Inhibition of Osteoclastogenesis by Thioredoxin-Interacting Protein-Derived Peptide (TN13)
Abstract
1. Introduction
2. Experimental Section
2.1. Mice
2.2. Cells, Media, and Reagents
2.3. Cell Culture
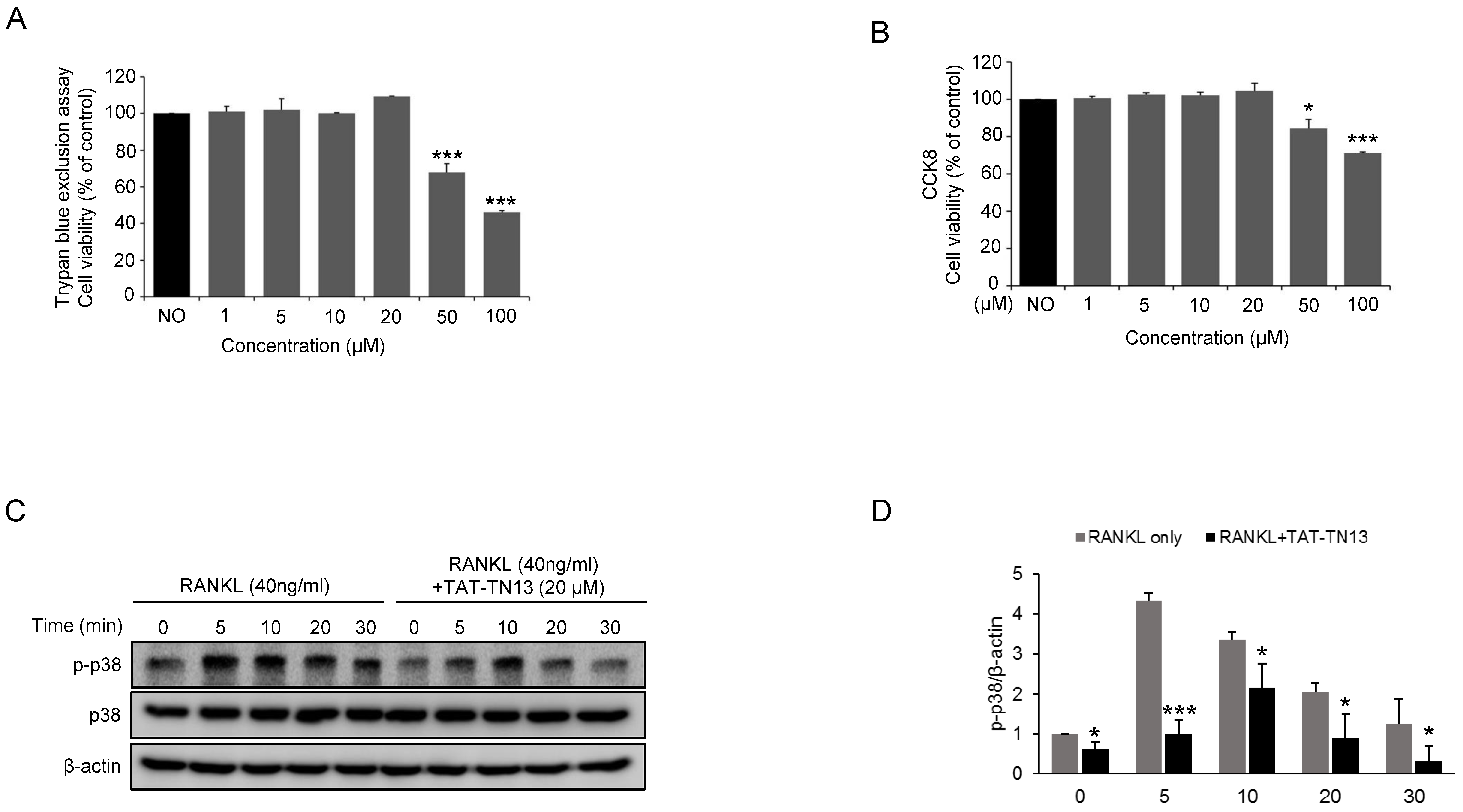
2.4. Cell Viability Assay
2.5. TRAP Staining
2.6. Resorption Pit Assay by Osteoclasts
2.7. RNA Isolation and Quantitative Real-Time PCR
2.8. Luciferase Reporter Assay
2.9. Western Blot Analysis
2.10. Immunofluorescence Analysis of NF-κB
2.11. Ovariectomized (OVX) Mouse Model
2.12. Histological Analysis
2.13. Statistical Analysis
3. Results
3.1. TAT-TN13 Inhibits RANKL-Induced Osteoclast Differentiation
3.2. TAT-TN13 Reduces the Number of Mature Osteoclasts
3.3. TAT-TN13 Suppresses RANKL-Induced Osteoclast-Related Genes Expression
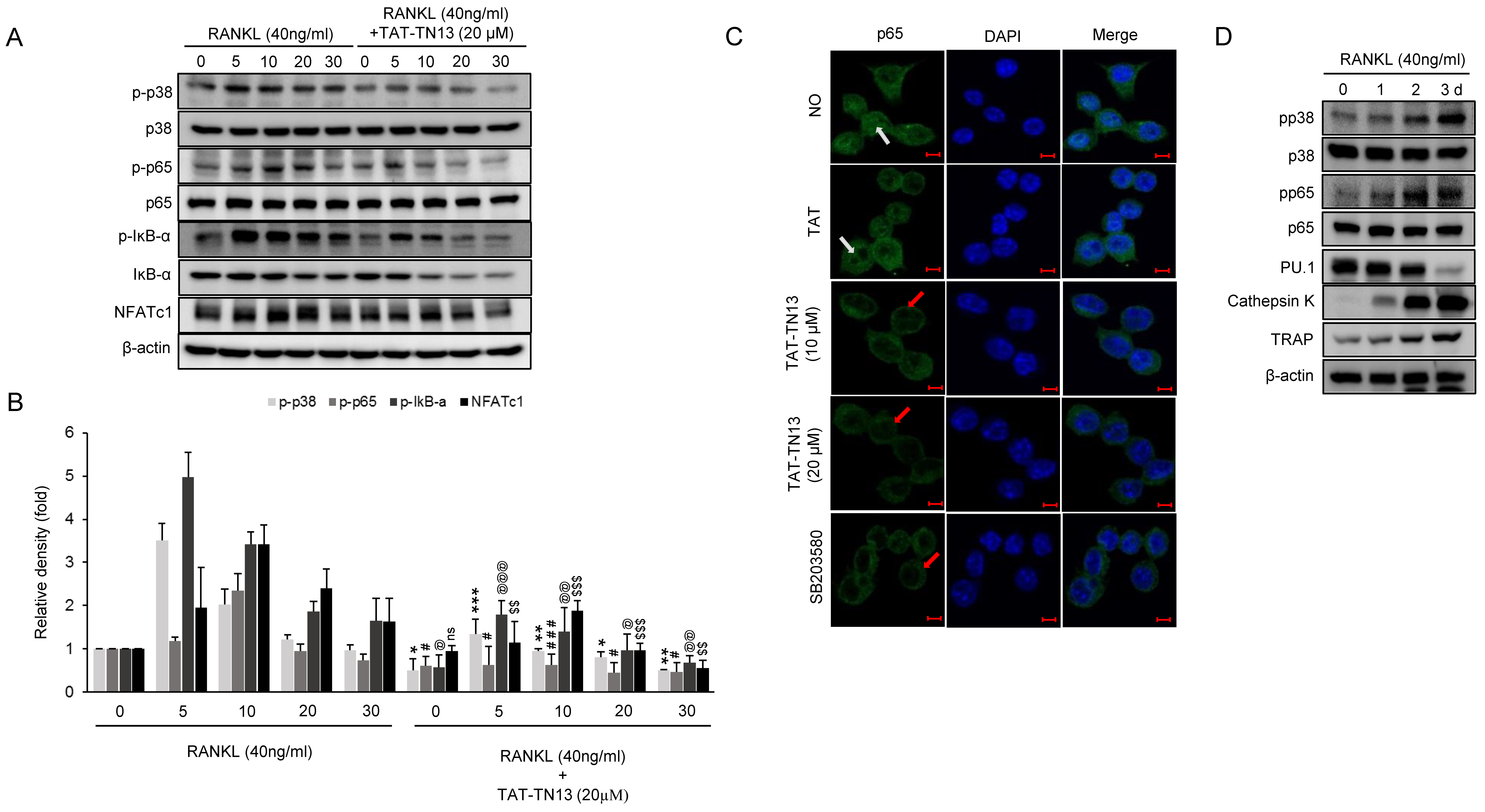
3.4. TAT-TN13 Suppresses RANKL-Induced NF-κB Activation
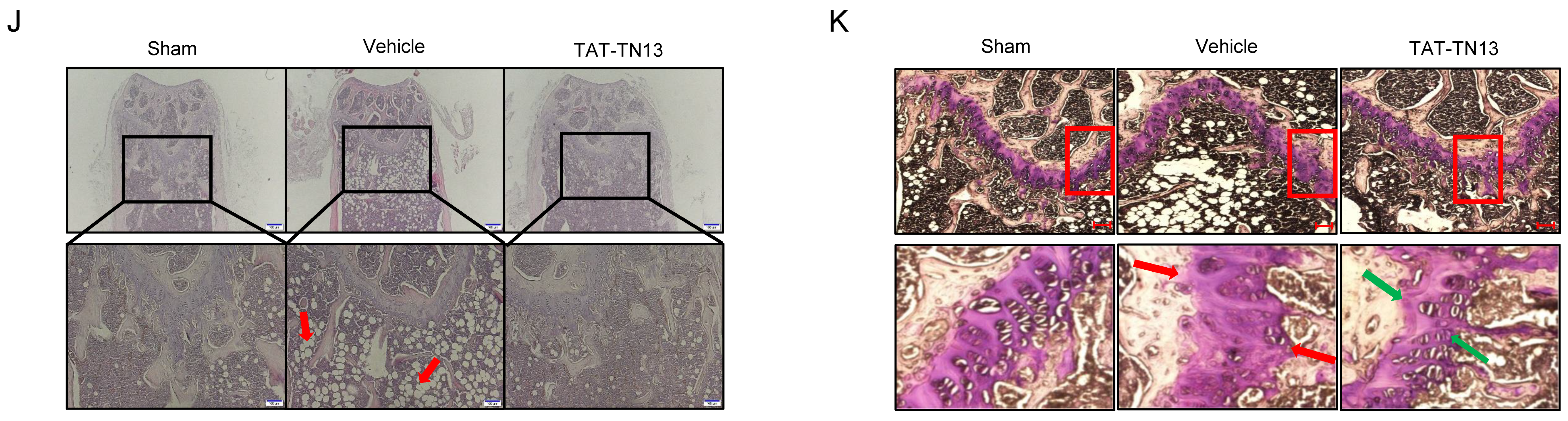
3.5. TAT-TN13 Prevented OVX-Induced Bone Loss In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, D.; Heo, D.N.; Kim, H.J.; Ko, W.K.; Lee, S.J.; Heo, M.; Bang, J.B.; Lee, J.B.; Hwang, D.S.; Do, S.H.; et al. Inhibition of Osteoclast Differentiation and Bone Resorption by Bisphosphonate-conjugated Gold Nanoparticles. Sci. Rep. 2016, 6, 27336. [Google Scholar] [CrossRef]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Takayanagi, H.; Iizuka, H.; Juji, T.; Nakagawa, T.; Yamamoto, A.; Miyazaki, T.; Koshihara, Y.; Oda, H.; Nakamura, K.; Tanaka, S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 259–269. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998, 273, 20551–20555. [Google Scholar] [CrossRef]
- Bharti, A.C.; Takada, Y.; Shishodia, S.; Aggarwal, B.B. Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB-independent and TRAF6-dependent mechanism. J. Biol. Chem. 2004, 279, 6065–6076. [Google Scholar] [CrossRef]
- Cong, Q.; Jia, H.; Li, P.; Qiu, S.; Yeh, J.; Wang, Y.; Zhang, Z.L.; Ao, J.; Li, B.; Liu, H. p38alpha MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Sci. Rep. 2017, 7, 45964. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.L.; Hammaker, D.; Edgar, M.; Zaiss, M.M.; Teufel, S.; David, J.P.; Schett, G.; Firestein, G.S. Differential roles of MAPK kinases MKK3 and MKK6 in osteoclastogenesis and bone loss. PLoS ONE 2014, 9, e84818. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sudo, T.; Saito, T.; Osada, H.; Tsujimoto, M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J. Biol. Chem. 2000, 275, 31155–31161. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ryu, J.; Ha, J.; Chang, E.J.; Kim, H.J.; Kim, H.M.; Kitamura, T.; Lee, Z.H.; Kim, H.H. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006, 13, 1879–1891. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef]
- Bohm, C.; Hayer, S.; Kilian, A.; Zaiss, M.M.; Finger, S.; Hess, A.; Engelke, K.; Kollias, G.; Kronke, G.; Zwerina, J.; et al. The alpha-isoform of p38 MAPK specifically regulates arthritic bone loss. J. Immunol. 2009, 183, 5938–5947. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.O.; Byun, J.E.; Kim, W.S.; Kim, M.J.; Song, H.Y.; Kim, Y.K.; Kang, D.K.; Park, Y.J.; Kim, T.D.; et al. Thioredoxin-interacting protein regulates haematopoietic stem cell ageing and rejuvenation by inhibiting p38 kinase activity. Nat. Commun. 2016, 7, 13674. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.S.; Jung, H.; Choi, I. Pharmacological Regulation of Oxidative Stress in Stem Cells. Oxidative Med. Cell. Longev. 2018, 2018, 13. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, W.S.; Kim, D.O.; Byun, J.E.; Huy, H.; Lee, S.Y.; Song, H.Y.; Park, Y.J.; Kim, T.D.; Yoon, S.R.; et al. Macrophage migration inhibitory factor interacts with thioredoxin-interacting protein and induces NF-kappaB activity. Cell. Signal. 2017, 34, 110–120. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, M.J.; Kim, D.O.; Byun, J.E.; Huy, H.; Song, H.Y.; Park, Y.J.; Kim, T.D.; Yoon, S.R.; Choi, E.J.; et al. Suppressor of Cytokine Signaling 2 Negatively Regulates NK Cell Differentiation by Inhibiting JAK2 Activity. Sci. Rep. 2017, 7, 46153. [Google Scholar] [CrossRef]
- Kawaida, R.; Ohtsuka, T.; Okutsu, J.; Takahashi, T.; Kadono, Y.; Oda, H.; Hikita, A.; Nakamura, K.; Tanaka, S.; Furukawa, H. Jun dimerization protein 2 (JDP2), a member of the AP-1 family of transcription factor, mediates osteoclast differentiation induced by RANKL. J. Exp. Med. 2003, 197, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-kappaB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. (Seoul) 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Verma, I.M.; Stevenson, J.K.; Schwarz, E.M.; Van Antwerp, D.; Miyamoto, S. Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev. 1995, 9, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 2007, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.J.; Li, H.W.; Liu, G.W.; Qu, X.H.; Tian, B.; Yan, W.; Lin, Z.; Tang, T.T.; Qin, A.; Dai, K.R. Andrographolide suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Br. J. Pharmacol. 2014, 171, 663–675. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tokimura, F.; Tanaka, S. A review of denosumab for the treatment of osteoporosis. Patient Prefer Adherence 2014, 8, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; Bjarnason, N.H.; Mitlak, B.H.; Ravoux, A.C.; Shah, A.S.; Huster, W.J.; Draper, M.; Christiansen, C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N. Engl. J. Med. 1997, 337, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D. Treatment of postmenopausal osteoporosis. Lancet 2002, 359, 2018–2026. [Google Scholar] [CrossRef]
- McClung, M.R.; Wasnich, R.D.; Hosking, D.J.; Christiansen, C.; Ravn, P.; Wu, M.; Mantz, A.M.; Yates, J.; Ross, P.D.; Santora, A.C., 2nd; et al. Prevention of postmenopausal bone loss: Six-year results from the Early Postmenopausal Intervention Cohort Study. J. Clin. Endocrinol. Metab. 2004, 89, 4879–4885. [Google Scholar] [CrossRef] [PubMed]
- Zwerina, J.; Hayer, S.; Redlich, K.; Bobacz, K.; Kollias, G.; Smolen, J.S.; Schett, G. Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum 2006, 54, 463–472. [Google Scholar] [CrossRef]
- Badger, A.M.; Griswold, D.E.; Kapadia, R.; Blake, S.; Swift, B.A.; Hoffman, S.J.; Stroup, G.B.; Webb, E.; Rieman, D.J.; Gowen, M.; et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum 2000, 43, 175–183. [Google Scholar] [CrossRef]
- Nishikawa, M.; Myoui, A.; Tomita, T.; Takahi, K.; Nampei, A.; Yoshikawa, H. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum 2003, 48, 2670–2681. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, S.; Ma, J.Y.; Mangadu, R.; Jiang, Y.; Zhao, J.J.; Almirez, R.; Kerr, I.; Stebbins, E.G.; O’Young, G.; Kapoun, A.M.; et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J. Pharmacol. Exp. Ther. 2006, 318, 132–141. [Google Scholar] [CrossRef]
- Mihara, K.; Almansa, C.; Smeets, R.L.; Loomans, E.E.; Dulos, J.; Vink, P.M.; Rooseboom, M.; Kreutzer, H.; Cavalcanti, F.; Boots, A.M.; et al. A potent and selective p38 inhibitor protects against bone damage in murine collagen-induced arthritis: A comparison with neutralization of mouse TNFalpha. Br. J. Pharmacol. 2008, 154, 153–164. [Google Scholar] [CrossRef]
- Saklatvala, J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004, 4, 372–377. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Zwerina, J.; Firestein, G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L.; Franzoso, G.; Siebenlist, U. Required and nonessential functions of nuclear factor-kappa B in bone cells. Bone 1999, 25, 137–139. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Kim, W.S.; Byun, J.-E.; Choi, J.H.; Yoon, S.R.; Choi, I.; Jung, H. Inhibition of Osteoclastogenesis by Thioredoxin-Interacting Protein-Derived Peptide (TN13). J. Clin. Med. 2019, 8, 431. https://doi.org/10.3390/jcm8040431
Kim MJ, Kim WS, Byun J-E, Choi JH, Yoon SR, Choi I, Jung H. Inhibition of Osteoclastogenesis by Thioredoxin-Interacting Protein-Derived Peptide (TN13). Journal of Clinical Medicine. 2019; 8(4):431. https://doi.org/10.3390/jcm8040431
Chicago/Turabian StyleKim, Mi Jeong, Won Sam Kim, Jae-Eun Byun, Jung Ha Choi, Suk Ran Yoon, Inpyo Choi, and Haiyoung Jung. 2019. "Inhibition of Osteoclastogenesis by Thioredoxin-Interacting Protein-Derived Peptide (TN13)" Journal of Clinical Medicine 8, no. 4: 431. https://doi.org/10.3390/jcm8040431
APA StyleKim, M. J., Kim, W. S., Byun, J.-E., Choi, J. H., Yoon, S. R., Choi, I., & Jung, H. (2019). Inhibition of Osteoclastogenesis by Thioredoxin-Interacting Protein-Derived Peptide (TN13). Journal of Clinical Medicine, 8(4), 431. https://doi.org/10.3390/jcm8040431






