“To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Chemical Vapor Deposition of Silver Nanoparticles (AgNPs)
2.3. The Wettability and Surface Free Energy of Biomaterials
2.4. Silver Ion Release in the Body Fluid Environment
2.5. Biointegration Studies
2.5.1. Cell Culture
2.5.2. Cell Adhesion and Proliferation Detected by the MTT Assay
2.5.3. Cell Morphology Evaluated by Scanning Electron Microscopy
2.5.4. Statistical Analysis in the MTT Assay
2.6. The Evaluation of the Antibacterial Properties of the Ti6Al4V/AgNPs and Ti6Al4V/TNT5/AgNPs Samples
3. Results
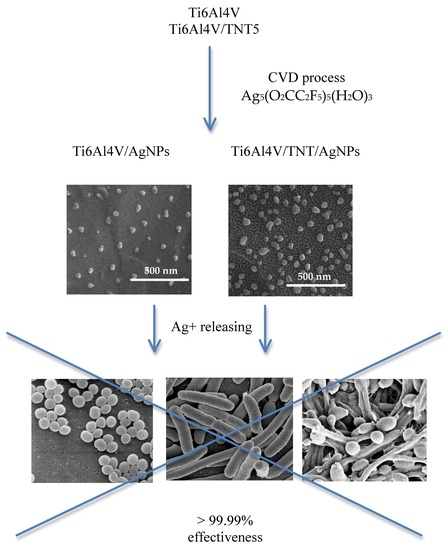
3.1. The Fabrication of Ti6Al4V/AgNPs and Ti6Al4V/TNT5/AgNPs Systems
3.2. Surface Morphology and Topography
3.3. Wettability and Surface Free Energy of Biomaterials
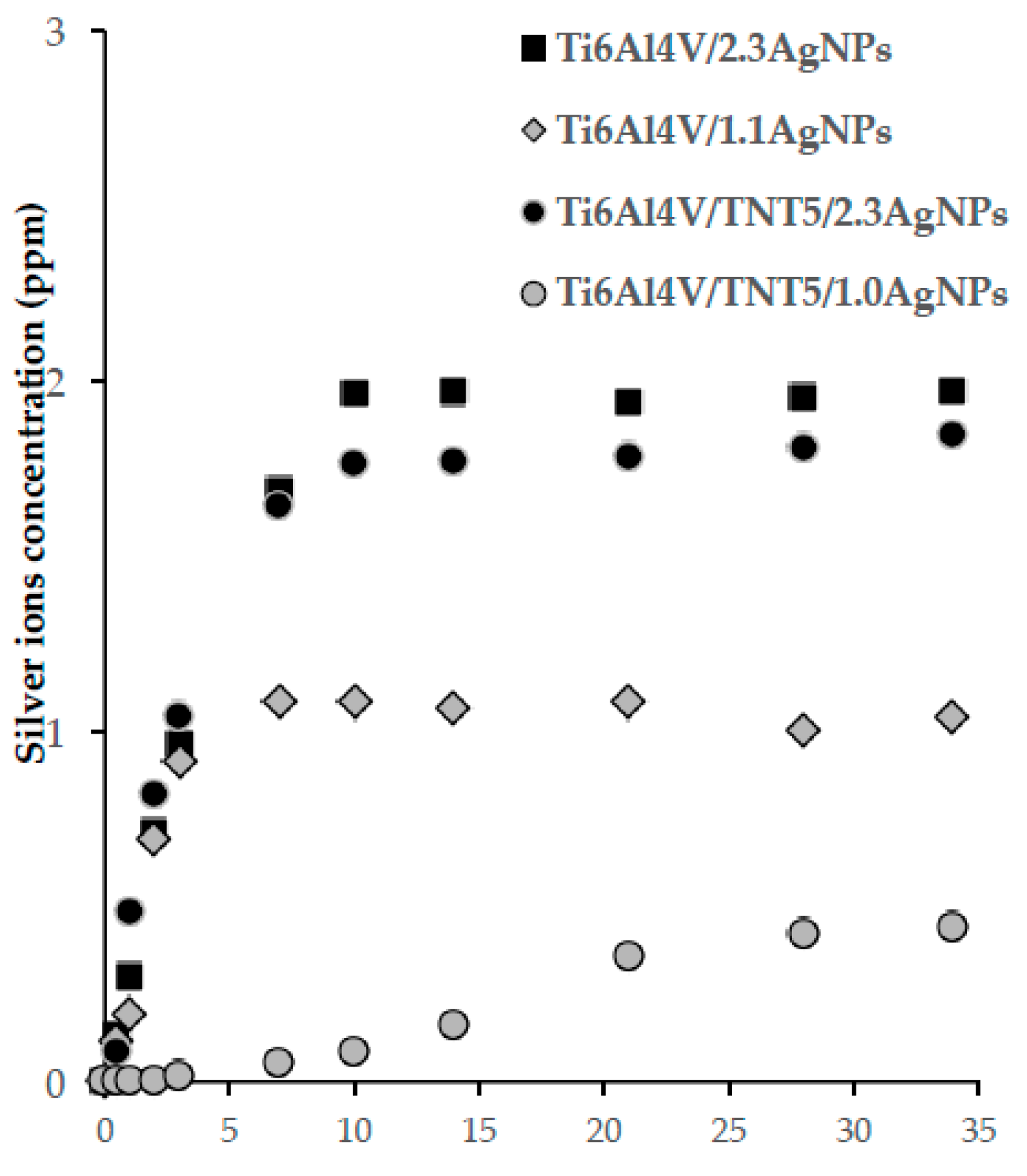
3.4. Silver Ion Release in the Body Fluid Environment
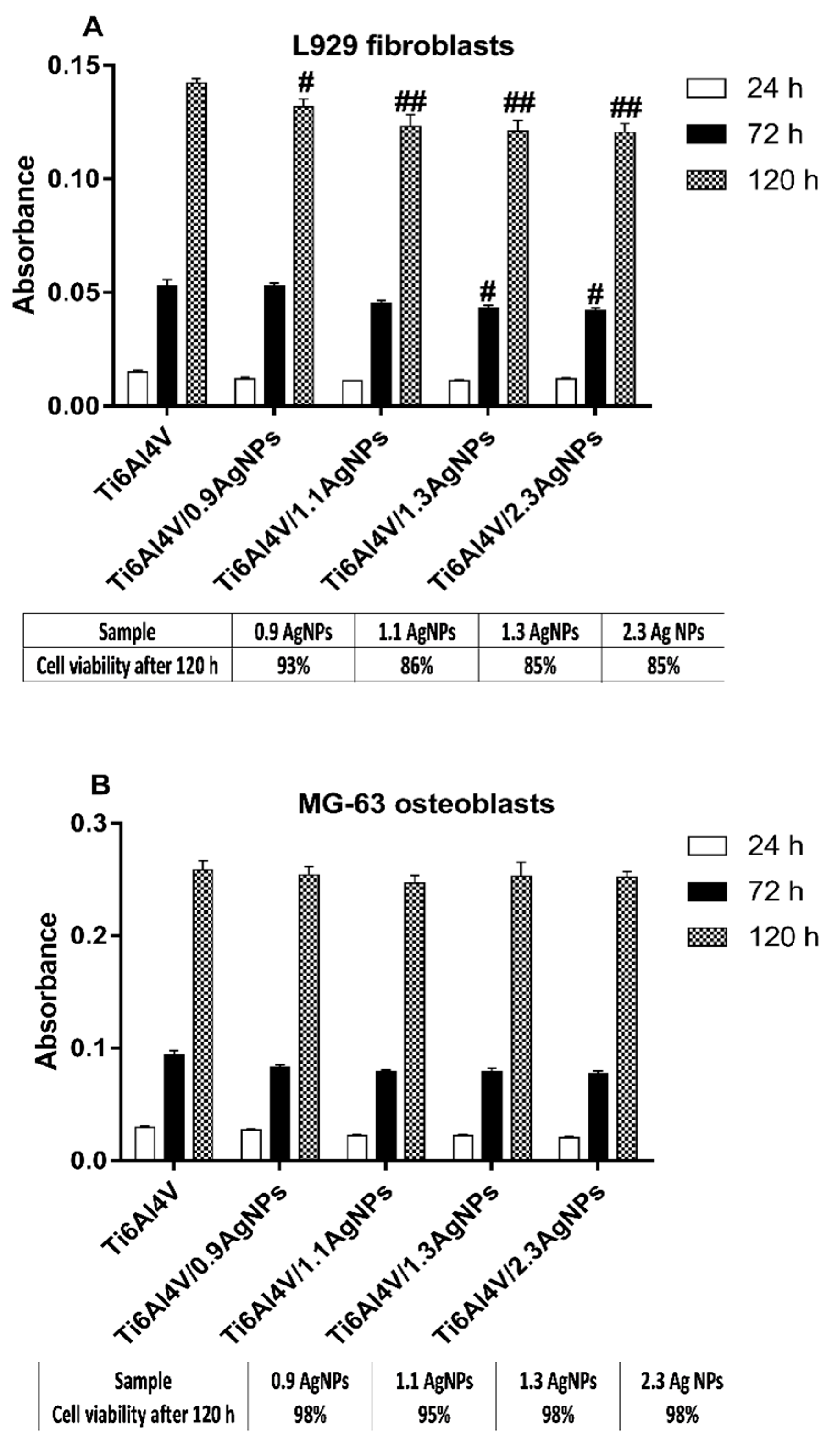
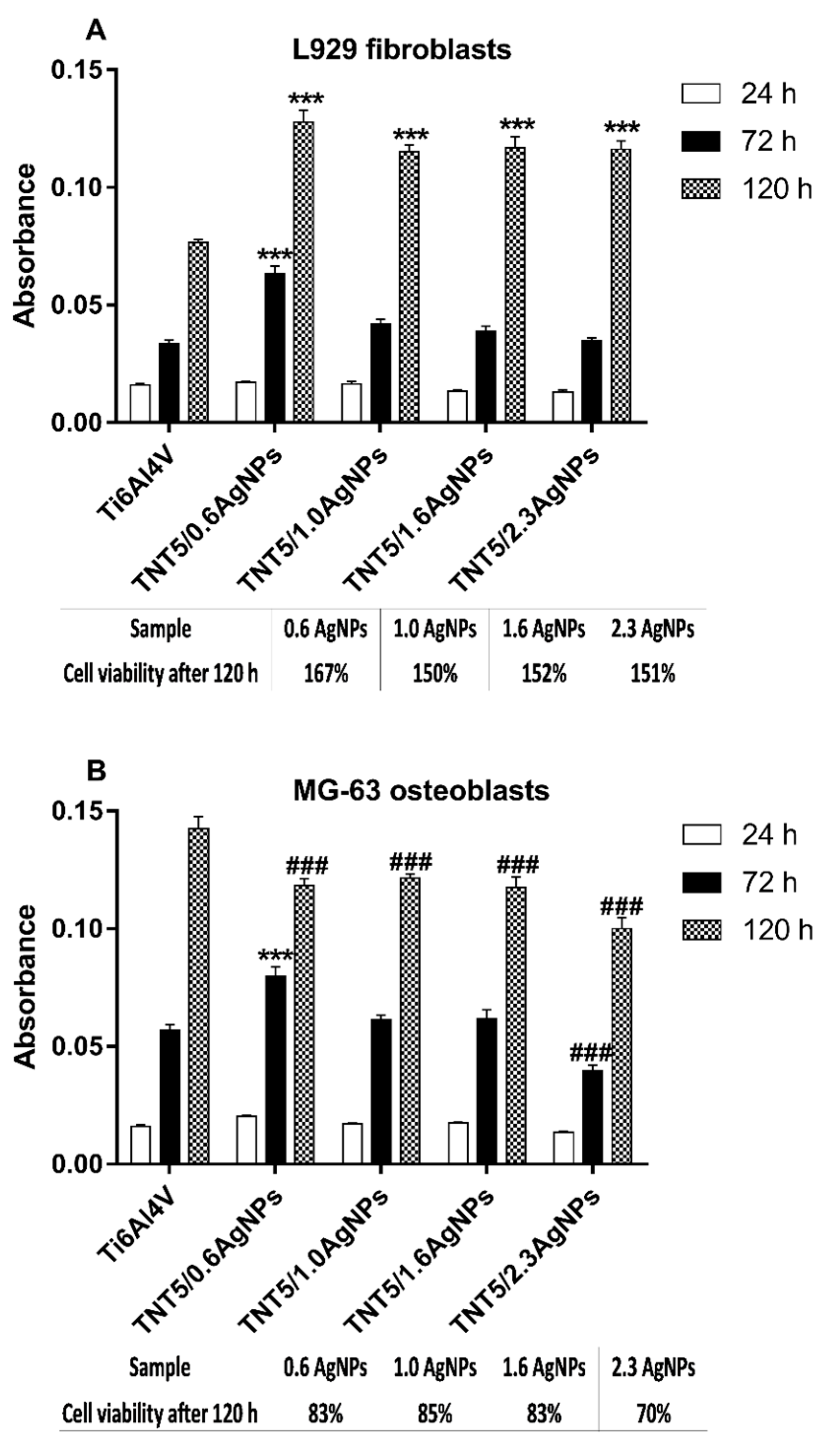
3.5. The Evaluation of the Biocompatibility of the Produced Titanium Alloy-Based Materials
3.6. Antimicrobial Activity of Silver-Coated Titanium Alloys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murr, L.E. Nanoparticulate materials in antiquity: The good, the bad and the ugly. Mater. Charact. 2009, 60, 261–270. [Google Scholar] [CrossRef]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar]
- Aleksander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Lemiere, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Solanki, J.N.; Murthy, Z.V.P. Controlled Size Silver Nanoparticles Synthesis with Water-in-Oil Microemulsion Method: A Topical Review. Ind. Eng. Chem. Res. 2011, 50, 12311–12323. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; De Conti, R.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef] [Green Version]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Young, X.; Yujie, X.; Byung Kwon, L.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics, Nanostructure. Angew. Chem. Int. Ed. 2009, 60–103. [Google Scholar]
- Abdeen, S.; Geo, S.; Sukanya, S.; Praseetha, K.P.; Dhanya, R.P. Biosynthesis of Silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155–162. [Google Scholar]
- Leśniak, W.; Bielińska, A.U.; Sun, K.; Janczak, K.W.; Shi, X.; Baker, J.R.; Balogh, L.P. Silver/dendrimer nanocomposites as biomarkers: Fabrication, characterization, in vitro toxicity, and intracellular detection. Nano Lett. 2005, 5, 2123–2130. [Google Scholar]
- Kobayashi, Y.; Katakami, H.; Mine, E.; Nagao, D.; Konno, M.; Liz-Marzan, L.M. Silica coating of silver nanoparticles using a modified Stober method. J. Colloid Interface Sci. 2005, 283, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2009, 12, 1531–1551. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Z.; Deng, B. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AshaRani, P.V.; Hande, M.P.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, X.; Wang, M.; Ran, D.; Xu, W.; Yang, J. Study on the inter-action between silver nanoparticles and nucleic acids in the presence of cetyltrimethylammonium bromide and its analytical application. Talanta 2008, 74, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxico. In Vitro 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Poortman, J.; Peters, R.J.; Wijma, E.; Kramer, E.; Makama, S.; Puspitaninganindita, K.; Marvin, H.J.P.; Peijnenburg, A.A.C.M.; Hendriksen, P.J.M. Characterization of Translocation of Silver Nanoparticles and Effects on Whole-Genome Gene Expression Using an In Vitro Intestinal Epithelium Coculture Model. ACS Nano 2011, 5, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Bligh, M.W.; Waite, T.D. Effects of aggregate structure on the dissolution kinetics of citrate-stabilized silver nanoparticles. Environ. Sci. Technol. 2013, 47, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Rigo, C.; Stocchero, M.; Vindigni, V.; Cairns, W.; Zavan, B. Silver nanoparticles and mitochondrial interaction. Int. J. Dent. 2013, 2013, 312747. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.A.; Muhsen, M.F. Ceramic Filters Impregnated with Silver Nanoparticles for Household Drinking Water Treatment. Am. J. Mater. Sci. 2017, 6, 232–239. [Google Scholar]
- Raghavendra, G.M.; Jayaramudu, T.; Varaprasad, K.; Sadiku, R.; Ray, S.S.; Rajua, K.M. Cellulose–polymer–Ag nanocomposite fibers for antibacterial fabrics/skin scaffolds. Carbohydr. Polym. 2013, 93, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jedrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Talik, E.; Mäkelä, M.; Leskelä, M.; Piszczek, P. Optimization of the Silver Nanoparticles PEALD Process on the Surface of 1-D Titania Coatings. Nanomaterials 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmanski, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 962. [Google Scholar] [CrossRef] [PubMed]
- Szłyk, E.; Piszczek, P.; Grodzicki, A.; Chaberski, M.; Goliński, A.; Szatkowski, J.; Błaszczyk, T. CVD of AgI Complexes with Tertiary Phosphines and Perfluorinated Carboxylates—A New Class of Silver Precursors. Chem. Vap. Depos. 2001, 7, 111–116. [Google Scholar] [CrossRef]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Nielsen, L.P.; Piszczek, P. The Bioactivity and Photocatalytic Properties of Titania Nanotube. Nanomaterials 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Burmester, A.; Luthringer, B.; Willumeit, R.; Feyerabend, F. Comparison of the reaction of bone-derived cells to enhanced MgCl2-salt concentrations. Biomatter 2014, 4, e967616. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Madras, G.; Basu, B. In vitro/In vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Pripatnanont, P.; Suttapreyasri, S.; Monmaturapoj, N. In vitro biocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.; Hofmann, M.C. In vitro cytotoxicity of nanoparticles in mammalian germ line stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrucke, P.; Wagener, M.; Seidel, P. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.Y.; Liu, C.P.; Huang, H.H.; Lee, S.W. Both enhanced biocompatibility and antibacterial activity in Ag-decorated TiO2 nanotubes. PLoS ONE 2013, 8, e75364. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Simchi, A.; Bagheri, R. Size tuning of Ag-decorated TiO2 nanotube arrays for improved bactericidal capacity of orthopedic implants. J. Biomed. Mater. Res. 2014, 102, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-1:2018 Standards. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Proces. Available online: https://www.iso.org/standard/68936.html (accessed on 10 January 2019).
- Kheur, S.; Singh, N.; Bodas, D.; Rauch, J.Y.; Jambhekar, S.; Kheur, M.; Rajwade, J. Nanoscale silver depositions inhibit microbial colonization and improve biocompatibility of titanium abutments. Colloids Surf. B Biointerfaces 2017, 159, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Controll. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, X.; Wang, X.; Qin, L. Review of antibacterial activity of titanium-based implants surfaces fabricated by micro-arc oxidation. Coatings 2017, 7, 45. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Li, W. Antibacterial TiO2 coating incorporating silver nanoparticles by microarc oxidation and ion implantation. J. Nanomater. 2013, 2013, 8. [Google Scholar]
- De Giglio, E.; Cometa, S.; Ricci, M.A.; Cafagna, D.; Savino, A.M.; Sabbatini, L.; Orciani, M.; Ceci, E.; Novello, L.; Tantillo, G.M.; et al. Ciprofloxacin modified electrosynthesized hydrogel coatings to prevent titanium-implant-associated infections. Acta Biomater. 2011, 7, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, O.; Stone, W.; Schemitsch, E.H.; Zalzal, P.; Waldman, S.; Papini, M.; Towler, M.R. Titanium addition influences antibacterial activity of bioactive glass coatings on metallic implants. Heliyon 2017, 3, e00420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, L.J.; Camacho, A.; Holt, K.; Kouri, B.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome. Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evolution of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar]
- Chauhan, R.; Kumar, A.; Abraham, J. A Biological approach to the synthesis of silver nanoparticles with Streptomyces sp. JAR1 and its antimicrobial activity. Sci. Pharm. 2013, 81, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver–nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Li, J.; Rong, K.; Zhao, H.; Li, F.; Lu, Z.; Chen, R. Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013, 13, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.O.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. Part A 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. Biol. Med. Res. Int. 2013, 1–9. [Google Scholar]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Wypij, M.; Czarnecka, J.; Swiecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 2. [Google Scholar] [CrossRef] [PubMed]








| Precursor | Ag5(O2CC2F5)5(H2O)3 |
|---|---|
| Precursor weight | 5, 10, 20, 50 |
| Vaporization temperature (TV) | 230 |
| Carrier gas | Ar |
| Total reactor pressure (p) | 3.0 |
| Flow of the precursor vapors above the substrate | 0.2–1.7 |
| Substrate temperature (TD) | 290 |
| Substrates | Ti6Al4V and Ti6Al4V/TNT5 |
| Deposition time | 30 |
| Ti6Al4V/AgNPs | Ti6Al4V/TNT5/AgNPs | |||
|---|---|---|---|---|
| Precursor Mass (mg) | wt% | d (nm) | wt% | d (nm) |
| 5 | 0.9 | 18 ± 8 | 0.6 | 38 ± 14 |
| 10 | 1.1 | 45 ± 15 | 1.0 | 43 ± 10 |
| 20 | 1.3 | 68 ± 32 | 1.6 | 57 ± 24 |
| 50 | 2.3 | 53 ± 18 | 2.3 | 115 ± 49 |
| Microorganisms | |||||
|---|---|---|---|---|---|
| Ti Alloys | E. coli ATCC8739 | E. coli ATCC25922 | S. aureus ATCC6538 | S. aureus ATCC25923 | C. albicans ATCC10231 |
| Ti6Al4V | - | - | - | - | - |
| Ti6Al4V/0.9 AgNPs | 99.57 | >99.99 | >99.99 | 99.93 | 99.96 |
| Ti6Al4V/1.1 AgNPs | 99.97 | 99.99 | >99.99 | 99.98 | 99.97 |
| Ti6Al4V/1.3 AgNPs | 99.94 | 99.80 | 99.93 | 99.96 | 99.67 |
| Ti6Al4V/2.3 AgNPs | 99.96 | 99.83 | 99.99 | 99.85 | 99.93 |
| Ti6Al4V/TNT5 | - | - | - | - | - |
| Ti6Al4V/TNT5/0.6 AgNPs | 99.90 | 99.94 | 99.94 | 99.99 | >99.99 |
| Ti6Al4V/TNT5/1.0 AgNPs | >99.99 | >99.99 | 99.61 | >99.99 | >99.99 |
| Ti6Al4V/TNT5/1.6 AgNPs | 99.95 | 99.90 | 99.46 | >99.99 | 99.99 |
| Ti6Al4V/TNT5/2.3 AgNPs | 99.70 | 99.99 | 99.95 | 99.71 | >99.99 |
| Microorganisms | |||||
|---|---|---|---|---|---|
| Ti Alloys | E. coli ATCC8739 | E. coli ATCC25922 | S. aureus ATCC6538 | S. aureus ATCC25923 | C. albicans ATCC10231 |
| Ti6Al4V | - | - | - | - | - |
| Ti6Al4V/0.9 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/1.1 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/1.3 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/2.3 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/TNT5 | - | - | - | - | - |
| Ti6Al4V/TNT5/0.6 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/TNT5/1.0 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/TNT5/1.6 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/TNT5/2.3 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Microorganisms | |||||
|---|---|---|---|---|---|
| Ti Alloys | E. coli ATCC8739 | E. coli ATCC25922 | S. aureus ATCC6538 | S. aureus ATCC25923 | C. albicans ATCC10231 |
| Ti6Al4V | - | - | - | - | - |
| Ti6Al4V/0.9 AgNPs | 99.94 | >99.99 | 99.78 | 99.58 | >99.99 |
| Ti6Al4V/1.1 AgNPs | 99.84 | 99.98 | 99.91 | 99.49 | >99.99 |
| Ti6Al4V/1.3 AgNPs | 99.91 | 99.98 | 99.84 | 99.87 | >99.67 |
| Ti6Al4V/2.3 AgNPs | 99.89 | 99.83 | 99.83 | 99.87 | >99.99 |
| Ti6Al4V/TNT5 | - | - | - | - | - |
| Ti6Al4V/TNT5/0.6 AgNPs | >99.99 | >99.99 | >99.99 | >99.99 | >99.99 |
| Ti6Al4V/TNT5/1.0 AgNPs | >99.99 | 99.99 | 99.99 | 99.99 | >99.99 |
| Ti6Al4V/TNT5/1.6 AgNPs | 99.90 | 99.84 | 99.99 | >99.99 | >99.99 |
| Ti6Al4V/TNT5/2.3 AgNPs | 99.66 | 99.88 | 99.81 | 99.76 | >99.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Grodzicka, M.; Ehlert, M.; Jędrzejewski, T.; Wypij, M.; Golińska, P. “To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants. J. Clin. Med. 2019, 8, 334. https://doi.org/10.3390/jcm8030334
Radtke A, Grodzicka M, Ehlert M, Jędrzejewski T, Wypij M, Golińska P. “To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants. Journal of Clinical Medicine. 2019; 8(3):334. https://doi.org/10.3390/jcm8030334
Chicago/Turabian StyleRadtke, Aleksandra, Marlena Grodzicka, Michalina Ehlert, Tomasz Jędrzejewski, Magdalena Wypij, and Patrycja Golińska. 2019. "“To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants" Journal of Clinical Medicine 8, no. 3: 334. https://doi.org/10.3390/jcm8030334
APA StyleRadtke, A., Grodzicka, M., Ehlert, M., Jędrzejewski, T., Wypij, M., & Golińska, P. (2019). “To Be Microbiocidal and Not to Be Cytotoxic at the Same Time…”—Silver Nanoparticles and Their Main Role on the Surface of Titanium Alloy Implants. Journal of Clinical Medicine, 8(3), 334. https://doi.org/10.3390/jcm8030334