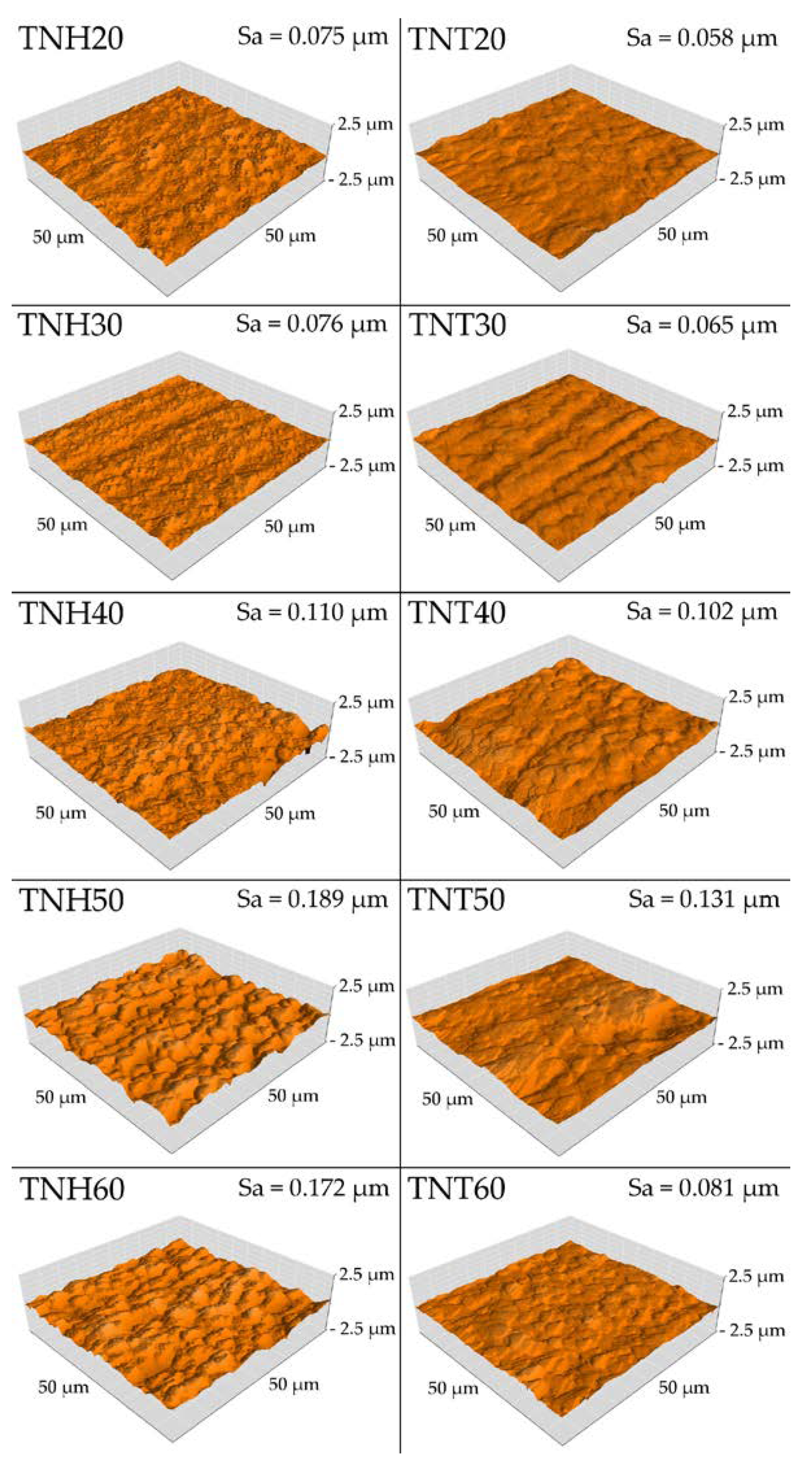
3.3.1. Surface Topography
3.3.2. Mechanical Properties (Hardness and Young’s Modulus) of Ti6Al4V/TNH20-60 and Ti6Al4V/TNT20-60 Systems
3.3.3. Adhesion Properties
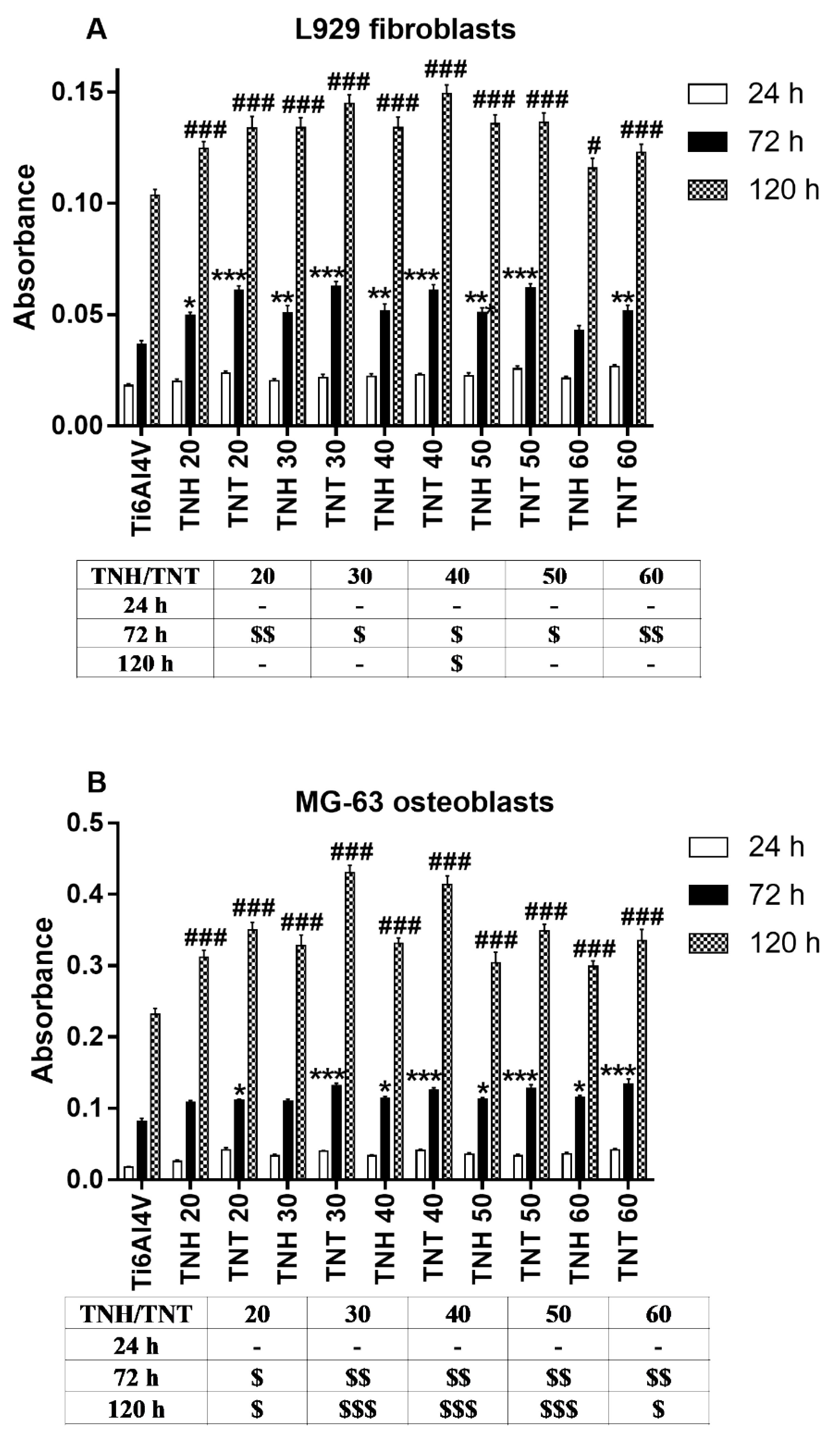
3.4. Cell Proliferation Detected by MTT Assay
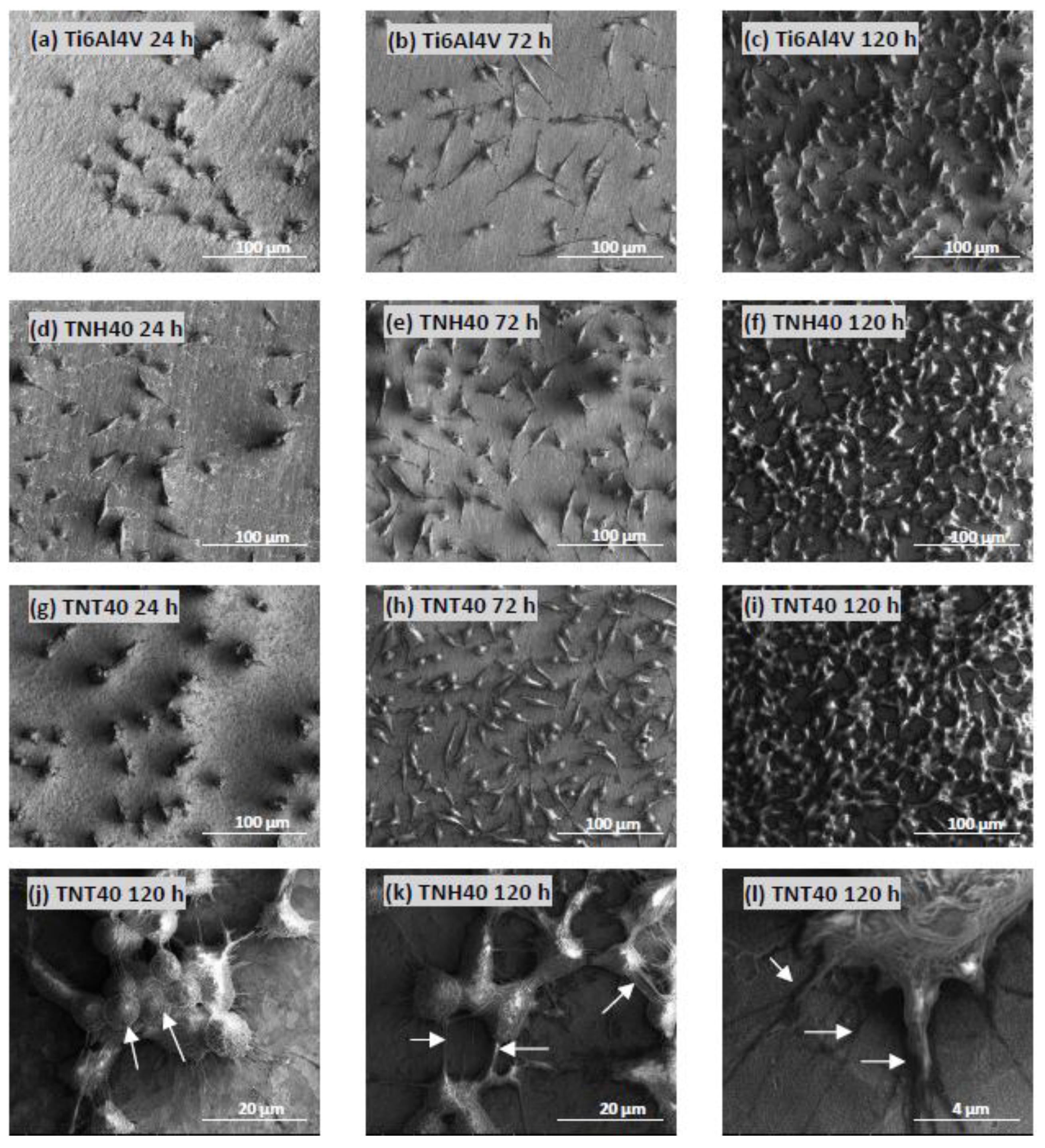
3.5. Cell Morphology Observed by Scanning Electron Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.; Su, B.; Dalby, M.J. Osteogenic and bactericidal surfaces from hydrothermal titania nanowires on titanium substrates. Sci. Rep. 2016, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial surface modification of titanium implants in orthopaedics. J. Tissue Eng. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.; Hooper, G.; Frampton, C.; Rothwell, A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J. Orthop. 2014, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, S.; Zhu, Y.; Zhong, M.; Lin, X.; Zhang, Y.; Tan, G.; Li, M.; Yin, Z.; Yu, P.; et al. Ti nanorod arrays with a medium density significantly promote osteogenesis and osteointegration. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawa, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, J.; Du, L.Q.; Jia, L.; Liu, H.; Ge, S. TiO2 nanorod arrays modified Ti substrates promote the adhesion, proliferation and osteogenic differentiation of human periodontal ligament stem cells. Mater. Sci. Eng. C 2017, 76, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Peng, R.; Ding, J. Cell-material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013, 25, 5257–5286. [Google Scholar] [CrossRef] [PubMed]
- Klokkevold, P.; Nishimura, R.D.; Adachi, M.; Caputo, A. Osseointegration enhanced by chemical etching of the titanium surface. A torque removal study in the rabbit. Clin. Oral Implant. Res. 1997, 8, 442–447. [Google Scholar] [CrossRef]
- Bächle, M.; Kohal, R.J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin. Oral Implant. Res. 2004, 15, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Piszczek, P. Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces. Nanomaterials 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Qin, J.; Yang, D.; Maher, S.; Lima-Marques, L.; Zhou, Y.; Chen, Y.; Atkins, G.J.; Losic, D. Micro- and Nano-structured 3D Printed Titanium Implants with Hydroxyapatite Coating for Improved Osseointegration. J. Mater. Chem. B 2018, 6, 3136–3144. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Radtke, A.; Bal, M.; Jędrzejewski, T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials 2018, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Yu, B.; Zhou, F.; Liu, W. TiO2 Nanotubes with Tunable Morphology, Diameter and Length: Synthesis and Photo-Elecrtical/Catalytic Performance. Chem. Mater. 2009, 21, 1198–1206. [Google Scholar] [CrossRef]
- Nyein, N.; Tanc, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. TiO2 nanotube arrays formation in fluoride/ethylene glycol electrolyte containing LiOH or KOH as photoanode for dye-sensitized solar cell. J. Photochem. Photobiol. A Chem. 2017, 343, 33–39. [Google Scholar] [CrossRef]
- Tai, M.A.; Razak, K.A.; Jaafar, M.; Lockma, Z. Initial growth study of TiO2 nanotube arrays anodised in KOH/fluoride/ethylene glycol electrolyte. Mater. Des. 2017, 128, 195–205. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Tavoosi, M.; Mozafarinia, R.; Ghasemi, A.; Doostmohammadi, A. Preparation and characterization of TiO2 nanotube arrays on Ti6Al4V surface for enhancement of cell treatment. Surf. Coat. Technol. 2017, 321, 409–415. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; Heyde, H.V.; Jin, S. Improved bone forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell–materials interaction. J. Biomed. Mater. Res. A 2009, 90, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Nielsen, L.P.; Piszczek, P. The bioactivity and photocatalytic properties of titania nanotube coatings produced with the use of the low-potential anodization of Ti6Al4V alloy surface. Nanomaterials 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Meia, S.; Wanga, W.; Chub, P.K.; Wua, Z.; Zhanga, Y. The role of sterilization in the cytocompatibility of titania nanotubes. Biomaterials 2010, 31, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of Using Sterilization by Gamma Radiation: The Other Side of the Coin. Int. J. Med. Sci. 2018, 15, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintani, H.; Sakudo, A.; Burke, P.; McDonnell, G. Gas plasma sterilization of microorganisms and mechanisms of action. Exp. Ther. Med. 2010, 1, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravikumar, M.; Hageman, D.J.; Tomaszewski, W.H.; Chandra, G.M.; Skousen, J.L.; Capadona, J.R. The effect of residual endotoxin contamination on the neuroinflammatory response to sterilized intracortical microelectrodes. J. Mater. Chem. B 2014, 2, 2517–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecker, M.; Danda, V.; Shoffstall, A.J.; Mahmood, S.F.; Joshi-Imre, A.; Frewin, C.L.; Voit, W.E. Sterilization of Thiol-ene/Acrylate Based Shape Memory Polymers for Biomedical Applications. Macromol. Mater. Eng. 2017, 302, 1600331. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Chapter 1 Contact Angle and Wetting Properties. In Surface Science Techniques, Springer Series in Surface Sciences; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The Evaluation of the Impact of Titania Nanotube Covers Morphology and Crystal Phase on Their Biological Properties. J. Mater. Sci. Mater. Med. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal. Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Radtke, A.T.; Jędrzejewski, W.; Kozak, B.; Sadowska, M.; Więckowska-Szakiel, E.; Talik, M.; Mäkelä, M.; Leskelä, P. Optimization of the silver clusters PEALD process on the surface of 1-D titania coatings. Nanomaterials 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Depositiom. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Albu, S.P.; Lee, K.; So, S.; Schmuki, P. Water annealing and other low temperature treatments of anodic TiO2 nanotubes: A comparison of properties and efficiencies in dye sensitized solar cells and for water splitting. Electrochim. Acta 2012, 82, 98–102. [Google Scholar] [CrossRef]
- Junkar, I.; Kulkarni, M.; Drašer, B.; Rugelj, N.; Mazare, A.; Flašker, A.; Drobne, D.; Humpoliček, P.; Resnik, M.; Schmuki, P.; et al. Influence of various sterilization procedures on TiO2 nanotubes used for bimedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, A.; Chidoni, A.; Shahzad, N.; Bianco, S.; Quaglio, M.; Pirri, C.F. Ultrafast room-temperature crystallization of TiO2 Nanotubes exploiting water-vapor treatment. Sci. Rep. 2015, 5, 7808. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Que, W.; Zhong, P.; Zhang, J.; He, Y. A facile method to crystallize amorphous anodized TiO2 nanotubes at low temperature. Acs Appl. Mater. Interfaces 2011, 3, 2800–2804. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Zahang, T.; Zhai, J.; Jiang, L. Low-temperature crystallization of anodized TiO2 nanotubes at the solid-gas interface and their photoelectrochemical properties. Nanoscale 2013, 5, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Weng, J.; Yang, B.C.; Qu, S.X.; Zhang, X.D. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials 2003, 24, 4663–4670. [Google Scholar] [CrossRef]
- Goodarzi, S.; Moztarzadeh, F.; Nezafati, N.; Omidvar, H. Titanium dioxide nanotube arrays: A novel approach into periodontal tissue regeneration on the surface of titanium implants. Adv. Mater. Lett. 2016, 7, 209–215. [Google Scholar] [CrossRef]
- Bezerra, H.; Inês, M.; Bernardi, B.; Maria, T.; Carlos, A.; Nara, A.; Rastelli, D.S. Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti-biofilm filler content for composite resins. Dent. Mater. 2018, 35, 36–46. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dikici, T.; Toparli, M. Microstructure and mechanical properties of nanostructured and microstructured TiO2 films. Mater. Sci. Eng. A 2016, 661, 19–24. [Google Scholar] [CrossRef]
- Munirathinam, B.; Neelakantan, L. Role of crystallinity on the nanomechanical and electrochemical properties of TiO2 nanotubes. J. Electroanal. Chem. 2016, 770, 73–83. [Google Scholar] [CrossRef]
- Rayón, E.; Bonache, V.; Salvador, M.D.; Bannier, E.; Sánchez, E.; Denoirjean, A.; Ageorges, H. Nanoindentation study of the mechanical and damage behaviour of suspension plasma sprayed TiO2 coatings. Surf. Coat. Technol. 2012, 206, 2655–2660. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, V.P.; Tyurin, A.I.; Loza, K.; Epple, M.; Surmenev, R.A. Hybrid biocomposites based on titania nanotubes and a hydroxyapatite coating deposited by RF-magnetron sputtering: Surface topography, structure, and mechanical properties. Appl. Surf. Sci. 2017, 426, 229–237. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Ossowska, A.; Beutner, R.; Scharnweber, D.; Zielinski, A. Properties of composite oxide layers on the Ti13Nb13Zr alloy. Surf. Eng. 2017, 33, 841–848. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Iventh Cedillo-Gonzalez, E.; Montorosi, M.; Mugoni, C.; Montorosi, M.; Siligardi, C. Improvement of the adhesion between TiO2 nanofilm and glass substrate by roughness modifications. Phys. Procedia 2013, 40, 19–29. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Lengner, C.J.; Steinman, H.A.; Gagnon, J.; Smith, T.W.; Henderson, J.E.; Kream, B.E.; Stein, G.S.; Lian, J.B.; Jones, S.N. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J. Cell. Biol. 2006, 172, 909–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, M.; Pripatnanont, P.; Suttapreyasri, S.; Monmaturapoj, N. In vitro biocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 52–61. [Google Scholar] [CrossRef] [PubMed]


| Biomaterial Sample | Hardness (GPa) | Young’s Modulus (GPa) | Maximum Depth of Indentation (nm) |
|---|---|---|---|
| Ti6Al4V | 16.17 ± 3.61 | 269.74 ± 40.10 | 162.14 ± 14.95 |
| TNH20 | 10.24 ± 2.59 | 293.01 ± 59.43 | 194.40 ± 24.46 |
| TNH30 | 9.15 ± 3.19 | 258.82 ± 57.44 | 212.80 ± 46.42 |
| TNH40 | 4.95 ± 2.78 | 213.74 ± 87.35 | 323.33 ± 141.30 |
| TNH50 | 4.43 ± 2.00 | 192.45 ± 56.42 | 312.17 ± 78.23 |
| TNH60 | 6.51 ± 2.80 | 214.97 ± 52.72 | 258.13 ± 68.94 |
| TNT20 | 19.38 ± 6.13 | 462.76 ± 245.91 | 143.99 ± 22.26 |
| TNT30 | 16.81 ± 5.80 | 370.23 ± 109.44 | 160.08 ± 38.65 |
| TNT40 | 9.42 ± 4.12 | 269.16 ± 79.77 | 212.39 ± 42.11 |
| TNT50 | 9.56 ± 5.12 | 269.14 ± 91.83 | 217.36 ± 51.49 |
| TNT60 | 14.32 ± 4.29 | 320.72 ± 77.26 | 169.28 ± 26.80 |
| Nanoscratch-Test Properties | ||
|---|---|---|
| Coating | Critical Load (mN) | Critical Friction (mN) |
| TNH20 | 234.86 ± 53.53 | 266.87 ± 59.73 |
| TNH30 | 254.14 ± 53.89 | 284.31 ± 73.77 |
| TNH40 | 293.23 ± 54.71 | 355.05 ± 73.27 |
| TNH50 | 268.78 ±83.19 | 316.54 ± 98.03 |
| TNH60 | 241.61 ± 68.00 | 246.25 ± 84.18 |
| TNT20 | 286.51 ± 77.35 | 307.92 ± 90.38 |
| TNT30 | 336.65 ± 41.21 | 397.86 ± 79.63 |
| TNT40 | 379.08 ± 46.38 | 417.66 ± 68.00 |
| TNT50 | 353.01 ± 12.82 | 388.39 ± 17.87 |
| TNT60 | 271.52 ± 46.79 | 311.04 ± 66.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Bartmański, M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. J. Clin. Med. 2019, 8, 272. https://doi.org/10.3390/jcm8020272
Radtke A, Ehlert M, Jędrzejewski T, Bartmański M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. Journal of Clinical Medicine. 2019; 8(2):272. https://doi.org/10.3390/jcm8020272
Chicago/Turabian StyleRadtke, Aleksandra, Michalina Ehlert, Tomasz Jędrzejewski, and Michał Bartmański. 2019. "The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process" Journal of Clinical Medicine 8, no. 2: 272. https://doi.org/10.3390/jcm8020272
APA StyleRadtke, A., Ehlert, M., Jędrzejewski, T., & Bartmański, M. (2019). The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. Journal of Clinical Medicine, 8(2), 272. https://doi.org/10.3390/jcm8020272