Cardioprotective Properties of Omecamtiv Mecarbil against Ischemia and Reperfusion Injury
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Protocol
2.2. Statistical Analysis
3. Results
3.1. Animal Characteristics
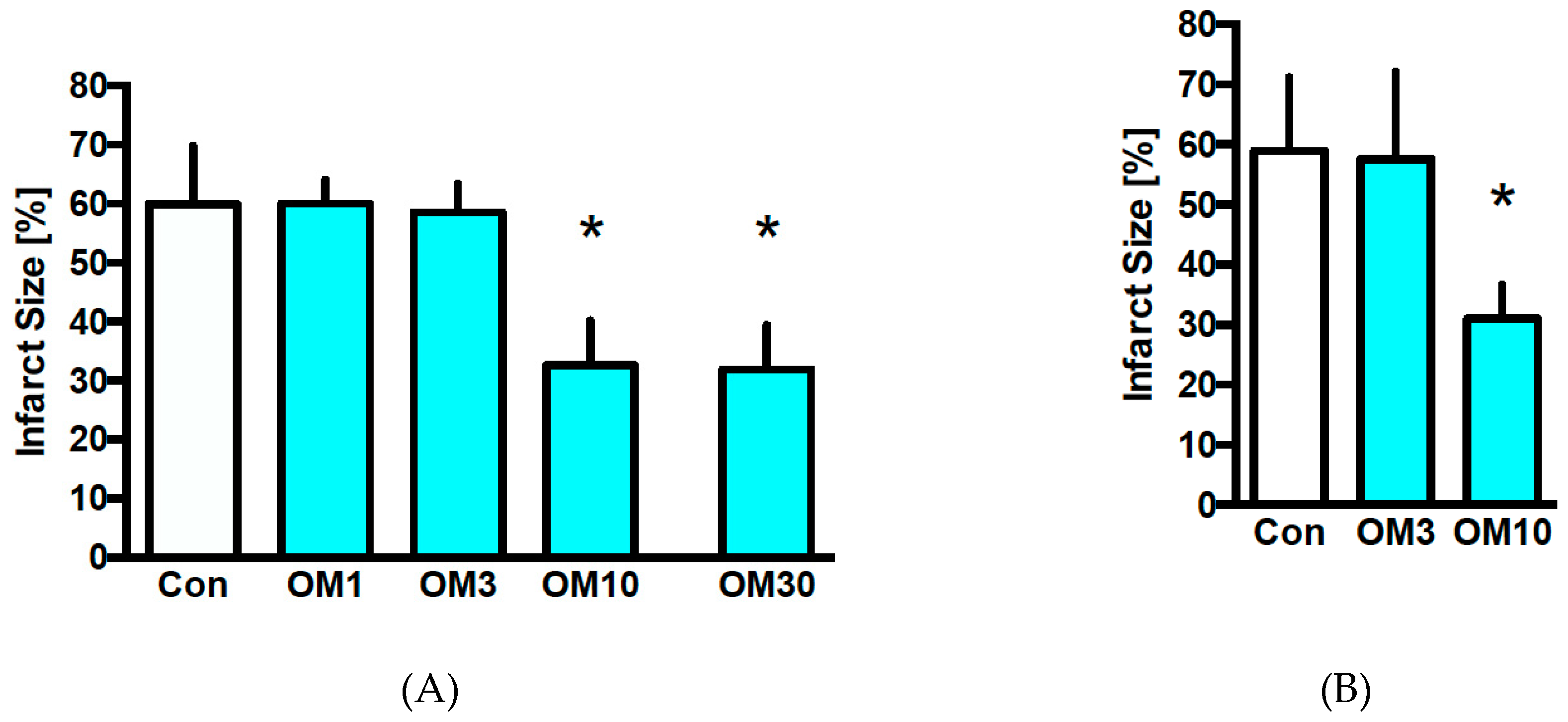
3.2. Infarct Size
3.3. Hemodynamics
4. Discussion
Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H579–H588. [Google Scholar] [CrossRef] [PubMed]
- Behmenburg, F.; Dorsch, M.; Huhn, R.; Mally, D.; Heinen, A.; Hollmann, M.W.; Berger, M.M. Impact of mitochondrial Ca2+-sensitive potassium (mBKCa) channels in sildenafil-induced cardioprotection in rats. PLoS ONE 2015, 10, e0144737. [Google Scholar] [CrossRef] [PubMed]
- Behmenburg, F.; Trefz, L.; Dorsch, M.; Strothoff, M.; Mathes, A.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Berger, M.M.; Huhn, R. Milrinone-induced postconditioning requires activation of mitochondrial Ca(2+)-sensitive Potassium (mBKCa) Channels. J. Cardiothorac. VASC Anesth. 2017. [Google Scholar] [CrossRef]
- Shen, Y.T.; Malik, F.I.; Zhao, X.; Depre, C.; Dhar, S.K.; Abarzua, P.; Morgans, D.J.; Vatner, S.F. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ. Heart Fail. 2010, 3, 522–527. [Google Scholar] [CrossRef]
- Planelles-Herrero, V.J.; Hartman, J.J.; Robert-Paganin, J.; Malik, F.I.; Houdusse, A. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Trial, G.-H. Registrational Study with Omecamtiv Mecarbil/AMG 423 to Treat Chronic Heart Failure with Reduced Ejection Fraction (GALACTIC-HF). ClinicalTrials.gov Identifier: NCT02678923. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02929329 (accessed on 18 February 2019).
- Liu, Y.; White, H.D.; Belknap, B.; Winkelmann, D.A.; Forgacs, E. Omecamtiv Mecarbil modulates the kinetic and motile properties of porcine beta-cardiac myosin. Biochemistry 2015, 54, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Malik, F.I.; Hartman, J.J.; Elias, K.A.; Morgan, B.P.; Rodriguez, H.; Brejc, K.; Anderson, R.L.; Sueoka, S.H.; Lee, K.H.; Finer, J.T.; et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science 2011, 331, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Kaplinsky, E.; Mallarkey, G. Cardiac myosin activators for heart failure therapy: Focus on omecamtiv mecarbil. Drugs Context 2018, 7, 212518. [Google Scholar] [CrossRef] [PubMed]
- Nanasi, P.; Komaromi, I.; Gaburjakova, M.; Almassy, J. Omecamtiv Mecarbil: A myosin motor activator agent with promising clinical performance and new in vitro results. Curr. Med. Chem. 2018, 25, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Bosnjak, Z.J.; Jiang, M.T. Myocardial protection by isoflurane preconditioning preserves Ca2+ cycling proteins independent of sarcolemmal and mitochondrial KATP channels. Anesth. Analg. 2007, 105, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, C.H.; Yao, Y.T.; Li, L.H. Downregulation of Na(+)/Ca(2+) exchanger isoform 1 protects isolated hearts by sevoflurane postconditioning but not by delayed remote ischemic preconditioning in rats. Chin. Med. J. 2017, 130, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies. Guide for the Care and Use of Laboratory Animals: National Research Council (US), 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Teerlink, J.R.; Senior, R.; Nifontov, E.M.; Mc Murray, J.J.; Lang, C.C.; Tsyrlin, V.A.; Greenberg, B.H.; Mayet, J.; Francis, D.P.; et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet 2011, 378, 676–683. [Google Scholar] [CrossRef]
- Bakkehaug, J.P.; Kildal, A.B.; Engstad, E.T.; Boardman, N.; Naesheim, T.; Ronning, L.; Aasum, E.; Larsen, T.S.; Myrmel, T.; How, O.J. Myosin activator omecamtiv mecarbil increases myocardial oxygen consumption and impairs cardiac efficiency mediated by resting myosin ATPase activity. Circ. Heart Fail. 2015, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Palaparthy, R.; Banfield, C.; Alvarez, P.; Yan, L.; Smith, B.; Johnson, J.; Monsalvo, M.L.; Malik, F. Relative bioavailability, food effect, and safety of the single-dose pharmacokinetics of omecamtiv mecarbil following administration of different modified-release formulations in healthy subjects. Int. J. Clin. Pharm. 2016, 54, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Szentandrassy, N.; Veress, R.; Almassy, J.; Magyar, J.; Banyasz, T.; Toth, A.; Papp, Z.; Nanasi, P.P. Frequency-dependent effects of omecamtiv mecarbil on cell shortening of isolated canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch. Pharm. 2017, 390, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.; Cros, C.; Oldman, K.L.; Harmer, A.R.; Pointon, A.; Pollard, C.E.; Abi-Gerges, N. Enhanced characterization of contractility in cardiomyocytes during early drug safety assessment. Toxicol. Sci. 2015, 145, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Szentandrassy, N.; Horvath, B.; Vaczi, K.; Kistamas, K.; Masuda, L.; Magyar, J.; Banyasz, T.; Papp, Z.; Nanasi, P.P. Dose-dependent electrophysiological effects of the myosin activator omecamtiv mecarbil in canine ventricular cardiomyocytes. J. Physiol. Pharm. 2016, 67, 483–489. [Google Scholar]

| n | Body Weight (g) | Heart Weight Dry (g) | Heart Weight Wet (g) | Time of Max. Ischemic Contracture (min) | Level of Max. Ischemic Contracture (mmHg) | |
|---|---|---|---|---|---|---|
| Con | 8 | 280 ± 23 | 0.11 ± 0.01 | 1.25 ± 0.12 | 16 ± 1 | 81 ± 15 |
| OM1 | 8 | 273 ± 33 | 0.11 ± 0.01 | 1.22 ± 0.13 | 16 ± 2 | 72 ± 14 |
| OM3 | 8 | 302 ± 31 | 0.12 ± 0.01 | 1.33 ± 0.18 | 21 ± 5 # | 60 ± 8 # |
| OM10 | 8 | 302 ± 34 | 0.11 ± 0.02 | 1.32 ± 0.11 | 23 ± 3 # | 45 ± 9 # |
| OM30 | 6 | 317 ± 34 | 0.13 ± 0.01 | 1.34 ± 0.13 | 22 ± 3 # | 42 ± 9 # |
| n | Body Weight (g) | Heart Weight Dry (g) | Heart Weight Wet (g) | Time of Max. Ischemic Contracture (min) | Level of Max. Ischemic Contracture (mmHg) | |
|---|---|---|---|---|---|---|
| Con | 8 | 316 ± 17 | 0.14 ± 0.02 | 1.42 ± 0.12 | 19 ± 6 | 68 ± 14 |
| OM3 | 8 | 306 ± 20 | 0.14 ± 0.01 | 1.38 ± 0.15 | 18 ± 5 | 67 ± 22 |
| OM10 | 8 | 299 ± 24 | 0.13 ± 0.01 | 1.29 ± 0.13 | 18 ± 2 | 63 ± 21 |
| Baseline | PC | Reperfusion | ||
|---|---|---|---|---|
| 30 | 60 | |||
| Heart Rate (bpm) | ||||
| Con | 309 ± 40 | 314 ± 31 | 275 ± 39 | 272 ± 29 |
| OM1 | 330 ± 39 | 314 ± 55 | 287 ± 61 | 261 ± 54 |
| OM3 | 328 ± 29 | 313 ± 27 | 281 ± 56 | 284 ± 53 |
| OM10 | 332 ± 45 | 292 ± 44 | 230 ± 56 | 287 ± 42 |
| OM30 | 320 ± 55 | 295 ± 62 | 222 ± 92 | 275 ± 66 |
| LVEDP (mmHg) | ||||
| Con | 4 ± 1 | 5 ± 2 | 116 ± 13 * | 102 ± 9 * |
| OM1 | 5 ± 1 | 14 ± 9 | 113 ± 17 * | 98 ± 13 * |
| OM3 | 5 ± 1 | 68 ± 17 *,# | 96 ± 30 * | 83 ± 25 * |
| OM10 | 6 ± 1 | 79 ± 8 *,# | 85 ± 19 *,# | 72 ± 15 *,# |
| OM30 | 4 ± 1 | 79 ± 8 *,# | 82 ± 25 *,# | 76 ± 20 *,# |
| Coronary flow (mL/min) | ||||
| Con | 13 ± 2 | 12 ± 2 | 7 ± 1 * | 6 ± 2 * |
| OM1 | 14 ± 2 | 16 ± 3 # | 7 ± 2 * | 6 ± 1 * |
| OM3 | 14 ± 3 | 14 ± 3 # | 9 ± 2 * | 8 ± 2 * |
| OM10 | 16 ± 2 # | 11 ± 2 | 10 ± 2 *,# | 9 ± 2 *,# |
| OM30 | 16 ± 2 # | 14 ± 1 | 10 ± 3 *,# | 10 ± 1 *,# |
| Baseline | Reperfusion | |||
|---|---|---|---|---|
| 30 | 45 | 60 | ||
| Heart Rate (bpm) | ||||
| Con | 323 ± 32 | 220 ± 78 * | 271 ± 33 | 278 ± 51 |
| OM3 | 320 ± 32 | 231 ± 51 | 257 ± 37 | 261 ± 56 |
| OM10 | 312 ± 23 | 205 ± 55 * | 211 ± 58 | 220 ± 72 |
| LVEDP (mmHg) | ||||
| Con | 4 ± 1 | 90 ± 23 * | 82 ± 19 * | 81 ± 19 * |
| OM3 | 5 ± 2 | 81 ± 27 * | 78 ± 23 * | 75 ± 22 * |
| OM10 | 5 ± 1 | 92 ± 18 * | 85 ± 17 * | 83 ± 14 * |
| Coronary flow (mL/min) | ||||
| Con | 16 ± 2 | 9 ± 2 * | 9 ± 2 * | 8 ± 2 * |
| OM3 | 16 ± 2 | 11 ± 4 * | 10 ± 4 * | 9 ± 4 * |
| OM10 | 17 ± 2 | 11 ± 2 * | 9 ± 2 * | 9 ± 2 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroethoff, M.; Behmenburg, F.; Meierkord, S.; Bunte, S.; Mayer, F.; Mathes, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Cardioprotective Properties of Omecamtiv Mecarbil against Ischemia and Reperfusion Injury. J. Clin. Med. 2019, 8, 375. https://doi.org/10.3390/jcm8030375
Stroethoff M, Behmenburg F, Meierkord S, Bunte S, Mayer F, Mathes A, Heinen A, Hollmann MW, Huhn R. Cardioprotective Properties of Omecamtiv Mecarbil against Ischemia and Reperfusion Injury. Journal of Clinical Medicine. 2019; 8(3):375. https://doi.org/10.3390/jcm8030375
Chicago/Turabian StyleStroethoff, Martin, Friederike Behmenburg, Simon Meierkord, Sebastian Bunte, Felix Mayer, Alexander Mathes, André Heinen, Markus W. Hollmann, and Ragnar Huhn. 2019. "Cardioprotective Properties of Omecamtiv Mecarbil against Ischemia and Reperfusion Injury" Journal of Clinical Medicine 8, no. 3: 375. https://doi.org/10.3390/jcm8030375
APA StyleStroethoff, M., Behmenburg, F., Meierkord, S., Bunte, S., Mayer, F., Mathes, A., Heinen, A., Hollmann, M. W., & Huhn, R. (2019). Cardioprotective Properties of Omecamtiv Mecarbil against Ischemia and Reperfusion Injury. Journal of Clinical Medicine, 8(3), 375. https://doi.org/10.3390/jcm8030375




