Abstract
High-Resolution Computed Tomography (HRCT) plays a central role in diagnosing Idiopathic Pulmonary Fibrosis (IPF) while its role in monitoring disease progression is not clearly defined. Given the variable clinical course of the disease, we evaluated whether HRCT abnormalities predict disease behavior and correlate with functional decline in untreated IPF patients. Forty-nine patients (with HRCT1) were functionally categorized as rapid or slow progressors. Twenty-one had a second HRCT2. Thirteen patients underwent lung transplantation and pathology was quantified. HRCT Alveolar (AS) and Interstitial Scores (IS) were assessed and correlated with Forced Vital Capacity (FVC) decline between HRCT1 and HRCT2. At baseline, AS was greater in rapids than in slows, while IS was similar in the two groups. In the 21 subjects with HRCT2, IS increased over time in both slows and rapids, while AS increased only in rapids. The IS change from HRCT1 to HRCT2 normalized per month correlated with FVC decline/month in the whole population, but the change in AS did not. In the 13 patients with pathology, the number of total lymphocytes was higher in rapids than in slows and correlated with AS. Quantitative estimation of HRCTs AS and IS reflects the distinct clinical and pathological behavior of slow and rapid decliners. Furthermore, AS, which reflects the immune/inflammatory infiltrate in lung tissue, could be a useful tool to differentiate rapid from slow progressors at presentation.
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease of unknown origin with a highly heterogeneous and unpredictable clinical course [1,2]. While in most cases the inexorable decline in lung function and symptoms occur over a period of years (slow progressors), 10–15% of individuals experience a much faster course, progressing from mild symptoms to respiratory failure and death over a period of months (rapid progressors) [1,3,4].
The identification of Usual Interstitial Pneumonia (UIP) pattern by High Resolution Computed Tomography (HRCT) plays a central role in the diagnosis of IPF, avoiding the need of lung biopsies in a high proportion of cases [2,5]. Furthermore, HRCT-derived scores for fibrosis extent have been widely shown to correlate with degree of physiological impairment and may be more sensitive to subtle changes in disease status than physiological metrics [6,7,8,9,10,11].
Although the crucial role of HRCT in staging and monitoring IPF over time has been emphasized [12], the big challenge for clinicians remains the possibility to forecast the disease course (slow or rapid) at the time of diagnosis [13,14,15]. To predict the variable and poorly defined natural history of IPF, composite scoring systems are increasingly being developed [6,7,16]. The Gender, Age, Physiology (GAP) index, which is based only on clinical and functional variables, was able to predict one-year mortality in a cohort of patients with IPF [16]. Moreover, integrating CT scores to the GAP model increased the accuracy of mortality prediction [7], indicating a potential role for the HRCT in the prediction schemes. However, these scoring systems, even if useful, are still neither able to foresee prospectively the highly heterogeneous and unpredictable disease behavior, nor able to guide treatment response.
We thought that a careful evaluation of the different HRCT patterns, including not only fibrotic changes but also ground glass opacities, might help to predict the future rate of functional decline in patients with IPF not conditioned by antifibrotic treatment. Taking advantage of our unique population of IPF patients not yet treated with antifibrotics, in this study, we assessed if HRCT pattern at diagnosis may: (a) predict disease behavior (slow or rapid progressors); (b) have a pathological basis; and (c) if changes of the HRCT pattern over time are linked to functional decline, without the confounding factor of treatment.
2. Methods
2.1. Study Population
In this longitudinal study, we analyzed a well-characterized cohort of Idiopathic Pulmonary Fibrosis (IPF) patients, with a long clinical functional and radiological follow up, referred between 2011 and 2014, naïve of antifibrotics.
All patients in our study, whether from our center or referred to our center, were offered antifibrotic therapy as soon as it became available, provided they met the Forced Vital Capacity (FVC), DLCO and age criteria for treatment and they had no clear contraindications to it. However, given that the aim of our study was to look at a population of patients off treatment, we considered only radiological and functional data before antifibrotic therapy was instituted. In addition, a minority of our patients belonged to an historical cohort from the pre-antifibrotic therapy era (before 2014) and they had no access to antifibrotic therapy.
Forty-nine patients from two Interstitial Lung Disease Centres in Italy (University of Padova, Italy, n = 43 and University of Foggia, Italy n = 6) were included.
The diagnosis of IPF was made in accordance with the latest guidelines [1,2] (Supplementary Materials). Clinical and functional data were collected at the time of diagnosis (Table 1).

Table 1.
Demographics and clinical characteristics of the entire population (n = 49), including 30 slow and 19 rapid progressors.
Based on their annual rate of decline in forced vital capacity percent (FVC%) predicted, patients were categorized as slow (<10%) or rapid progressors (≥10%). The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital of Padova (4280/AO/17). Informed consent was obtained for all study participants.
2.2. Study Design and Radiological Analysis
A HRCT was obtained at diagnosis (HRCT1) in all patients. Twenty-one patients had a second HRCT (HRCT2), after a median of 17 months of follow-up. The clinical and functional data of this subgroup are shown in Table S1. HRCT1 and HRCT2 were scored blindly and independently by two expert thoracic radiologists by using a quantitative scale, as previously described [8]. Briefly, this score consists of the assessment of ground glass opacities (GGO) (alveolar score, AS%) and fibrotic extent (interstitial score, IS%) for each lung lobe. After each individual lobe was scored for both IS and AS, the final result was expressed as mean value of the five lobes for the whole lung and in different lung regions (upper and lower). The inter-observer agreement between the two radiologists was good (Cohen’s kappa 0.7), a value similar to that reported in previous studies [11].
In the twenty-one IPF patients in whom a second HRCT was available, we studied the correlation between radiological changes and FVC% decline by calculating the change of Alveolar Score (ΔAS/month), the change of Interstitial Score (ΔIS/month) and the change in FVC (ΔFVC mL/month) in the period from HRCT1 to HRCT2. We expressed the radiological changes per month to normalize the differences in timing between HRCT1 and HRCT2 in the slow and rapid progressors.
2.3. Pathological Analysis
Thirteen of the 49 patients underwent lung transplantation during the follow up (for clinical-functional data, see Table S2). In all cases, the presence of UIP pattern was histologically confirmed by our expert pathologist (FC) [1]. The native lungs were fixed in formalin by airway perfusion and samples from upper and lower lobes were obtained and embedded in paraffin. Sections with a thickness of 5 μm were cut and stained for histological and immunohistochemical analysis, as previously described [3].
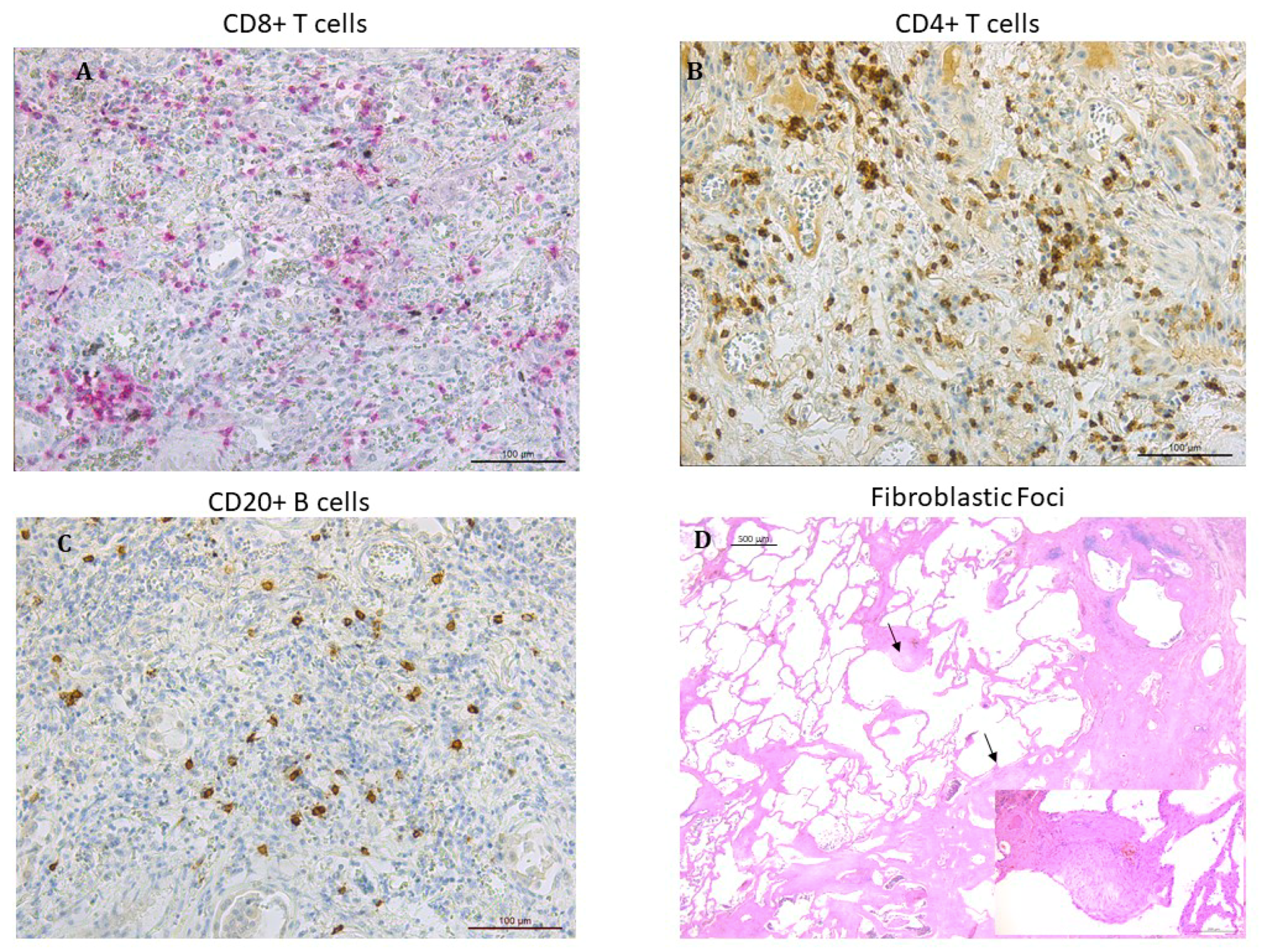
Fibroblastic foci were counted in sections stained with hematoxylin–eosin and expressed as number of fibroblastic foci/mm2 of area examined (Figure 1). Cellular infiltrate including total leukocytes (CD45+), neutrophils, macrophages (CD68+), and total lymphocytes calculated as sum of CD4+, CD8+ T lymphocytes as well as B lymphocytes (CD20+) was identified by immunohistochemistry as previously described [3,17,18,19] (Figure 1). Each inflammatory cell type was quantified in 20 non-overlapping high-power fields per slide and expressed as cells/mm2 of area examined.
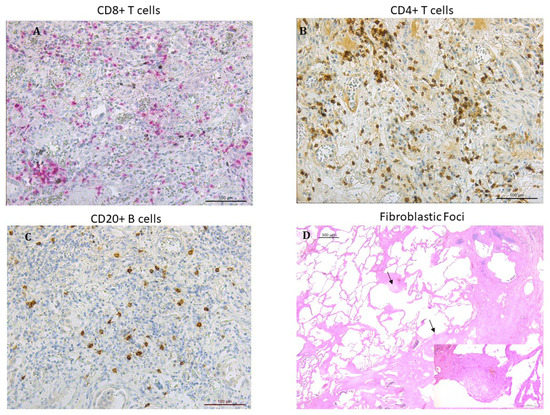
Figure 1.
Microphotographs showing lymphocytes infiltrating the lung tissue in patients with IPF: (A) CD8+ T lymphocytes stained in red (B) CD4+ T lymphocytes stained in brown. (C) CD20+ B lymphocytes stained in brown. Immunostaining with monoclonal antibodies anti-human CD8, anti-human CD4 and anti-human CD20. (D) fibroblastic foci (arrows) in the transition zone between the normal lung (on the left) and the dense remodeled parenchyma with microhoneycombing (on the right) (hematoxylin and eosin staining). Insert at higher magnification: detail showing a fibroblastic focus composed of spindle cells with overlying hyperplastic pneumocytes (hematoxylin and eosin staining).
In the thirteen IPF patients in whom the histological tissue and a HRCT performed at time close to the transplantation were available, we studied the correlation between the radiological changes and the cellular inflammatory infiltrate and between the radiological changes and the fibroblastic foci count.
2.4. Statistical Analysis
Statistical analyses were performed as previously described [20] (Supplementary Materials). To compare clinical and pathological data between rapids and slows, Chi square test or Fisher’s exact test and Mann–Whitney U test were used when appropriate. To evaluate the difference between HRCT1 and HRCT2, Wilcoxon analysis was performed.
Correlation coefficients between radiological, functional and pathological findings were calculated using the nonparametric Spearman’s rank method. Adjusted p-values for multiple comparisons were calculated using the Holm method. The inter-observer agreement between the two radiologists was evaluated by kappa statistic measure [21]. All data were analyzed using SPSS Software version 25.0 (New York, NY, US: IBM Corp. USA) p-values < 0.05 were considered statistically significant.
3. Results
3.1. Clinical and Radiological Characteristics at Baseline
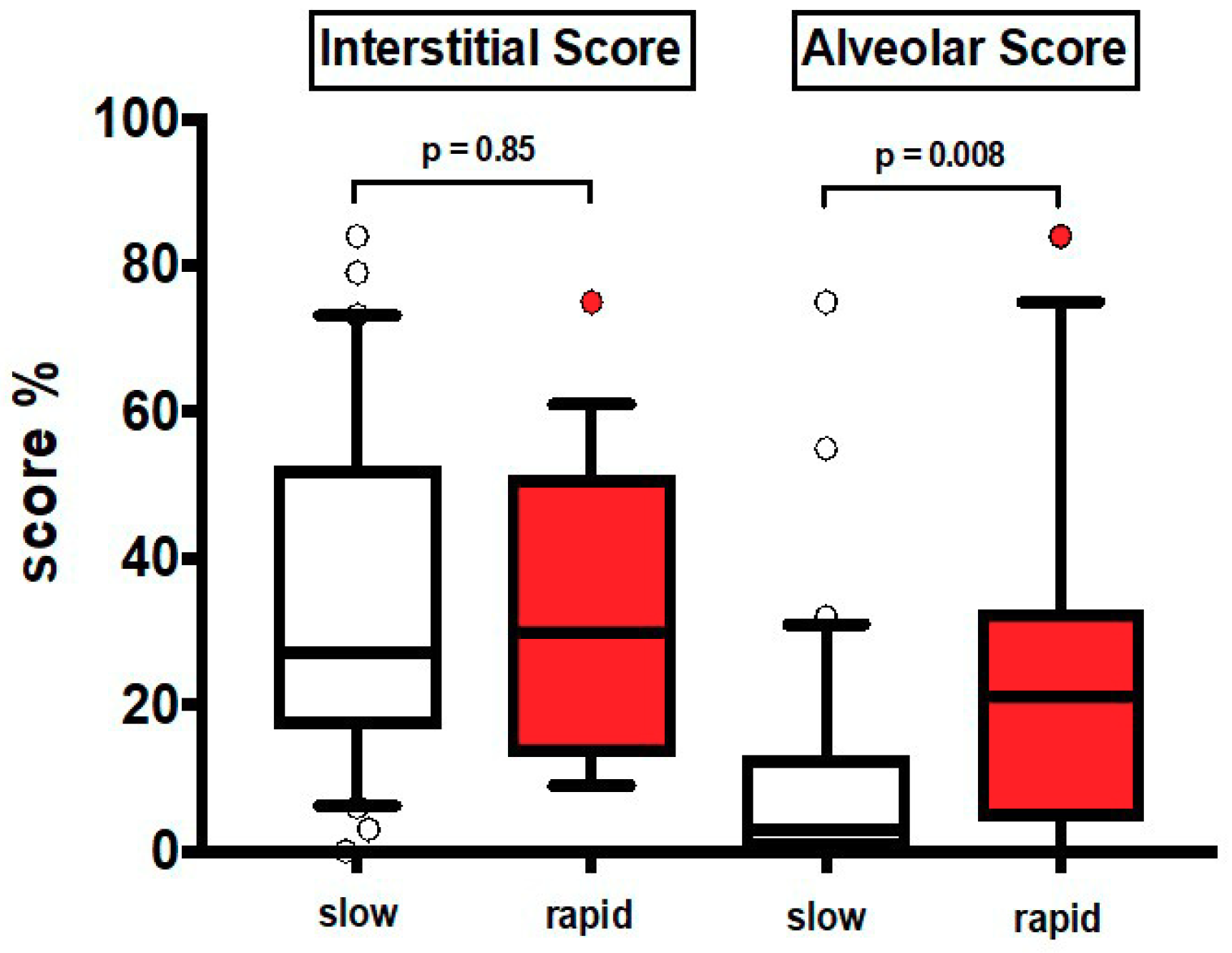
The clinical characteristics at baseline are shown in Table 1. Most patients were males and former smokers. Thirty patients were slow and 19 rapid progressors (median annual FVC decline: 130 mL and 689 mL respectively). None of the patients was treated with antifibrotics and 60% (equally distributed between the two groups) were treated with low dose prednisone with or without azathioprine according to previous guidelines [22]. In HRCT1, AS was significantly greater in rapid than in slow progressors (p = 0.008), while IS was similar in the two groups, either in the entire lung (Figure 2) or in different lung regions, upper and lower zones (Table S3).
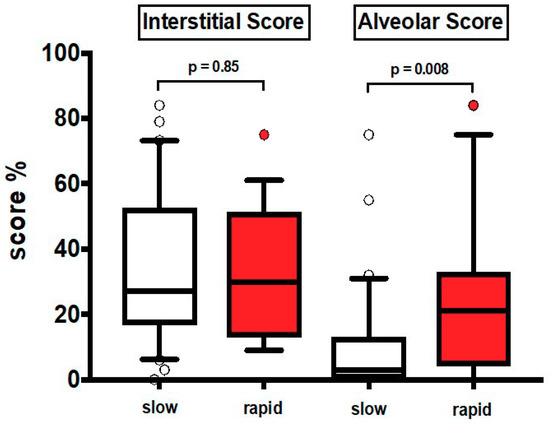
Figure 2.
Values of HRCT1 Interstitial Score and Alveolar Score at baseline in slow progressors (slow) and rapid progressors (rapid). Horizontal bars represent median values; bottom and top of each box plot 25th and 75th; brackets show 10th and 90th percentiles; and circles represent outliers. White boxes indicate slow progressors and red boxes rapid progressors.
To corroborate the findings observed in previous analyses, we obtained a ROC curve on Alveolar Score data in rapid and slow progressors. We found that the area under the curve was 0.72, (95% Confidence Interval 0.57–0.87; p = 0.008). On the other hand, in ROC curve for Interstitial Score, we did not observe any statistically significant results (95% Confidence Interval 0.35–0.67; p = 0.88).
3.2. Pathological Analysis
In the 13 patients who were transplanted (Table S2), we quantified the lung pathology (Figure 1A–C). The number of CD20+ B lymphocytes, CD4+ and CD8+ T lymphocytes (considered both individually or all together as total lymphocytes) was significantly increased in rapids than in slows (Table 2). No significant difference in the number of CD45+, neutrophils, macrophages and fibroblastic foci was found between rapid and slow progressors.

Table 2.
Inflammatory cells numbers of the entire population with lung pathology (n = 13), including six slow and seven rapid progressors.
3.3. Pathological-Radiological Correlations
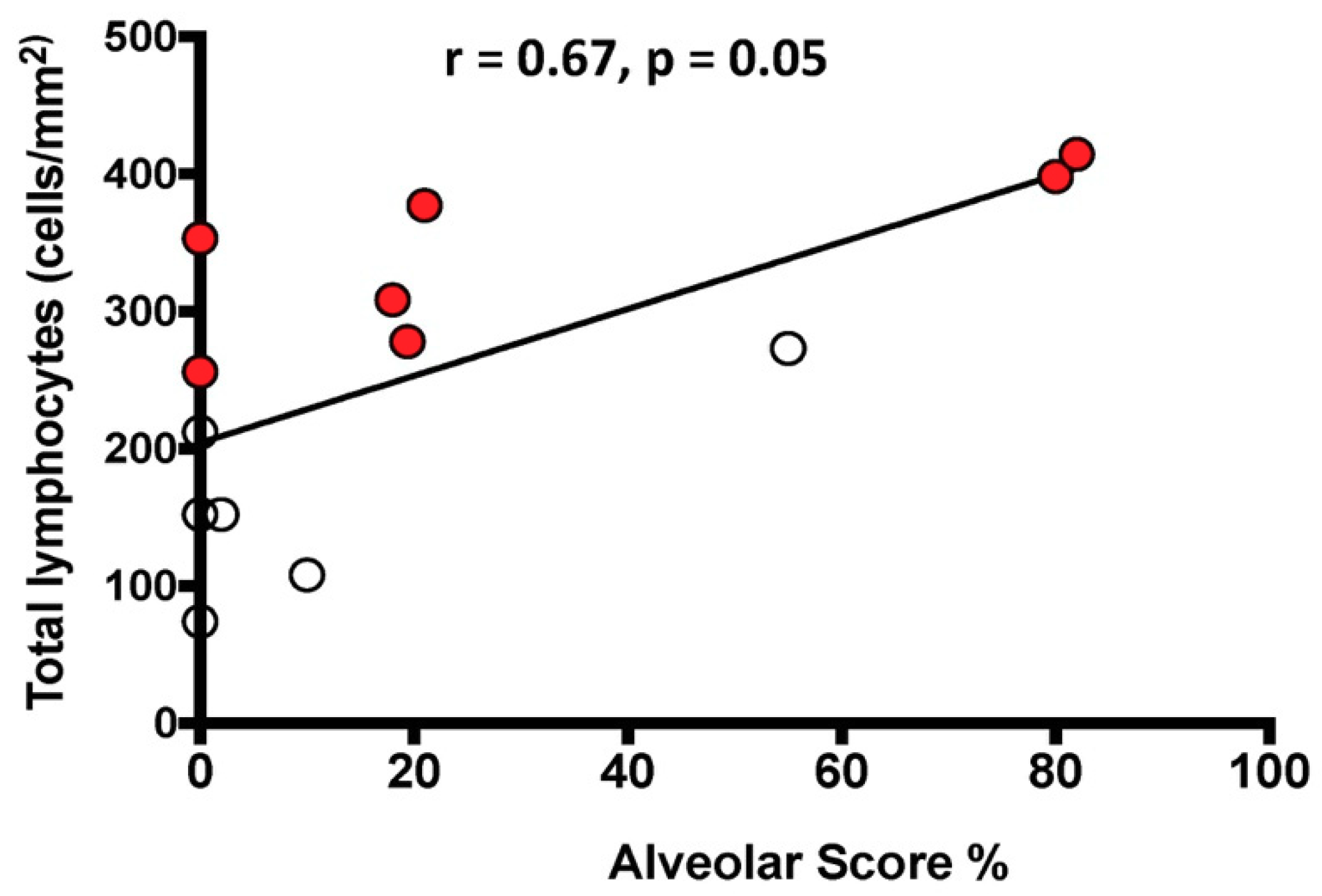
The total number of lymphocytes/mm2 was positively correlated with the HRCT AS in the whole population (Figure 3).
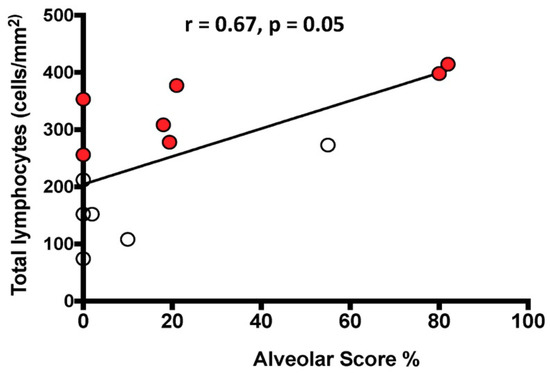
Figure 3.
Relationship between the number of total lymphocytes infiltrating the lung tissue and the HRCT Alveolar Score. The black line represents the correlation in the entire population. White circles indicate slow progressors and red circles rapid progressors. Spearman’s rank correlation: r = 0.67, p = 0.01 in the entire population; r = 0.33, p = 0.48 in slow progressors alone; r = 0.81, p = 0.03 in rapid progressors alone.
The number of FF/mm2 did not correlate with the HRCT IS in rapid progressors, slow progressors, or when considering the entire population.
3.4. Functional and Radiological Characteristics at Follow Up
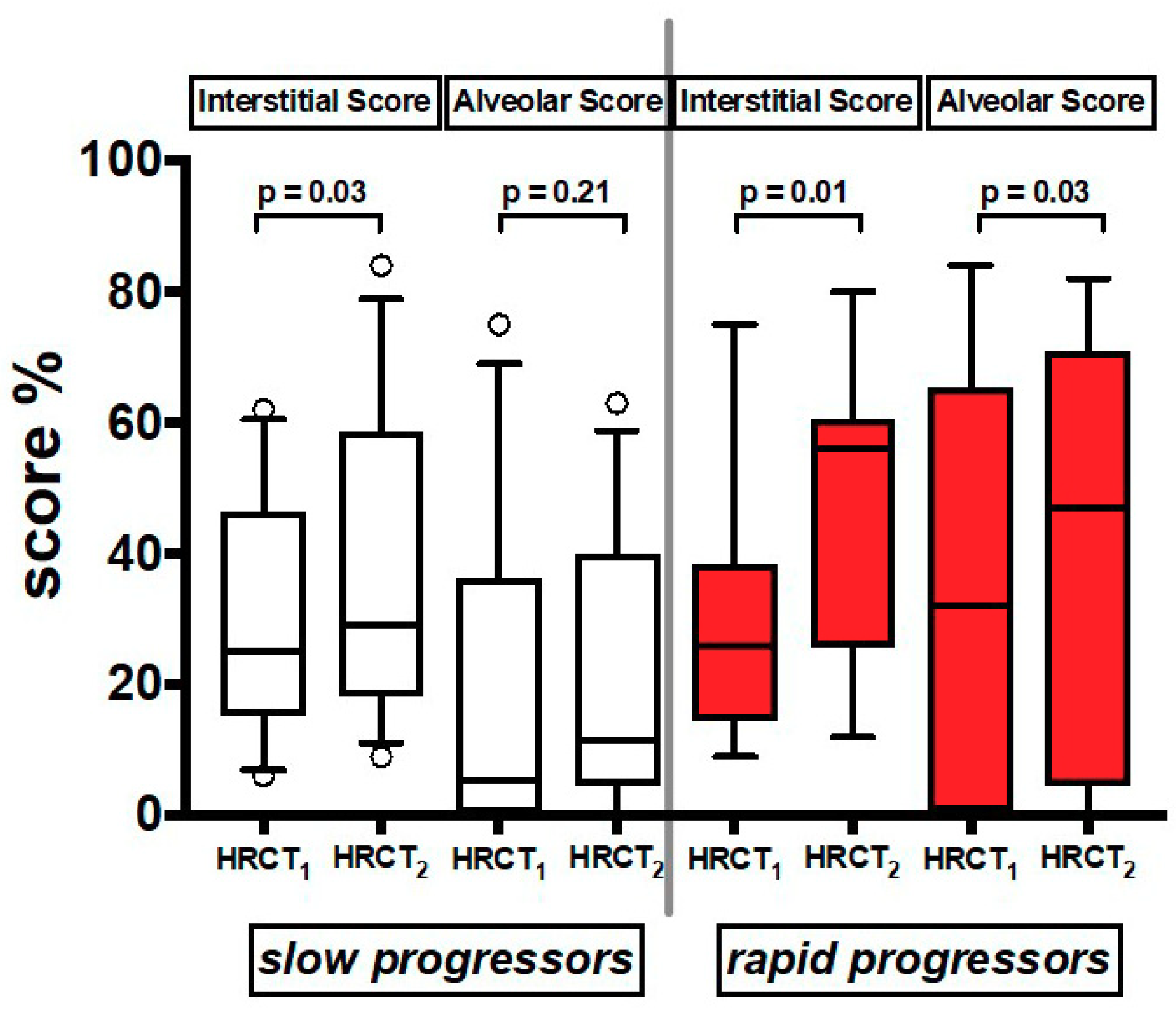
In the 21 patients who had a follow up HRCT2 (Table S1), we found that both AS and IS increased significantly over time in both groups together (Table S4). When the patients were divided by rate of decline, IS increased over time in both slows and rapids, while AS increased significantly only in rapids (Figure 4) (Table S4).
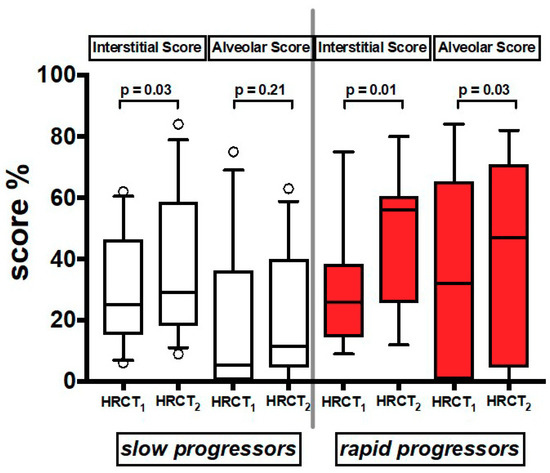
Figure 4.
Values of Interstitial Score and Alveolar Score of the two serial HRCT scans (HRCT1 at baseline and HRCT2 at follow up). Horizontal bars represent median values; bottom and top of each box plot 25th and 75th; brackets show 10th and 90th percentiles; and circles represent outliers. White boxes indicate slow progressors and red boxes rapid progressors.
3.5. Functional-Radiological Correlations
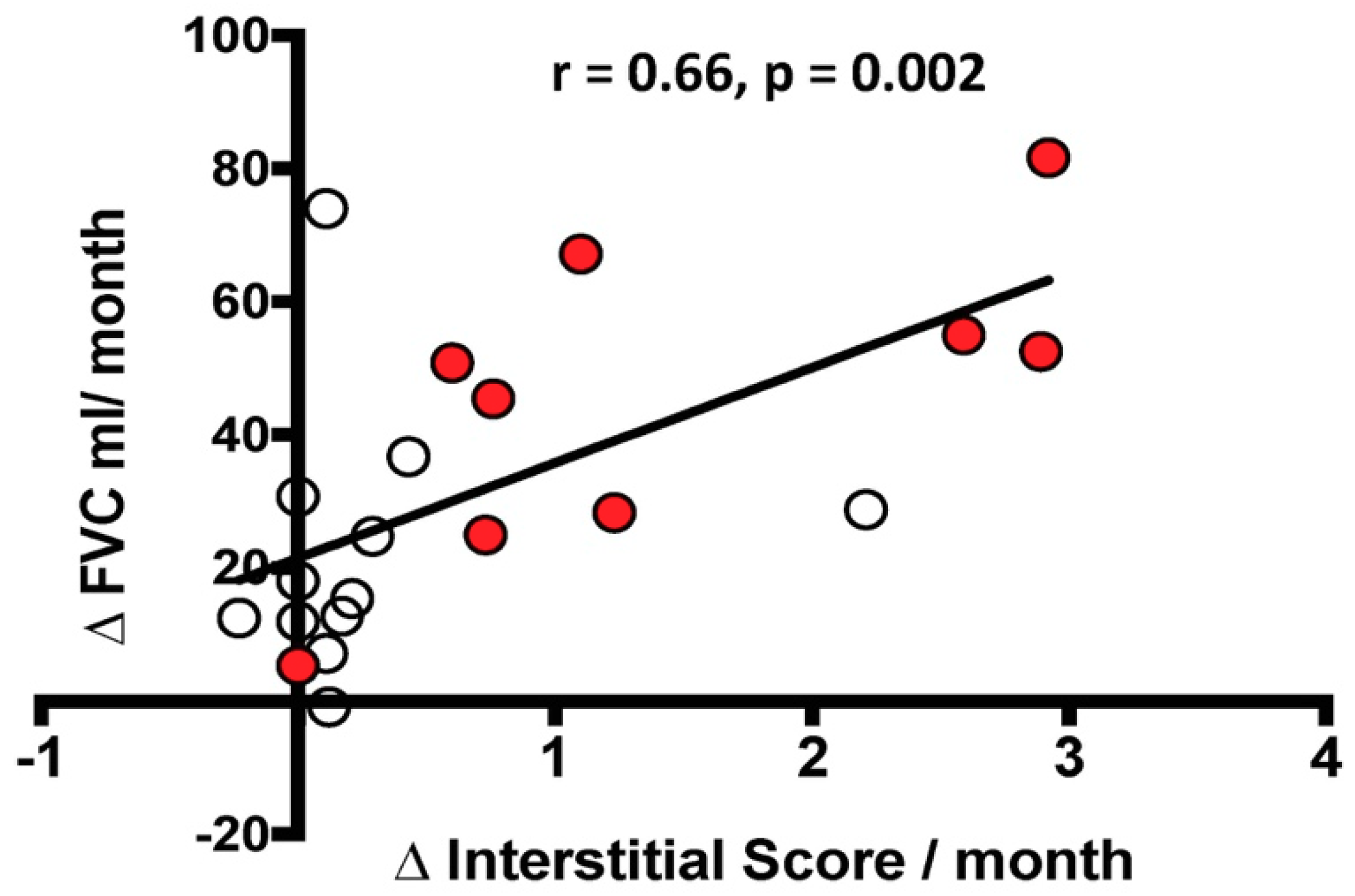
There was a significant correlation between the functional decline, defined as ΔFVC mL/month, and the radiological changes in IS, defined as ΔIS/month, but not with ΔAS/month. However, when stratified by the rate of decline, the correlation between ΔFVC mL/month and ΔIS/month was no longer significant in the rapid or slow decliners (Figure 5).
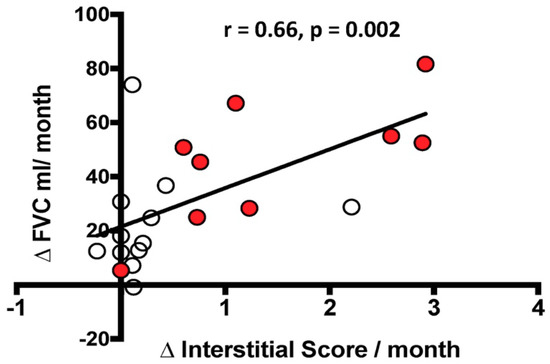
Figure 5.
Relationship between the change over time in FVC mL (ΔFVC mL/month) and the change over time in Interstitial Score (ΔInterstitial Score/month). The black line represents the correlation in the entire population. White circles indicate slow progressors and red circles represent rapid progressors. Spearman’s rank correlation: r = 0.66, p = 0.002 in the entire population; r = 0.31, p = 0.6 in slow progressors alone; r = 0.73, p = 0.06 in rapid progressors alone.
When the delta IS was normalized by IS at the beginning, the correlation with the delta FVC was maintained in the patient population as a whole (p = 0.01, r = 0.57) but not in the slow and rapid subgroups.
4. Discussion
High-Resolution Computed Tomography (HRCT) plays a central role in diagnosing and staging the severity of IPF [2,5,7,8,10,12]. The HRCT relevance in IPF underlines its potential usefulness as a tool to predict future disease behavior at the time of diagnosis and to design future clinical trials [12].
In this study, we investigated in a group of IPF patients, followed long term (prior to available anti-fibrotic treatment), whether radiological quantification of fibrotic abnormalities and ground glass opacities in HRCT at diagnosis may predict disease behavior over time. Our results showed that, in the HRCT performed at diagnosis, patients who had experienced a rapid functional decline, rapid progressors, had a higher alveolar score (AS) than slow progressors, while the extent of fibrosis (Interstitial Score, IS) was similar in the two groups. Furthermore, in a second HRCT at follow up, changes in IS over time was correlated with functional decline.
Idiopathic Pulmonary Fibrosis is a heterogeneous disease, the outcome of which is determined by the rapidity of the longitudinal loss of forced vital capacity, a major prognostic predictor linked to an increase in mortality risk [16,23,24,25]. The diagnosis of IPF can be done in most cases by HRCT, considered a good reflection of UIP, the pathological counterpart of IPF. Based on its diagnostic accuracy, the scoring of the different abnormalities seen in HRCT has been used in an attempt to improve the prediction of IPF outcomes [7,10]. In general, these studies show an important variability in individual disease progression and an association between mortality and increased fibrosis score over time, especially when combined with changes in FVC [7,8,10,11,12,26].
According to the annual FVC decline greater or lower than 10%pred, two clinical IPF phenotypes have been repeatedly reported in the literature, the rapid and the slow progressors [3,4,5,27]. These phenotypes have been shown to have a distinct gene expression profile, including the activation of important pro-inflammatory pathways that may potentially play a role in disease progression of rapid decliners [4,27]. Previous studies assessing the value of HRCT in the prediction of IPF behavior did not differentiate their patients by the predetermined rate of progression, rapid or slow, a factor of crucial significance for the understanding of disease prognosis. In the present study, we analyzed a group of patients naïve to antifibrotics classified as slow or rapid progressors, based on FVC decay over time, with the aim to identify HRCT features that could possibly differentiate at presentation rapid from slow decliners, a major prognostic predictor in IPF.
Differently from previous studies [6,7], we evaluated not only the degree of interstitial score (IS) but also the degree of ground glass opacities, alveolar score (AS). Although pure ground glass opacity is not usually a feature of UIP, many patients with fibrotic lung disease have ground glass opacity admixed with reticular abnormality and/or traction bronchiectasis. In this context, the ground glass opacity should be regarded as part of the fibrotic process, as indicated by the recent Fleischner Society white paper [5] and, as such, we believe it needed to be assessed [2]. The HRCT findings in our study, showing that at baseline rapid progressors had an AS significantly greater (almost double) than slow progressors, is of high interest since it might help to identify, early in the course of the disease, the more aggressive phenotype with worse prognosis.
The significance of ground glass opacities in IPF is not clear, but it might be related to parenchymal inflammatory/exudative infiltrates, probably more evident in cases with more aggressive disease and rapid progression. In support of this possibility are our recently published findings that the different clinical course (rapid or slow) of an IPF population undergoing lung transplantation was associated with distinct underlying pathology in the explanted lungs [3]. Rapid progressors showed an extensive cellular immune infiltrate, both innate and adaptive, more prominent than slow progressors [3]. The possibility that AS might represent an alveolar inflammatory/exudative infiltrate is supported by the correlation observed in the present study between AS and the total number of lymphocytes in the 13 explanted lungs. Because the timing to the second HRCT was different in the 21 patients with two consecutive HRCTs, to compare the results, we calculated the changes over time in IS, AS, and FVC and expressed them as change/per month. As suggested by others [26], the change in FVC/month in the whole group (rapids and slows together) correlated significantly with IS change/month.
Of interest and fitting with our previous findings, the AS, plausibly representing a cellular/exudative inflammatory response, increased significantly at follow up in rapid progressors while it remained stable in slow progressors. The exact explanation of this feature needs to be elucidated, however we can speculate that the AS signals a more exudative and thus unstable disease, rich in fibroblast foci, and more likely to rapidly progress towards fibrotic changes and consequently rapid functional worsening. These findings seem in line with our previous evidence on lung pathology that showed the presence of an intense lung immune infiltrate in the rapid progressors, but not in the slow progressors [3]. These results would support a role of inflammation and of adaptive immune response in determining disease behavior [28] and might account, at least in part, for the different responses to anti-fibrotic drugs among IPF patients. In favor of this possibility is our recent report showing that pirfenidone reduces FVC decline in IPF patients with a more pronounced beneficial effect in rapid than in slow progressors [29].
A strength of our study is the unique opportunity to investigate a population of IPF patients naïve of anti-fibrotic treatment, followed for at least one year, in which the disease decline phenotype, rapid or slow, could be determined by changes in FVC. Knowing the rate of decline allowed us to investigate if patterns of HRCT abnormalities could separate the rapid from slow phenotype, since the rate of FVC decay signals the worse prognosis of the disease and the response to treatment. Another important and unique feature of the study is that both AS and IS, radiological parameters which correlated with FVC decay, had a pathological confirmation. Due to the availability of effective anti-fibrotic drugs [30,31], studies on IPF patients naïve of antifibrotics will become progressively less common, if at all possible (and ethical). Thus, the HRCT patterns described could help to predict the long-term disease behavior and prognosis and be the bases for further studies in treated patients.
A limitation of our study is the relative low number of cases and the fact that the time interval between HRCT1 and HRCT2 was not the same in all patients. We corrected for this difference in timing by expressing AS and IS as changes per month. Since the pulmonary function tests were performed at the same time of HRCT, we could correlate the radiological changes with the functional changes. The quantitative estimation of HRCTs disease extent was independently evaluated by two expert radiologists with a good inter-observer agreement. It may appear a limitation not having used the automated quantitative imaging analysis [32,33,34], however visual lung scores are and have been used as “gold standard” to validate software analyses [11,35] that, in any case, have themselves some limitations and disadvantages such as the applicability to retrospective CT dataset [34,36].
5. Conclusion
In conclusion, quantitative estimation of High-Resolution Computed Tomography alveolar (AS) and interstitial (IS) scores reflects the distinct clinical and pathological behavior of Idiopathic Pulmonary Fibrosis slow and rapid decliners. Furthermore, the alveolar score, which reflects the immune/inflammatory infiltrate found in lung tissue, could be a useful tool to differentiate rapid from slow progressors at presentation.
Supplementary Materials
The following material is available online at https://www.mdpi.com/2077-0383/8/3/399/s1, Methods; Table S1: Demographics and clinical characteristics of the 21 subjects with available follow up HRCT2 (of which 12 slow and 9 rapid progressors); Table S2: Demographics and clinical features of the 13 subjects undergoing lung transplantation (of which 6 slow and 7 rapid progressors); Results; Table S3: Alveolar Score (AS) and Interstitial Score (IS) of HRCT1 in the entire population (n = 49), of which 30 slow and 19 rapid progressors; Table S4. Alveolar Score (AS) and Interstitial Score (IS) of both h HRCT scans (HRCT1 and HRCT2) in the entire population (n = 49), of which 30 slow and 19 rapid progressors.
Author Contributions
Conceptualization, E.B. (Elisabetta Balestro); Data curation, E.C., D.B., F.P. and D.L.; Formal analysis, E.B. (Erica Bazzan); Funding acquisition, P.S.; Investigation, G.T.; Resources, G.B., R.P., F.R. and M.S. (Marco Schiavon); Validation, F.C. and M.P.F.B.; and Writing—review and editing, E.C., E.B. (Elisabetta Balestro), P.S., M.G.C. and M.S. (Marina Saetta).
Funding
University of Padova; Department of Cardiac, Thoracic, Vascular Sciences and Public Health, grant BIRD163522.
Acknowledgements
All authors take responsibility for the content of the manuscript, including data and analysis.
Conflicts of Interest
Financial/nonfinancial disclosures: P.S. has received personal fees and non-financial support from Roche, Boehringer-Ingelheim, Zambon, and PPM Services. Elisabetta Balestro has received personal fees from Roche and Boehringer-Ingelheim. Marina Saetta has received research grants for the Department (not personal) to her Institution from Takeda Ltd., Chiesi Farmaceutici and Laboratori Guidotti SpA. These funds were not used to support this project. All other authors declare no competing interests.
Abbreviations
| IPF | Idiopathic Pulmonary Fibrosis |
| UIP | Usual Interstitial Pneumonia |
| HRCT | High Resolution Computed Tomography |
| GGO | Ground Glass Opacities |
| FVC | Forced Vital Capacity |
| AS | Alveolar Score |
| IS | Interstitial Score |
| FF | Fibroblastic Foci |
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morelli, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Balestro, E.; Calabrese, F.; Turato, G.; Lunardi, F.; Bazzan, E.; Marulli, G.; Biondini, D.; Rossi, E.; Sanduzzi, A.; Rea, F.; et al. Immune inflammation and disease progression in idiopathic pulmonary fibrosis. PLoS ONE 2016, 11, e0154516. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Carrillo, G.; Estrada, A.; Mejia, M.; Becerril, C.; Cisneros, J.; Gaxiola, M.; Pérez-Padilla, R.; Navarro, C.; Richards, T.; et al. Accelerated variant of idiopathic pulmonary fibrosis: Clinical behavior and gene expression pattern. PLoS ONE 2007, 2, e482. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Wells, A.U.; Desai, S.R.; Rubens, M.B.; Goh, N.S.; Cramer, D.; Nicholson, A.G.; Colby, T.V.; du Bois, R.M.; Hansell, D.M. Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003, 167, 962–969. [Google Scholar] [CrossRef]
- Ley, B.; Elicker, B.M.; Hartman, T.E.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Lee, J.S.; Jones, K.D.; Richeldi, L.; King, T.E., Jr.; Collard, H.R. Idiopathic Pulmonary Fibrosis: CT and Risk of Death. Radiology 2014, 273, 570–579. [Google Scholar] [CrossRef]
- Fell, C.D.; Martinez, F.J.; Liu, L.X.; Murray, S.; Han, M.K.; Kazerooni, E.A.; Gross, B.H.; Myers, J.; Travis, W.D.; Colby, T.V. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 832–837. [Google Scholar] [CrossRef]
- Lynch, D.A.; Godwin, J.D.; Safrin, S.; Starko, K.M.; Hormel, P.; Brown, K.K.; Raghu, G.; King, T.E., Jr.; Bradford, W.Z.; et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: Diagnosis and prognosis. Am. J. Respir. Crit. Care Med. 2005, 172, 488–493. [Google Scholar] [CrossRef]
- Best, A.C.; Meng, J.; Lynch, A.M.; Bozic, C.M.; Miller, D.; Grunwald, G.K.; Lynch, D.A. Idiopathic pulmonary fibrosis: Physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology 2008, 246, 935–940. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Lynch, D.A.; van Beek, E.J.; Kazerooni, E.A.; Guo, J.; Xia, M.; Murray, S.; Anstrom, K.J.; Yow, E.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis: The Association between the Adaptive Multiple Features Method and Fibrosis Outcomes. Am. J. Respir. Crit. Care Med. 2017, 195, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Goldin, J.G.; King, T.E., Jr.; Lynch, D.A.; Richeldi, L.; Wells, A.U. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: A Position Paper from the Fleischner society. Lancet Respir. Med. 2015, 3, 483–496. [Google Scholar] [CrossRef]
- Ley, B.; Bradford, W.; Vittinghoff, E.; Weycker, D.; du Bois, R.M.; Collard, H.R. Predictors of Mortality Poorly Predict Common Measures of Disease Progression in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.L.; Nabihah Tayob, M.; Han, M.K.; Zappala, C.; Kervitsky, D.; Murray, S.; Wells, A.U.; Brown, K.K.; Martinez, F.J.; Flaherty, K.R. Predicting Pulmonary Fibrosis Disease Course From Past Trends in Pulmonary Function. Chest 2014, 145, 57–585. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, M.L.; Xia, M.; Zhou, Y.; Murray, S.; Tayob, N.; Brown, K.K.; Wells, A.U.; Schmidt, S.L.; Martinez, F.J.; Flaherty, K.R. Idiopathic Pulmonary Fibrosis: Gender-Age-Physiology Index Stage for Predicting Future Lung Function Decline. Chest 2016, 149, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Ballarin, A.; Bazzan, E.; Zenteno, R.H.; Turato, G.; Baraldo, S.; Zanovello, D.; Mutti, E.; Hogg, J.C.; Saetta, M.; Cosio, M.G. Mast cell infiltration discriminates between histopathological phenotypes of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 233–239. [Google Scholar] [CrossRef]
- Bazzan, E.; Saetta, M.; Turato, G.; Borroni, E.M.; Cancellieri, C.; Baraldo, S.; Savino, B.; Calabrese, F.; Ballarin, A.; Balestro, E.; et al. Expression of the atypical chemokine receptor D6 in human alveolar macrophages in COPD. Chest 2013, 143, 98–106. [Google Scholar] [CrossRef]
- Saetta, M.; Baraldo, S.; Corbino, L.; Turato, G.; Braccioni, F.; Rea, F.; Cavallesco, G.; Tropeano, G.; Mapp, C.E.; Maestrelli, P.; et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 711–717. [Google Scholar] [CrossRef]
- Bonato, M.; Bazzan, E.; Snijders, D.; Tinè, M.; Biondini, D.; Turato, G.; Balestro, E.; Papi, A.; Cosio, M.G.; Barbato, A.; et al. Clinical and Pathologic Factors Predicting Future Asthma in Wheezing Children. A Longitudinal Study. Am. J. Respir. Cell Mol. Biol. 2018, 59, 458–466. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991; Volume 10, pp. 1635–1636. [Google Scholar]
- American Thoracic Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am. J. Respir. Crit. Care Med. 2000, 161, 646–664. [Google Scholar] [CrossRef]
- Zappala, C.J.; Latsi, P.I.; Nicholson, A.G.; Colby, T.V.; Cramer, D.; Renzoni, E.A.; Hansell, D.M.; du Bois, R.M.; Wells, A.U. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 830–835. [Google Scholar] [CrossRef]
- Du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; King, T.E., Jr.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: Test properties and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 184, 1382–1389. [Google Scholar] [CrossRef]
- Ley, B.; Collard, H.R.; King, T.E., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Oda, K.; Ishimoto, H.; Yatera, K.; Naito, K.; Ogoshi, T.; Yamasaki, K.; Imanaga, T.; Tsuda, T.; Nakao, H.; Kawanami, T.; Mukae, H. High-resolution CT scoring system-based grading scale predicts the clinical outcomes in patients with idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 1–9. [Google Scholar] [CrossRef]
- Boon, K.; Bailey, N.W.; Yang, J.; Steel, M.P.; Groshong, S.; Kervitsky, D.; Brown, K.K.; Schwarz, M.I.; Schwartz, D.A. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PLoS ONE 2009, 4, e5134. [Google Scholar] [CrossRef]
- Kwapiszewska, G.; Gungl, A.; Wilhelm, J.; Marsh, L.M.; Thekkekara Puthenparampil, H.; Sinn, K.; Didiasova, M.; Klepetko, W.; Kosanovic, D.; et al. Transcriptome profiling reveals the complexityof pirfenidone effects in idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 52, 1800564. [Google Scholar] [CrossRef]
- Biondini, D.; Balestro, E.; Lacedonia, D.; Cerri, S.; Milaneschi, R.; Luppi, F.; Cocconcelli, E.; Bazzan, E.; Clini, E.; Foschino Barbaro, M.P.; et al. Pretreatment rate of decay in forced vital capacity predicts long-term response to pirfenidone in patients with idiopathic pulmonary fibrosis. Sci. Rep. 2018, 8, 5961. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- King, T.E.J.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Humphries, S.M.; Swigris, J.J.; Brown, K.K.; Strand, M.; Gong, Q.; Sundy, J.S.; Raghu, G.; Schwarz, M.I.; Flaherty, K.R.; Sood, R.; et al. Quantitative high-resolution computed tomography fibrosis score: Performance characteristics in idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef]
- Maldonado, F.; Moua, T.; Rajagopalan, S.; Karwoski, R.A.; Raghunath, S.; Decker, P.A.; Hartman, T.E.; Bartholmai, B.J.; Robb, R.A.; Ryu, J.H. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 204–212. [Google Scholar] [CrossRef]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; van Moorsel, C.H.M.; van Es, H.W.; van Beek, F.T.; Struik, M.H.L.; Kokosi, M.; Egashira, R.; Brun, A.L.; Nair, A.; Walsh, S.L.F.; et al. Predicting Outcomes in Idiopathic Pulmonary Fibrosis Using Automated Computed Tomographic Analysis. Am. J. Respir. Crit. Care Med. 2018, 198, 767–776. [Google Scholar] [CrossRef]
- Humphries, S.M.; Schroeder, J.D.; Huckleberry, J.; Rho, B.H.; Schroeder, J.D.; Strand, M.; Schwarz, M.I.; Flaherty, K.R.; Kazerooni, E.A.; van Beek, E.J.R.; et al. Idiopathic Pulmonary Fibrosis: Data-driven Textural Analysis of Extent of Fibrosis at Baseline and 15-Month Follow-up. Radiology 2017, 285, 270–278. [Google Scholar] [CrossRef]
- Wu, X.; Kim, G.H.; Huckleberry, J.; Rho, B.H.; Schroeder, J.D.; Strand, M.; Schwarz, M.I.; Flaherty, K.R.; Kazerooni, E.A.; van Beek, E.J.R.; et al. Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis: The Future of Quantitative Analysis. Am. J. Respir. Crit. Care Med. 2019, 199, 12–21. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).