Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis
Abstract
:1. Introduction
2. Matherials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Nitrous Oxide Exposure
2.5. Main Outcomes and Measures
2.6. Data Synthesis and Analysis
2.7. Assessment of Bias
3. Results
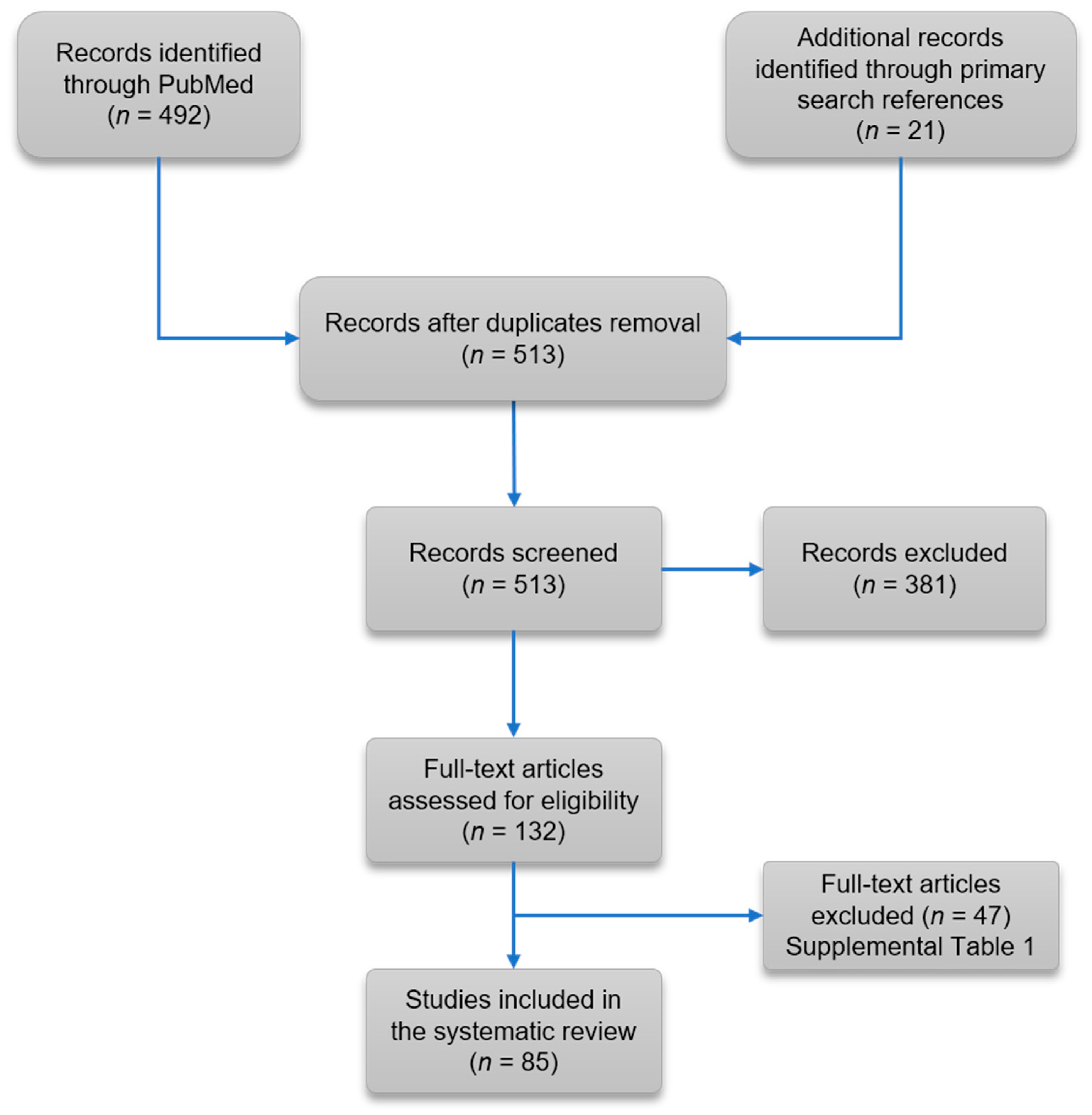
3.1. Literature Review
3.2. Prevalence of Clinical, Laboratory and Magnetic Resonance Findings in Association with N2O Exposure
3.3. Secondary Outcomes
3.3.1. Predictors of Short Nitrous Oxide Exposure
Univariate Analysis
Multivariate Analysis
3.3.2. Association Between the Amount of Nitrous Oxide Exposure and Outcomes
3.3.3. Assessment of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erving, H.W. The discoverer of anaesthesia: Dr. Horace wells of hartford. Yale J. Biol. Med. 1933, 5, 421–430. [Google Scholar] [PubMed]
- Finder, S.G. Lessons from history: Horace wells and the moral features of clinical contexts. Anesth. Prog. 1995, 42, 1–6. [Google Scholar]
- Randhawa, G.; Bodenham, A. The increasing recreational use of nitrous oxide: History revisited. Br. J. Anaesth. 2016, 116, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Garakani, A.; Jaffe, R.J.; Savla, D.; Welch, A.K.; Protin, C.A.; Bryson, E.O.; McDowell, D.M. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: A systematic review of the case literature. Am. J. Addict. 2016, 25, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Lassen, H.C.; Henriksen, E.; Neukirch, F.; Kristensen, H.S. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet (Lond. Engl.) 1956, 270, 527–530. [Google Scholar] [CrossRef]
- Amess, J.A.; Burman, J.F.; Rees, G.M.; Nancekievill, D.G.; Mollin, D.L. Megaloblastic haemopoiesis in patients receiving nitrous oxide. Lancet (Lond. Engl.) 1978, 2, 339–342. [Google Scholar] [CrossRef]
- Reuters, T. Endnote x7; Thomson Reuters: Philadelphia, PA, USA, 2013. [Google Scholar]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Brito, A.; Miller, J.W.; Green, R.; Allen, L.H. Combined indicator of vitamin b12 status: Modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin. Chem. Lab. Med. 2015, 53, 1215–1225. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An inTroduction to the Bootstrap; Taylor & Francis: Abingdon, UK, 1994. [Google Scholar]
- Organization of Word Health. Iron Deficiency Anaemia Assessment, Prevention, and Control a Guide for Programme Managers. Available online: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf (accessed on 27 September 2018).
- Bosco, P.; Gueant-Rodriguez, R.M.; Anello, G.; Barone, C.; Namour, F.; Caraci, F.; Romano, A.; Romano, C.; Gueant, J.L. Methionine synthase (mtr) 2756 (a -->g) polymorphism, double heterozygosity methionine synthase 2756 ag/methionine synthase reductase (mtrr) 66 ag, and elevated homocysteinemia are three risk factors for having a child with down syndrome. Am. J. Med. Genet. Part A 2003, 121, 219–224. [Google Scholar] [CrossRef]
- Brunaud, L.; Alberto, J.M.; Ayav, A.; Gerard, P.; Namour, F.; Antunes, L.; Braun, M.; Bronowicki, J.P.; Bresler, L.; Gueant, J.L. Vitamin b12 is a strong determinant of low methionine synthase activity and DNA hypomethylation in gastrectomized rats. Digestion 2003, 68, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ortiou, S.; Alberto, J.M.; Gueant, J.L.; Merten, M. Homocysteine increases methionine synthase mrna level in caco-2 cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2004, 14, 407–414. [Google Scholar]
- Bosco, P.; Gueant-Rodriguez, R.M.; Anello, G.; Spada, R.; Romano, A.; Fajardo, A.; Caraci, F.; Ferri, R.; Gueant, J.L. Association of homocysteine (but not of mthfr 677 c > t, mtr 2756 a > g, mtrr 66 a > g and tcn2 776 c>g) with ischaemic cerebrovascular disease in sicily. Thromb. Haemost. 2006, 96, 154–159. [Google Scholar]
- Alberto, J.M.; Hamelet, J.; Noll, C.; Blaise, S.; Bronowicki, J.P.; Gueant, J.L.; Delabar, J.M.; Janel, N. Mice deficient in cystathionine beta synthase display altered homocysteine remethylation pathway. Mol. Genet. Metab. 2007, 91, 396–398. [Google Scholar] [PubMed]
- Candito, M.; Rivet, R.; Herbeth, B.; Boisson, C.; Rudigoz, R.C.; Luton, D.; Journel, H.; Oury, J.F.; Roux, F.; Saura, R.; et al. Nutritional and genetic determinants of vitamin b and homocysteine metabolisms in neural tube defects: A multicenter case-control study. Am. J. Med. Genet. Part A 2008, 146, 1128–1133. [Google Scholar]
- Fofou-Caillierez, M.B.; Mrabet, N.T.; Chery, C.; Dreumont, N.; Flayac, J.; Pupavac, M.; Paoli, J.; Alberto, J.M.; Coelho, D.; Camadro, J.M.; et al. Interaction between methionine synthase isoforms and mmachc: Characterization in cblg-variant, cblg and cblc inherited causes of megaloblastic anaemia. Hum. Mol. Genet. 2013, 22, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Pooya, S.; Lorentz, S.; Gauchotte, G.; Arnold, C.; Gueant, J.L.; Battaglia-Hsu, S.F. Decreased vitamin b12 availability induces er stress through impaired sirt1-deacetylation of hsf1. Cell Death Dis. 2013, 4, e553. [Google Scholar] [CrossRef] [PubMed]
- Bassila, C.; Ghemrawi, R.; Flayac, J.; Froese, D.S.; Baumgartner, M.R.; Gueant, J.L.; Coelho, D. Methionine synthase and methionine synthase reductase interact with mmachc and with mmadhc. Biochim. Et Biophys. Acta 2017, 1863, 103–112. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin b12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Green, R. Vitamin b12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef]
- Hathout, L.; El-Saden, S. Nitrous oxide-induced b(1)(2) deficiency myelopathy: Perspectives on the clinical biochemistry of vitamin b(1)(2). J. Neurol. Sci. 2011, 301, 1–8. [Google Scholar] [CrossRef]
- Dinn, J.J.; McCann, S.; Wilson, P.; Reed, B.; Weir, D.; Scott, J. Animal model for subacute combined degeneration. Lancet (Lond. Engl.) 1978, 2, 1154. [Google Scholar] [CrossRef]
- van der Westhuyzen, J.; Fernandes-Costa, F.; Metz, J. Cobalamin inactivation by nitrous oxide produces severe neurological impairment in fruit bats: Protection by methionine and aggravation by folates. Life Sci. 1982, 31, 2001–2010. [Google Scholar] [CrossRef]
- Metz, J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu. Rev. Nutr. 1992, 12, 59–79. [Google Scholar] [CrossRef]
- Cantrill, R.C.; Oldfield, M.; van der Westhuyzen, J.; McLoughlin, J. Protein profile of the myelin membrane of the fruit bat rousettus aegyptiacus. Comp. Biochem. Physiol. B 1983, 76, 881–884. [Google Scholar] [CrossRef]
- Deacon, R.; Purkiss, P.; Green, R.; Lumb, M.; Perry, J.; Chanarin, I. Vitamin b12 neuropathy is not due to failure to methylate myelin basic protein. J. Neurol. Sci. 1986, 72, 113–117. [Google Scholar] [CrossRef]
- Veber, D.; Mutti, E.; Galmozzi, E.; Cedrola, S.; Galbiati, S.; Morabito, A.; Tredici, G.; La Porta, C.A.; Scalabrino, G. Increased levels of the cd40:Cd40 ligand dyad in the cerebrospinal fluid of rats with vitamin b12(cobalamin)-deficient central neuropathy. J. Neuroimmunol. 2006, 176, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G.; Nicolini, G.; Buccellato, F.R.; Peracchi, M.; Tredici, G.; Manfridi, A.; Pravettoni, G. Epidermal growth factor as a local mediator of the neurotrophic action of vitamin b(12) (cobalamin) in the rat central nervous system. FASEB J. 1999, 13, 2083–2090. [Google Scholar] [CrossRef]
- Scalabrino, G.; Tredici, G.; Buccellato, F.R.; Manfridi, A. Further evidence for the involvement of epidermal growth factor in the signaling pathway of vitamin b12 (cobalamin) in the rat central nervous system. J. Neuropathol. Exp. Neurol. 2000, 59, 808–814. [Google Scholar] [PubMed]
- Scalabrino, G.; Mutti, E.; Veber, D.; Aloe, L.; Corsi, M.M.; Galbiati, S.; Tredici, G. Increased spinal cord ngf levels in rats with cobalamin (vitamin b12) deficiency. Neurosci. Lett. 2006, 396, 153–158. [Google Scholar] [CrossRef]
- Scalabrino, G.; Carpo, M.; Bamonti, F.; Pizzinelli, S.; D’Avino, C.; Bresolin, N.; Meucci, G.; Martinelli, V.; Comi, G.C.; Peracchi, M. High tumor necrosis factor-alpha [corrected] levels in cerebrospinal fluid of cobalamin-deficient patients. Ann. Neurol. 2004, 56, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Osborne, M.L.; Kolhouse, J.F.; Binder, M.J.; Podell, E.R.; Utley, C.S.; Abrams, R.S.; Allen, R.H. Nitrous oxide has multiple deleterious effects on cobalamin metabolism and causes decreases in activities of both mammalian cobalamin-dependent enzymes in rats. J. Clin. Investig. 1981, 67, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Chan, M.T.; Leslie, K.; Peyton, P.; Paech, M.; Forbes, A. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br. J. Anaesth. 2008, 100, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Home Office. Drug Misuse: Findings from the 2016/17 Crime Survey for England and Wales. Available online: https://assets.Publishing.Service.Gov.Uk/government/uploads/system/uploads/attachment_data/file/642738/drug-misuse-2017-hosb1117.Pdf (accessed on 28 July 2018).
- Kaar, S.J.; Ferris, J.; Waldron, J.; Devaney, M.; Ramsey, J.; Winstock, A.R. Up: The rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J. Psychopharmacol. 2016, 30, 395–401. [Google Scholar] [CrossRef]
- Survey, G.D. Key Findings from the Global Drug Survey 2016 (data collected nov 15 January 16). Available online: https://www.Globaldrugsurvey.Com/past-findings/the-global-drug-survey-2016-findings/ (accessed on 28 July 2018).
- Imberger, G.; Orr, A.; Thorlund, K.; Wetterslev, J.; Myles, P.; Moller, A.M. Does anaesthesia with nitrous oxide affect mortality or cardiovascular morbidity? A systematic review with meta-analysis and trial sequential analysis. Br. J. Anaesth. 2014, 112, 410–426. [Google Scholar] [CrossRef] [Green Version]
- Amouzou, E.K.; Chabi, N.W.; Adjalla, C.E.; Rodriguez-Gueant, R.M.; Feillet, F.; Villaume, C.; Sanni, A.; Gueant, J.L. High prevalence of hyperhomocysteinemia related to folate deficiency and the 677c-->t mutation of the gene encoding methylenetetrahydrofolate reductase in coastal west africa. Am. J. Clin. Nutr. 2004, 79, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bosco, P.; Anello, G.; Ferri, R.; Gueant-Rodriguez, R.M.; Gueant, J.L. Heterogeneity of association between mthfr and stroke among european regions: Additional population studies are needed in italy. Stroke 2006, 37, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Gueant-Rodriguez, R.M.; Juilliere, Y.; Nippert, M.; Abdelmouttaleb, I.; Herbeth, B.; Aliot, E.; Danchin, N.; Gueant, J.L. Left ventricular systolic dysfunction is an independent predictor of homocysteine in angiographically documented patients with or without coronary artery lesions. J. Thromb. Haemost. Jth 2007, 5, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Rodriguez-Gueant, R.M.; Chamaillard, M.; Desreumaux, P.; Xia, B.; Bronowicki, J.P.; Bigard, M.A.; Gueant, J.L. Vascular and cellular stress in inflammatory bowel disease: Revisiting the role of homocysteine. Am. J. Gastroenterol. 2007, 102, 1108–1115. [Google Scholar] [CrossRef]
- Spada, R.S.; Stella, G.; Calabrese, S.; Bosco, P.; Anello, G.; Gueant-Rodriguez, R.M.; Romano, A.; Benamghar, L.; Fontaine, T.; Gueant, J.L. Association of vitamin b12, folate and homocysteine with functional and pathological characteristics of the elderly in a mountainous village in sicily. Clin. Chem. Lab. Med. 2007, 45, 136–142. [Google Scholar] [CrossRef]
- Gueant-Rodriguez, R.M.; Spada, R.; Moreno-Garcia, M.; Anello, G.; Bosco, P.; Lagrost, L.; Romano, A.; Elia, M.; Gueant, J.L. Homocysteine is a determinant of apoa-i and both are associated with ankle brachial index, in an ambulatory elderly population. Atherosclerosis 2011, 214, 480–485. [Google Scholar] [CrossRef] [PubMed]
- den Heijer, M.; Koster, T.; Blom, H.J.; Bos, G.M.; Briet, E.; Reitsma, P.H.; Vandenbroucke, J.P.; Rosendaal, F.R. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N. Engl. J. Med. 1996, 334, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Juni, P.; Holenstein, F.; Sterne, J.; Bartlett, C.; Egger, M. Direction and impact of language bias in meta-analyses of controlled trials: Empirical study. Int. J. Epidemiol. 2002, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]


| Setting of nitrous oxide exposure | N | n | Percentage (95% CI) |
| Recreational | 100 | 57 | 57.0 (47.1–66.9) |
| Surgery | 100 | 25 | 25.0 (16.4–33.6) |
| Occupational exposure | 100 | 9 | 9.0 (3.3–14.7) |
| Pain management | 100 | 6 | 6.0 (1.3–10.7) |
| Manipulation under general anesthesia | 100 | 1 | 1.0 (0–3.0) |
| Munchausen syndrome | 100 | 1 | 1.0 (0–3.0) |
| Management of sleep disturbance | 100 | 1 | 1.0 (0–3.0) |
| Frequency of nitrous oxide exposure | N | n | Percentage (95% CI) |
| Regular | 100 | 76 | 76.0 (67.5–84.5) |
| Once | 100 | 24 | 24.0 (15.5–32.5) |
| Quantitative estimates of nitrous oxide exposure* | n | Median | IQR (25th–75th) |
| Number of nitrous oxide cartridge per day | 30 | 25 | 8–85 |
| Duration of nitrous oxide duration (year) | 52 | 0.7 | 0.3–1.5 |
| Quantification of nitrous oxide exposure (cartridge-years) | 28 | 18.5 | 1.4–99.9 |
| Magnetic resonance imaging findings | N | n | Percentage (95% CI) |
| Presence of T2 signal hyperintensity in the spinal cord | 75 | 51 | 68.0 (57.2–78.8) |
| Reported diagnoses* | N | n | Percentage (95% CI) |
| Subacute combined degeneration | 100 | 28 | 28.0 (19.0–37.0) |
| Myelopathy | 100 | 26 | 26.0 (17.3–34.7) |
| Generalized demyelinating polyneuropathy | 100 | 23 | 23.0 (14.6–31.4) |
| Vitamin B12 deficiency | 100 | 14 | 14.0 (7.1–20.9) |
| Axonal polyneuropathy | 100 | 11 | 11.0 (4.8–17.2) |
| Encephalopathy | 100 | 2 | 2.0 (0–4.8) |
| Recurrent paraparesis | 100 | 1 | 1.0 (0–3) |
| MTHFR deficiency | 100 | 1 | 1.0 (0–3) |
| Toxicity due to N2O with no specific diagnosis applied | 100 | 19 | 19.0 (11.2–26.8) |
| Clinical Findings | N | n | Percentage (95% CI) |
|---|---|---|---|
| Paresthesia in extremities, numbness, tingling | 100 | 80 | 80.0 (72.0–88.0) |
| Unsteady gait, walking difficulty | 100 | 58 | 58.0 (48.2–67.8) |
| Weakness | 100 | 43 | 43.0 (33.1–52.9) |
| Fallings or equilibrium disorders | 100 | 24 | 24.0 (15.5–32.5) |
| Lhermitte’s sign | 100 | 15 | 15.0 (7.9–22.1) |
| Ataxia | 100 | 12 | 12.0 (5.5–18.5) |
| Cognitive decline | 100 | 9 | 9.0 (3.3–14.7) |
| Urinary incontinence | 100 | 8 | 8.0 (2.6–13.4) |
| Quadriparesis or paralysis | 100 | 7 | 7.0 (1.9–12.1) |
| Behavior alteration | 100 | 6 | 6.0 (1.3–10.7) |
| Urine retention | 100 | 5 | 5.0 (0.7–9.4) |
| Impaired memory | 100 | 5 | 5.0 (0.7–9.4) |
| Headache | 100 | 4 | 4.0 (0.1–7.9) |
| Depression | 100 | 4 | 4.0 (0.1–7.9) |
| Thrombo-occlusive event | 100 | 3 | 3.0 (0–6.4) |
| Mental confusion | 100 | 3 | 3.0 (0–6.4) |
| Constipation | 100 | 3 | 3.0 (0–6.4) |
| Paranoid behavior | 100 | 3 | 3.0 (0–6.4) |
| Foot pain | 100 | 3 | 3.0 (0–6.4) |
| Hyperpigmentation | 100 | 3 | 3.0 (0–6.4) |
| Abdominal pain | 100 | 3 | 3.0 (0–6.4) |
| Agitation | 100 | 2 | 2.0 (0–4.8) |
| Fecal incontinence | 100 | 2 | 2.0 (0–4.8) |
| Lethargy | 100 | 2 | 2.0 (0–4.8) |
| Seizures | 100 | 2 | 2.0 (0–4.8) |
| Decreased libido | 100 | 2 | 2.0 (0–4.8) |
| Visual hallucination | 100 | 2 | 2.0 (0–4.8) |
| Anorexia | 100 | 1 | 1.0 (0–3) |
| Apnea | 100 | 1 | 1.0 (0–3) |
| Athetoid movement | 100 | 1 | 1.0 (0–3) |
| Bulbar paralysis | 100 | 1 | 1.0 (0–3) |
| Chest pain | 100 | 1 | 1.0 (0–3) |
| Disorientation | 100 | 1 | 1.0 (0–3) |
| Hypotonia | 100 | 1 | 1.0 (0–3) |
| Neck pain | 100 | 1 | 1.0 (0–3) |
| Painful erection | 100 | 1 | 1.0 (0–3) |
| Paraplegia | 100 | 1 | 1.0 (0–3) |
| Polyneuropathy | 100 | 1 | 1.0 (0–3) |
| Respiratory paralysis | 100 | 1 | 1.0 (0–3) |
| Spasm | 100 | 1 | 1.0 (0–3) |
| Suicidal thought | 100 | 1 | 1.0 (0–3) |
| Syncope | 100 | 1 | 1.0 (0–3) |
| Tachypnea | 100 | 1 | 1.0 (0–3) |
| Vertigo | 100 | 1 | 1.0 (0–3) |
| Vomiting | 100 | 1 | 1.0 (0–3) |
| Laboratory findings (continuous) | n | Median | IQR (25th–75th) |
| Hemoglobin (g/dL)* | 43 | 12.0 | 9.1–13.3 |
| Males | 23 | 12.8 | 10.8–14.2 |
| Females | 20 | 10.7 | 8.3–12.4 |
| Hematocrit (%)† | 21 | 38 | 33–42 |
| Males | 13 | 40 | 33–44 |
| Females | 8 | 35 | 32–39 |
| Mean corpuscular volume (fL) | 55 | 100 | 94–103 |
| Vitamin B12 (pmol/L) | 82 | 101 | 74–161 |
| Folate (serum) (µg/L) | 20 | 12.8 | 7.3–14.6 |
| Homocysteine (µmol/L) | 31 | 55 | 29–111 |
| Methylmalonic acid (µmol/L) | 16 | 5.0 | 1.1–6.6 |
| Combined indicator of vitamin B12 status | 33 | −2.802 | −3.368–−1.924 |
| Laboratory findings (dichotomized) | N | n | Percentage (95%, CI) |
| Low hemoglobin status* | 43 | 24 | 55.8 (40.3–71.3) |
| Low hematocrit status† | 21 | 21 | 52.4 (29.1–75.7) |
| Mean corpuscular volume >100 fL | 55 | 23 | 41.8 (28.4–55.3) |
| Vitamin B12 < 150 pmol/L | 82 | 58 | 70.7 (60.7–80.8) |
| Folate (serum) <7 µg/L | 20 | 5 | 25.0 (4.2–45.8) |
| Homocysteine >15 µmol/L | 31 | 28 | 90.3 (79.3–100) |
| Methylmalonic acid >0.4 µmol/L | 16 | 15 | 93.8 (80.4–100) |
| Combined indicator of vitamin B12 status‡ | |||
| 2 = elevated B12 (cB12 ≥ 1.5) | 33 | 0 | 0.0 (—) |
| 1 = adequate B12 status (cB12: −0.5 to 1.5) | 33 | 3 | 9.1 (0–19.4) |
| −1 = decreased B12 (cB12: −1.5 to -0.5) | 33 | 2 | 6.1 (0–14.7) |
| −2 = possibly B12 deficient (cB12: −2.5 to −1.5) | 33 | 9 | 27.3 (11.2–43.3) |
| −3 = probably B12 deficient (cB12 < −2.5) | 33 | 19 | 57.6 (39.8–75.4) |
| Predictor | Short Exposure to N2O, Median (IQR) | Regular Exposure to N2O, Median (IQR) | AUROC† Defined Cut-Off | AUROC, p-Value | Univariate LR*, OR (95% CI) | Univariate LR*, p-Value | Multivariate LR‡, OR (95% CI) | Multivariate LR‡, p-Value |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 47 (25–58) | 26 (22–33) | ≥40 | 0.0076 | 23.33 (6.84–79.61) | < 0.0001 | 23.95 (3.62–158.61) | 0.001 |
| Vitamin B12 (pmol/L) | 74 (33–104) | 110 (81–194) | ≤74 | 0.0002 | 6.06 (2.05–17.90) | 0.001 | 10.57 (1.70–65.90) | 0.01 |
| MCV (fL) | 104 (101–110) | 97 (92–101) | >100 | <0.0001 | 9.75 (1.93–49.15) | 0.006 | Not retained§ | Not retained§ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oussalah, A.; Julien, M.; Levy, J.; Hajjar, O.; Franczak, C.; Stephan, C.; Laugel, E.; Wandzel, M.; Filhine-Tresarrieu, P.; Green, R.; et al. Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis. J. Clin. Med. 2019, 8, 551. https://doi.org/10.3390/jcm8040551
Oussalah A, Julien M, Levy J, Hajjar O, Franczak C, Stephan C, Laugel E, Wandzel M, Filhine-Tresarrieu P, Green R, et al. Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis. Journal of Clinical Medicine. 2019; 8(4):551. https://doi.org/10.3390/jcm8040551
Chicago/Turabian StyleOussalah, Abderrahim, Mélissa Julien, Julien Levy, Olivia Hajjar, Claire Franczak, Charlotte Stephan, Elodie Laugel, Marion Wandzel, Pierre Filhine-Tresarrieu, Ralph Green, and et al. 2019. "Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis" Journal of Clinical Medicine 8, no. 4: 551. https://doi.org/10.3390/jcm8040551
APA StyleOussalah, A., Julien, M., Levy, J., Hajjar, O., Franczak, C., Stephan, C., Laugel, E., Wandzel, M., Filhine-Tresarrieu, P., Green, R., & Guéant, J.-L. (2019). Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis. Journal of Clinical Medicine, 8(4), 551. https://doi.org/10.3390/jcm8040551






