New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Design
2.2. Interventional Study
2.3. Electroretinogram
2.4. Tissue Processing
2.5. RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) Assay
2.6. Western Blotting
2.7. Immunohistochemical Analysis
2.8. Retinal Vascular Permeability
2.9. Statistical Analysis
3. Results
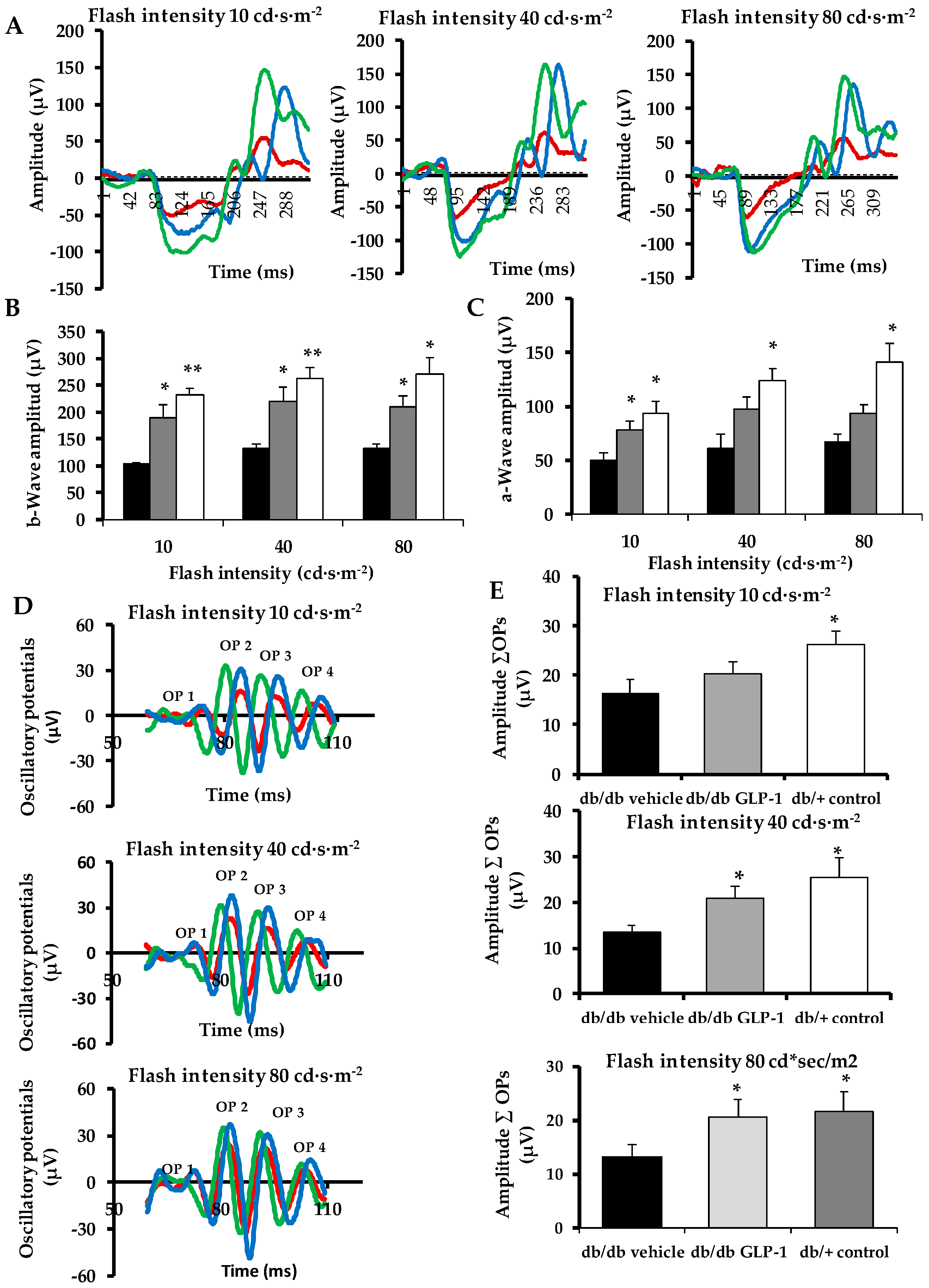
3.1. Topical Administration of Glucagon-Like Peptide-1 (GLP-1) Ameliorates Electroretinogram Abnormalities Induced by Diabetes
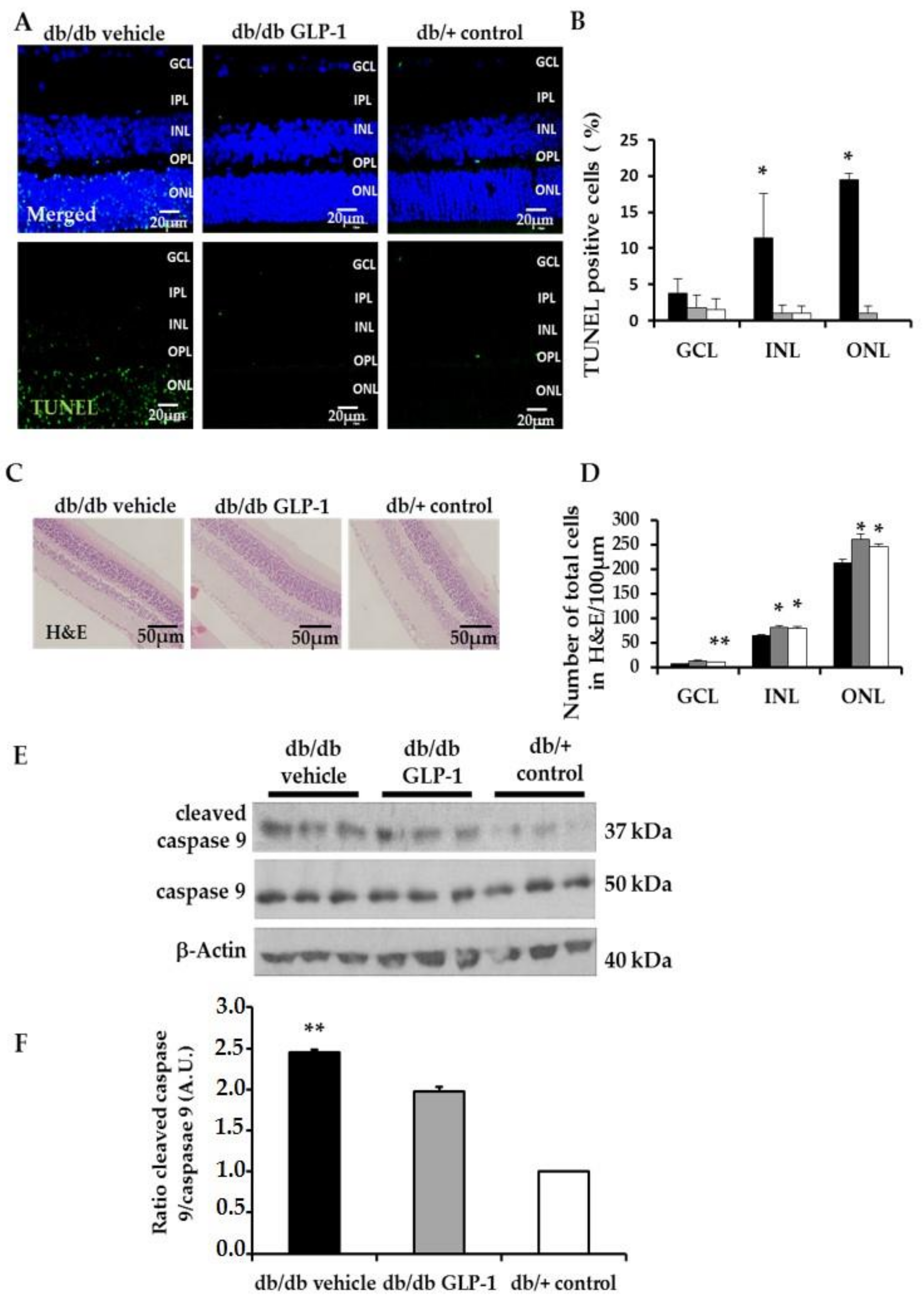
3.2. Neurodegeneration Is Inhibited by Glucagon-Like Peptide-1 (GLP-1) Eye-Drops in Diabetic Mice
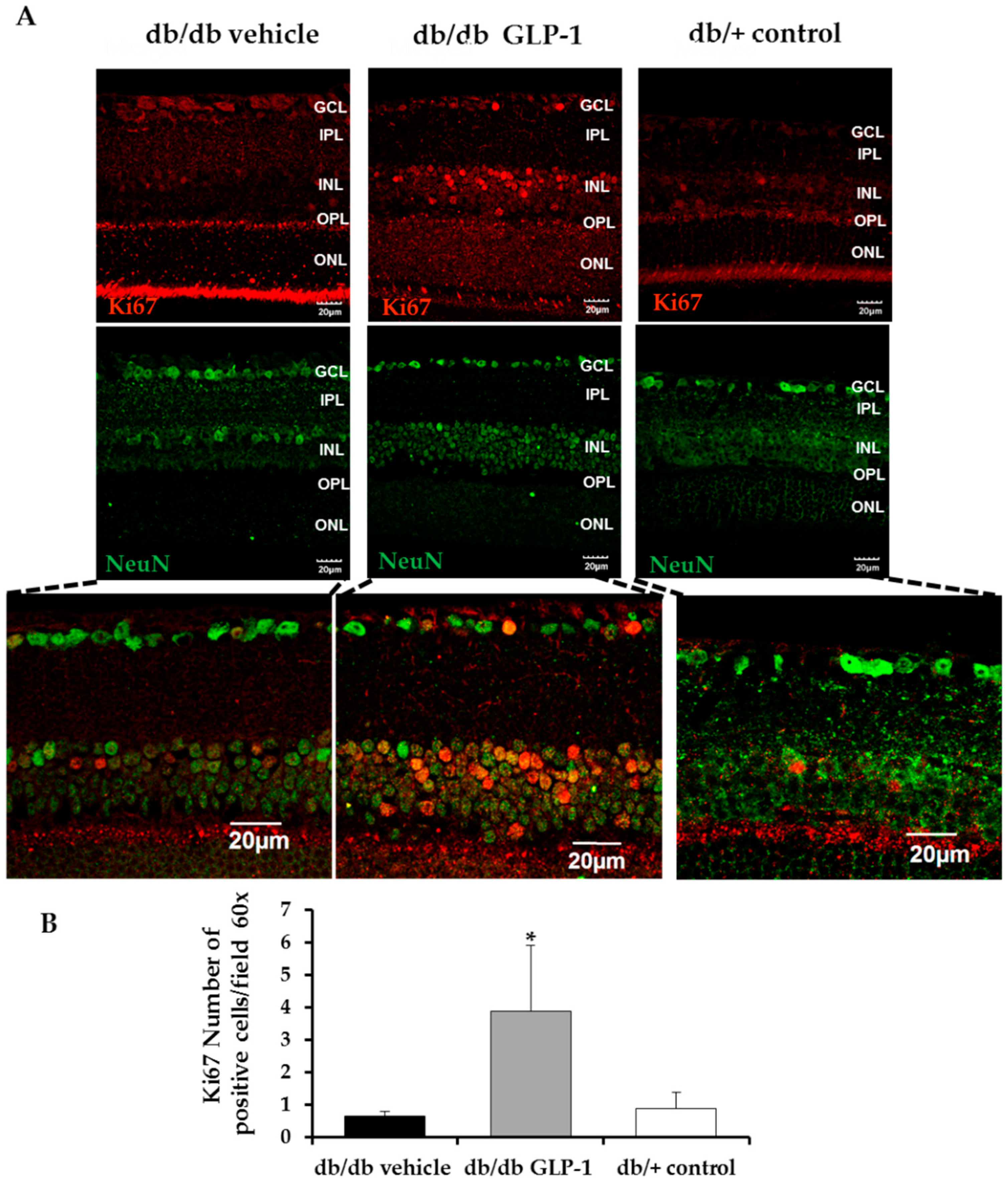
3.3. Topical Administration of Glucagon-Like Peptide-1 (GLP-1) Preserves Retinal Thickness and Promotes Neurogenesis
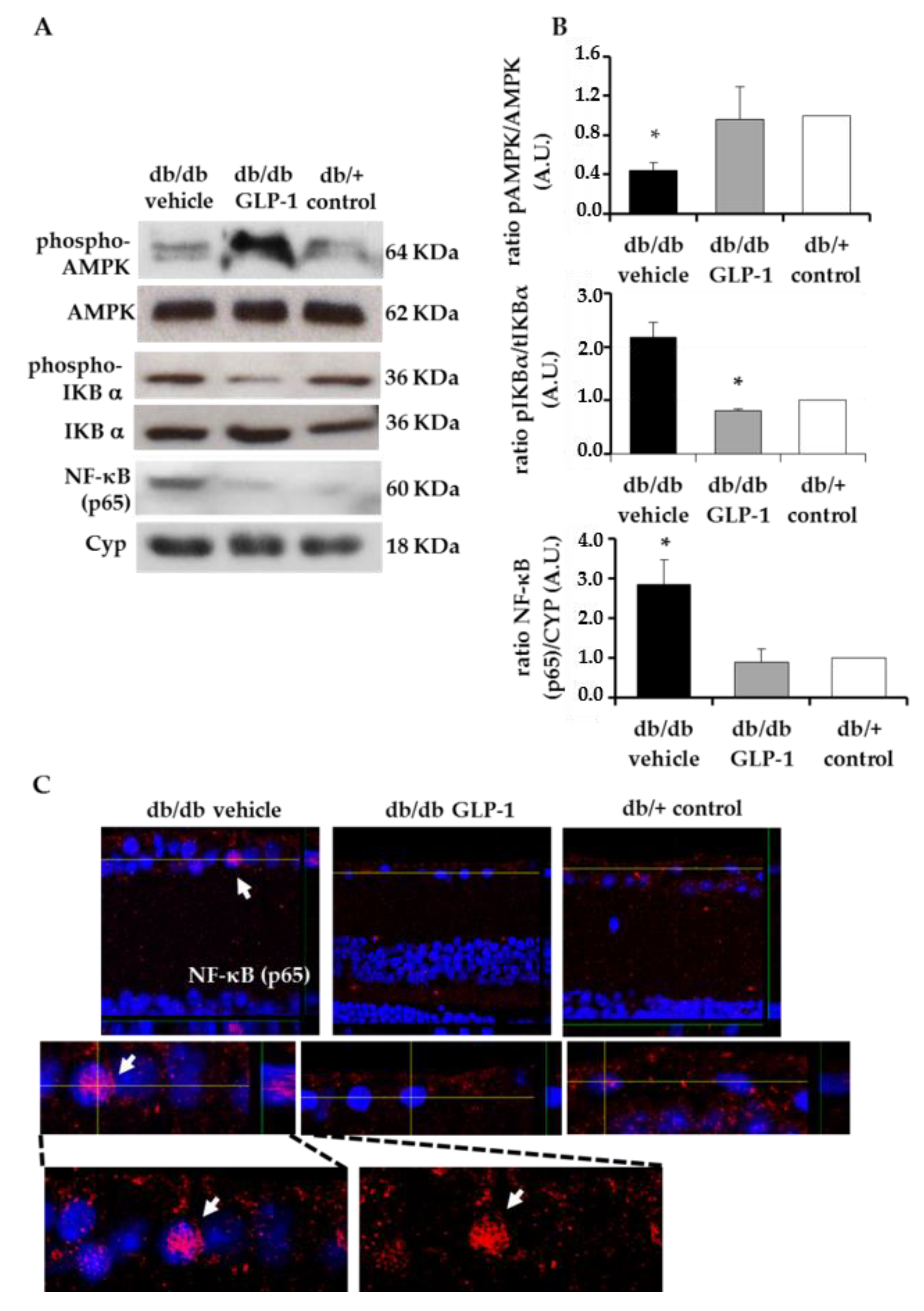
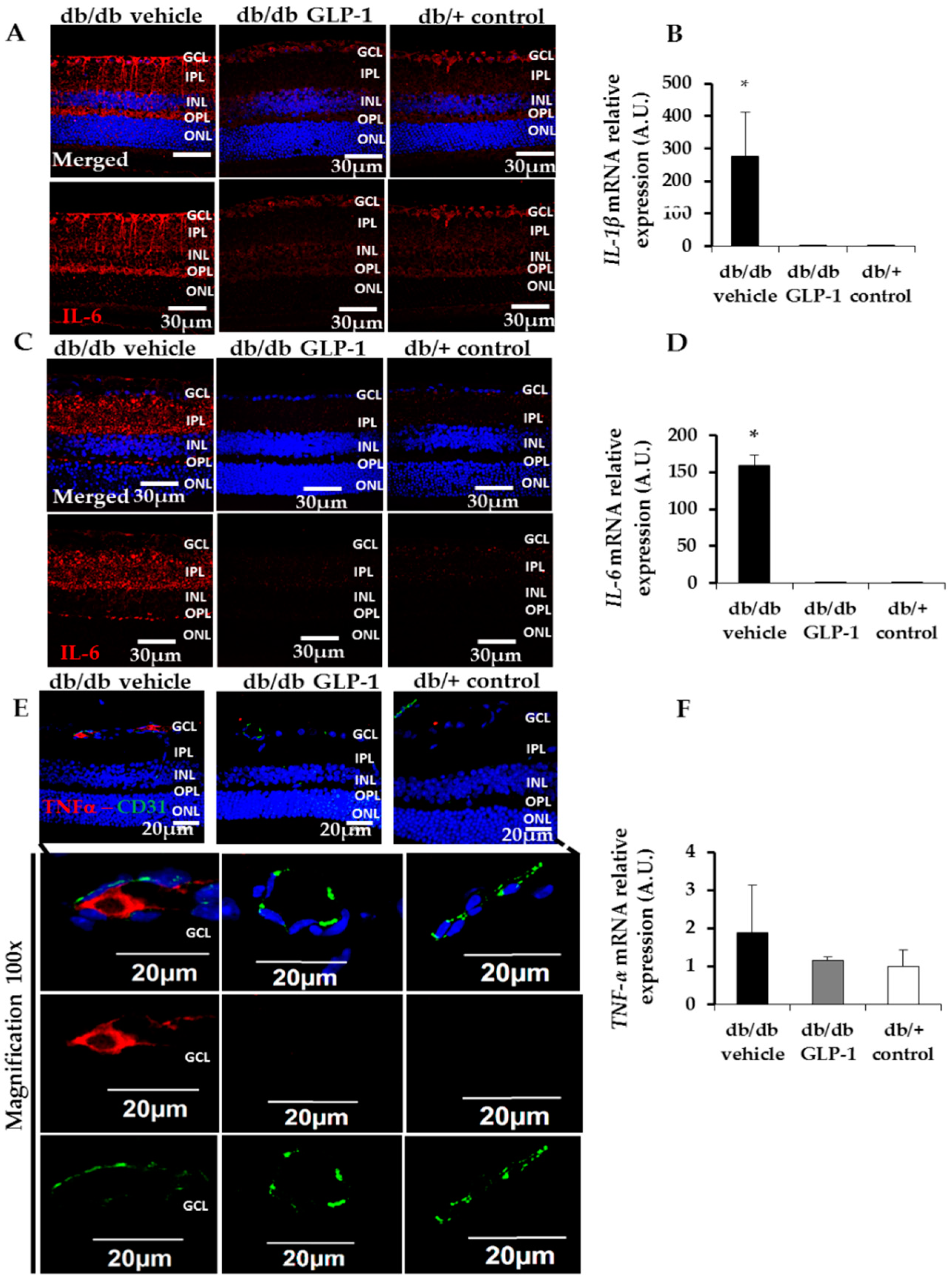
3.4. Treatment with Glucagon-Like Peptide-1 (GLP-1) down-Regulates Inflammation Induced by Diabetes through the Inhibition of NF-κB, Proinflammatory Cytokines and the NLRP3 Inflammasome Pathway
3.5. Treatment with Glucagon-Like Peptide-1 (GLP-1) up-Regulates Survival Pathways in the Diabetic Retina
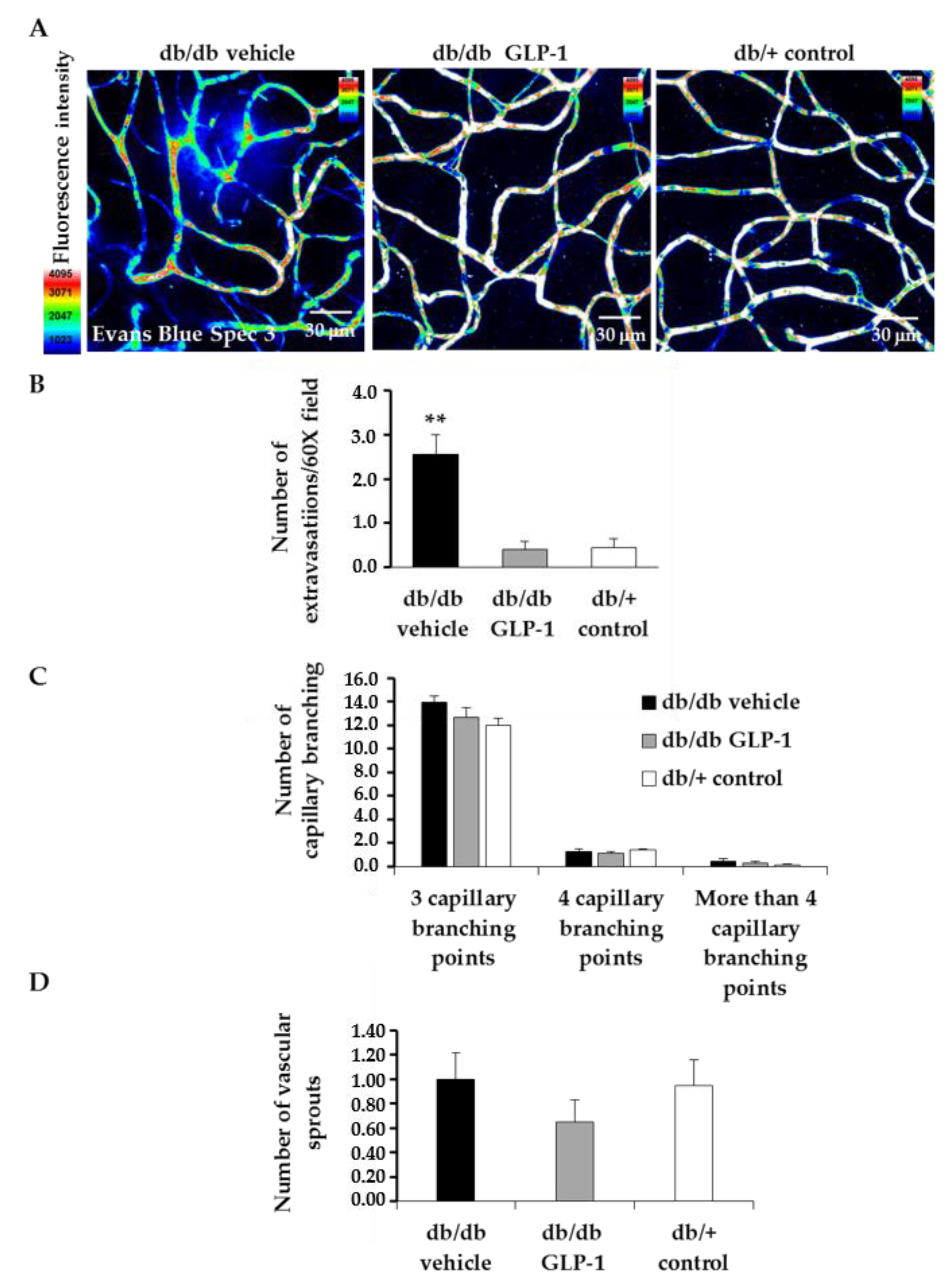
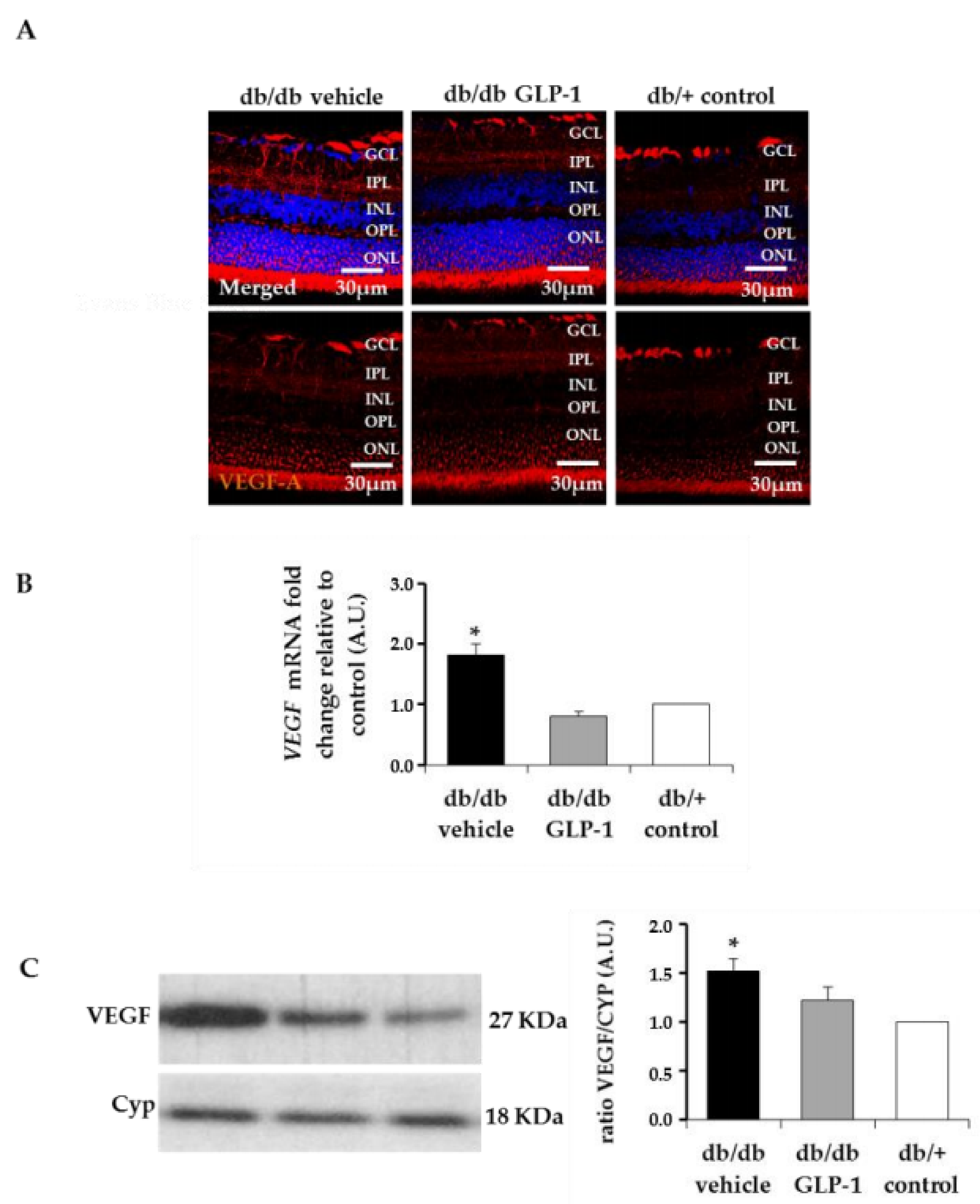
3.6. Treatment with Glucagon-Like Peptide-1 (GLP-1) Reduces Diabetes-Induced VEGF Overexpression and Vascular Leakage
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BRB | Blood retinal barrier |
| DR | Diabetic retinopathy |
| NPDR | Non-proliferative Diabetic retinopathy |
| GFAP | Glial fibrillar acidic protein |
| GLAST | Glutamate−aspartate transporter |
| GLP-1 | Glucagon-like peptide 1 |
| GLP1-R | Glucagon-like peptide 1 receptor |
| GLP-1RA | Glucagon-like peptide 1 receptor agonist |
References
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 17, 16012. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A.; Penn State Retina Research Group. Retinal neurodegeneration: Early pathology in diabetes. Clin. Exp. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 16, 9. [Google Scholar] [CrossRef]
- Marmor, M.F.; Holder, G.E.; Seeliger, M.W.; Yamamoto, S. International Society for Clinical Electrophysiology of Vision. Standard for clinical electroretinography. Doc. Ophthalmol. 2004, 108, 107–114. [Google Scholar] [CrossRef]
- Anderson, P.J.; Watts, H.; Hille, C.; Philpott, K.; Clark, P.; Gentleman, M.C.S.; Jen, L.-S. Glial and endothelial blood-retinal barrier responses to amyloid-beta in the neural retina of the rat. Clin. Ophthalmol. 2008, 2, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Solà-Adell, C.; Sampedro, J.; Valeri, M.; Genís, X.; Simó-Servat, O.; García-Ramírez, M.; Simó, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Dahl Ejby Jensen, L.; Söll, I.; Hauptmann, G.; Cao, Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS ONE 2008, 3, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jun, H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 1–11. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, C.; Guan, M.; Zheng, Z.; Li, J.; Xu, W.; Wang, L.; He, F.; Xue, Y. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc. Diabetol. 2014. [Google Scholar] [CrossRef]
- Tang, S.T.; Su, H.; Zhang, Q.; Tang, H.Q.; Wang, C.J.; Zhou, Q.; Wei, W.; Zhu, H.Q.; Wang, Y. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int. J. Mol. Med. 2016, 37, 1558–1566. [Google Scholar] [CrossRef]
- Nakano, N.; Matsuda, S.; Ichimura, M.; Minami, A.; Ogino, M.; Murai, T.; Kitagishi, Y. PI3K/AKT signaling mediated by G protein-coupled receptors is involved in neurodegenerative Parkinson’s disease. Int. J. Mol. Med. 2017, 39, 253–260. [Google Scholar] [CrossRef]
- Ahn, J.Y. Neuroprotection signaling of nuclear akt in neuronal cells. Exp. Neurobiol. 2014, 23, 200–206. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Hernández-Damián, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef]
- Abbasi, M.; Gupta, V.; Chitranshi, N.; You, Y.; Dheer, Y.; Mirzaei, M.; Graham, S.L. Regulation of brain-derived neurotrophic factor and growth factor signaling pathways by tyrosine phosphatase shp2 in the retina: A brief review. Front. Cell Neurosci. 2018, 1–10. [Google Scholar] [CrossRef]
- Barber, A.J.; Nakamura, M.; Wolpert, E.B.; Reiter, C.E.; Seigel, G.M.; Antonetti, D.A.; Gardner, T.W. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 2001, 276, 32814–32821. [Google Scholar] [CrossRef]
- Reiter, C.E.; Wu, X.; Sandirasegarane, L.; Nakamura, M.; Gilbert, K.A.; Singh, R.S.; Fort, P.E.; Antonetti, D.A.; Gardner, T.W. Diabetes reduces basal retinal insulin receptor signaling: Reversal with systemic and local insulin. Diabetes 2006, 55, 1148–1156. [Google Scholar] [CrossRef]
- Rajala, A.; Gupta, V.K.; Anderson, R.E.; Rajala, R.V. Light activation of the insulin receptor regulates mitochondrial hexokinase. A possible mechanism of retinal neuroprotection. Mitochondrion 2013, 13, 566–576. [Google Scholar] [CrossRef]
- Lopez-Carballo, G.; Moreno, L.; Masia, S.; Perez, P.; Barettino, D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J. Biol. Chem. 2002, 277, 25297–25304. [Google Scholar] [CrossRef]
- Bergmann, M.W.; Rechner, C.; Freund, C.; Baurand, A.; El Jamali, A.; Dietz, R. Statins inhibit reoxygenation-induced cardiomyocyte apoptosis: Role for glycogen synthase kinase 3beta and transcription factor beta-catenin. J. Mol. Cell. Cardiol. 2004, 37, 681–690. [Google Scholar] [CrossRef]
- Venkatesan, B.; Prabhu, S.D.; Venkatachalam, K.; Mummidi, S.; Valente, A.J.; Clark, R.A.; Delafontaine, P.; Chandrasekar, B. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010, 22, 809–820. [Google Scholar] [CrossRef]
- Ying, Y.; Zhu, H.; Liang, Z.; Ma, X.; Li, S. GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J. Mol. Endocrinol. 2015, 55, 245–262. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Yang, S.; Zhang, J. The Wnt/β-catenin signaling pathway in the adult neurogenesis. Eur. J. Neurosci. 2011, 33, 1–8. [Google Scholar] [CrossRef]
- Porter, W.D.; Flatt, P.R.; Hölscher, C.; Gault, V.A. Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in OB/OB mice. Int. J. Obes. 2013, 37, 678–684. [Google Scholar] [CrossRef]
- Darsalia, V.; Mansouri, S.; Ortsäter, H.; Olverling, A.; Nozadze, N.; Kappe, C.; Iverfeldt, K.; Tracy, L.M.; Grankvist, N.; Sjöholm, Å.; Patrone, C. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in type 2 diabetic rats. Clin. Sci. 2012, 122, 473–483. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]




| Gen Symbol | Sequence Primer |
|---|---|
| IL-1 Forward (5′-3′) | 5′-GCAACTGTTCCTGAACTCAACT-3′ |
| IL-1 Reverse (5′-3′) | 5′-ATCTTTTGGGGTCCGTCAACT-3′ |
| IL-6 Forward (5′-3′) | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ |
| IL-6 Reverse (5′-3′) | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| TNF Forward (5′-3′) | 5′-CCCTCACACTCAGATCATCTTCT-3′ |
| TNF Reverse (5′-3′) | 5′-GCTACGACGTGGGCTACAG-3′ |
| VEGFa Forward (5′-3′) | 5′-GAGTACCCCGACGAGATAGA-3′ |
| VEGFa Reverse (5′-3′) | 5′-GGCTTTGGTGAGGTTTGAT-3′ |
| NLRP-3 Forward (5′-3′) | 5′-ATTACCCGCCCGAGAAAGG-3′ |
| NLRP-3 Reverse (5′-3′) | 5′-TCGCAGCAAAGATCCACACAG-3′ |
| IL-18 Forward (5′-3′) | 5′-AGCAGTCCCAACTAAGCAGTA-3′ |
| IL-18 Reverse (5′-3′) | 5′-CAGCCAGTAGAGGATGCTGA-3′ |
| Actin Forward (5′-3′) | 5′-CTAAGGCCAACCGTGAAAG-3′ |
| Actin Reverse (5′-3′) | 5′-ACCAGAGGCATACAGGGACA-3′ |
| Antibody | Description |
|---|---|
| p-AMPK | 1:1000; ab80039; Abcam, Cambridge, UK |
| AMPK | 1:1000; ab133448; Abcam, Cambridge, UK |
| p-IKBα | 1:1000; ab133462; Abcam, Cambridge, UK |
| IKBα | 1:5000; ab32518; Abcam, Cambridge, UK |
| NF-κB (p65) | 1:1000; sc-8008; Santa Cruz, Dallas, Texas, USA |
| p-AKT | 1:1000; #2965; Cell Signaling, Leiden, The Netherlands |
| AKT | 1:5000; #9272; Cell Signaling, Leiden, The Netherlands |
| p-GSK3β | 1:1000; ab75745; Abcam, Cambridge, UK |
| β-catenin | 1:1000; GTX132611; Genetex, California, USA |
| Bcl-xL | 1:1000; 610211; BD Biosciences, Sparks, MD, USA |
| VEGF | 1:5000; ab46154; Abcam, Cambridge, UK |
| Antibody | Description |
|---|---|
| GFAP | rabbit monoclonal; 1:500; ab7260 (Abcam, Cambridge, UK) |
| Ki67 | rabbit polyclonal; 1:500; ab15580 (Abcam, Cambridge, UK) |
| NFκB | mouse monoclonal; 1:100; sc-8008 (Santa Cruz, Dallas, Texas, USA) |
| IL-1β | rabbit polyclonal; 1:100; ab9722 (Abcam, Cambridge, UK) |
| IL-6 | rabbit polyclonal; 1:100; ab7737 (Abcam, Cambridge, UK) |
| TNF-α | mouse monoclonal; 1:100; ab8348 (Abcam, Cambridge, UK) |
| Collagen IV | rabbit polyclonal; 1:200; ab6586 (Abcam, Cambridge, UK) |
| NLRP3 | rabbit polyclonal; 1:200; ab214185 (Abcam, Cambridge, UK) |
| Bcl-xL | rabbit polyclonal; 1:100; 610211 (BD Biosciences, Sparks, MD, USA) |
| VEGFA | rabbit monoclonal; 1:100; ab52917 (Abcam, Cambridge, UK) |
| Glial Fibrillary Acidic Protein (GFAP) Score | Description |
|---|---|
| 1 | Müller cell endfeet region/GCL only |
| 2 | Müller cell endfeet region/GCL plus a few proximal processes |
| 3 | Müller cell endfeet plus many processes, but not extending to ONL |
| 4 | Müller cell endfeet plus processes throughout with some in the ONL |
| 5 | Müller cell endfeet plus lots of dark processes from GCL to the outer margin of ONL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampedro, J.; Bogdanov, P.; Ramos, H.; Solà-Adell, C.; Turch, M.; Valeri, M.; Simó-Servat, O.; Lagunas, C.; Simó, R.; Hernández, C. New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 339. https://doi.org/10.3390/jcm8030339
Sampedro J, Bogdanov P, Ramos H, Solà-Adell C, Turch M, Valeri M, Simó-Servat O, Lagunas C, Simó R, Hernández C. New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy. Journal of Clinical Medicine. 2019; 8(3):339. https://doi.org/10.3390/jcm8030339
Chicago/Turabian StyleSampedro, Joel, Patricia Bogdanov, Hugo Ramos, Cristina Solà-Adell, Mireia Turch, Marta Valeri, Olga Simó-Servat, Carmen Lagunas, Rafael Simó, and Cristina Hernández. 2019. "New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy" Journal of Clinical Medicine 8, no. 3: 339. https://doi.org/10.3390/jcm8030339
APA StyleSampedro, J., Bogdanov, P., Ramos, H., Solà-Adell, C., Turch, M., Valeri, M., Simó-Servat, O., Lagunas, C., Simó, R., & Hernández, C. (2019). New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy. Journal of Clinical Medicine, 8(3), 339. https://doi.org/10.3390/jcm8030339






