Acid-Base Disturbances in Patients with Asthma: A Literature Review and Comments on Their Pathophysiology
Abstract
1. Introduction
2. Respiratory Alkalosis
3. Respiratory Acidosis
4. Metabolic acidosis
4.1. Non-Anion Gap Metabolic Acidosis
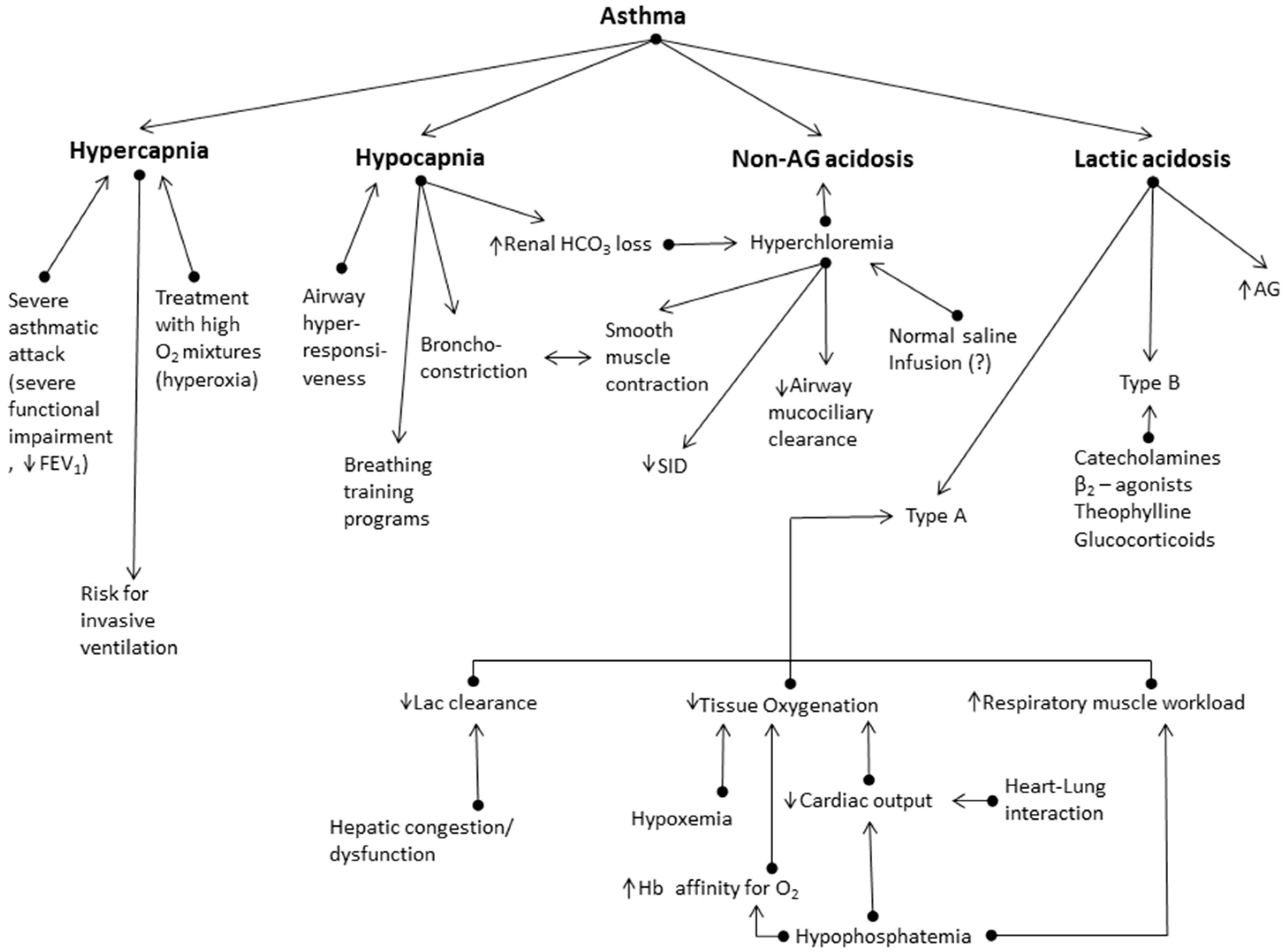
- Insofar as chronic hypocapnia in these patients is accompanied by hyperchloremia, the role of Cl− channels in vascular and non-vascular smooth muscle contraction, as in the human airways, must be stressed, e.g., an alteration of Cl− concentration changes the myogenic tone in the blood vessels [44,45]. Additionally, Cl− channels in epithelial cells may affect mucus hydration on the airway surfaces [46]. Overall, Cl− may have a critical role in asthma pathophysiology.
- In conditions like asthma exacerbations, where an acute acid-base disorder complicates a chronic respiratory disorder, such as chronic hypocapnia in asthmatics, the use of base excess (or base deficit) method [47] to assess the severity of metabolic acidosis can lead to ‘erroneous assessment’ of the patient’s acid-base status, lacking any clinical relevance. Thus, in the study of Okrent et al. [36], in patients with acute severe asthma, metabolic acidosis was diagnosed by the increase of base deficit > 2 mEq/L. In this study, authors supported that this indicated a true loss of the body’s alkaline reserve. Nevertheless, one of the patients with the more severe metabolic acidosis, diagnosed with the base excess criterion (−4.9 mEq/L), had hypocapnia (PCO2 = 27 mmHg), pH higher than the mean physiological value (7.43), and HCO3− concentration lower than the normal value (19 mEq/L), for which, however, no treatment is indicated, and which actually corresponds to the expected metabolic compensation for a chronic respiratory alkalosis (the expected HCO3− concentration reduction (Δ(HCO3−)) equals 0.4 × ΔPCO2, i.e., (HCO3−) = 18.8 mEq/L) [37]. Thus, the physiologic compensation for an uncomplicated acid-base disturbance has been viewed as a serious metabolic acidosis superimposed on the chronic respiratory disorder. Overall, caution is needed in assessing the metabolic component of these acid-base disorders by utilizing the base excess values; diagnostic errors and therapeutic ill-practices may occur when they are not considered alongside the required clinical information. Criticism on the subject has long been made by Schwartz and Relman [48], which even took the form of a ‘transatlantic debate’ with arguments from both sides [49].
- Finally, regarding the increased clinical risk demonstrated in asthmatics with non-AG acidosis (accompanied by hyperchloremia) [32], it should be noted that there are several studies suggesting that hyperchloremia per se is associated with poor outcome in hospitalized and critically ill patients [50,51,52]. Hyperchloremia induced by intravenous administration of crystalloid solutions with high Cl− concentration is not to be overlooked [53,54], although there is no study investigating this issue exclusively in patients with acute severe asthma. In addition, hypocapnia, besides the acid-base balance, can have serious effects on the organs and systems in the body, and can adversely affect outcome in the critically ill [55].
4.2. Lactic Acidosis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McFadden, E. Acute Severe Asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 740–759. [Google Scholar] [CrossRef]
- Tai, E.; Read, J. Blood-gas tensions in bronchial asthma. Lancet 1967, 1, 644–646. [Google Scholar] [CrossRef]
- McFadden, E.R., Jr.; Lyons, H.A. Arterial blood gas tensions in asthma. N. Eng. J. Med. 1968, 278, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Roisin, R.; Roca, J. Contributions of multiple inert gas elimination technique to pulmonary medicine. 3. Bronchial asthma. Thorax 1994, 49, 1027–1033. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Roisin, R. Gas exchange abnormalities in asthma. Lung 1990, 168, 599–605. [Google Scholar] [CrossRef]
- Osborne, C.A.; O’Connor, B.J.; Lewis, A.; Kanabar, V.; Gardner, W.N. Hyperventilation and asymptomatic chronic asthma. Thorax 2000, 55, 1016–1022. [Google Scholar] [CrossRef][Green Version]
- Van den Elshout, F.J.; van Herwaarden, C.L.; Folgering, H.T. Effects of hypercapnia and hypocapnia on respiratory resistance in normal and asthmatic subjects. Thorax 1991, 46, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, G.A.; Gonzalez, S.; Zaltsman, J.; Menga, G.; Adrogué, H.J. Acid–base patterns in acute severe asthma. J. Asthma 2013, 50, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Twort, C.H.; Cameron, I.R. Effects of PCO2, pH and extracellular calcium on contraction of airway smooth muscle from rats. Respir. Physiol. 1986, 66, 259–267. [Google Scholar] [CrossRef]
- Lindeman, K.S.; Croxton, T.L.; Lande, B.; Hirshman, C.A. Hypocapnia-induced contraction of porcine airway smooth muscle. Eur. Respir. J. 1998, 12, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Calderini, I.S.; Tavola, M. The effects of CO2 on respiratory mechanics in anesthetized paralyzed humans. Anesthesiology 2001, 94, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Bruton, A.; Lewith, G.T. The Buteyko breathing technique for asthma: A review. Complement. Ther. Med. 2005, 13, 41–46. [Google Scholar] [CrossRef]
- Bruton, A.; Lee, A.; Yardley, L.; Raftery, J.; Arden-Close, E.; Kirby, S.; Zhu, S.; Thiruvothiyur, M.; Webley, F.; Taylor, L.; et al. Physiotherapy breathing retraining for asthma: A randomised controlled trial. Lancet Respir. Med. 2018, 6, 19–28. [Google Scholar] [CrossRef]
- Mountain, R.D.; Sahn, S.A. Clinical features and outcome in patients with acute asthma presenting with hypercapnia. Am. Rev. Respir. Dis. 1988, 138, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Tan, W.C.; Lim, T.K. Severe asthma. Singap. Med. J. 1997, 38, 238–240, 243. [Google Scholar]
- Cham, G.W.; Tan, W.P.; Earnest, A.; Soh, C.H. Clinical predictors of acute respiratory acidosis during exacerbation of asthma and chronic obstructive pulmonary disease. Eur. J. Emerg. Med. 2002, 9, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.; Forfar, J.O.; Grubb, D.J. Arterial blood gas tensions and ph in acute asthma in childhood. Br. Med. J. 1968, 3, 460–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weng, T.R.; Langer, H.M.; Featherby, E.A.; Levison, H. Arterial blood gas tensions and acid-base balance in symptomatic and asymptomatic asthma in childhood. Am. Rev. Respir. Dis. 1970, 101, 274–282. [Google Scholar]
- Rosenzweig, J.R.C.; Edwards, L.; Lincourt, W.; Dorinsky, P.; ZuWallack, R.L. The relationship between health-related quality of life, lung function and daily symptoms in patients with persistent asthma. Respir. Med. 2004, 98, 1157–1165. [Google Scholar] [CrossRef][Green Version]
- Waddell, J.A.; Emerson, P.A.; Gunstone, R.F. Hypoxia in bronchial asthma. Br. Med. J. 1967, 2, 402–404. [Google Scholar] [CrossRef][Green Version]
- Miyamoto, T.; Mizuno, K.; Furuya, K. Arterial blood gases in bronchial asthma. J. Allergy 1970, 45, 248–254. [Google Scholar] [CrossRef]
- Perrin, K.; Wijesinghe, M.; Healy, B.; Wadsworth, K.; Bowditch, R.; Bibby, S.; Baker, T.; Weatherall, M.; Beasley, R. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax 2011, 66, 937–941. [Google Scholar] [CrossRef]
- Tobin, M.J. Principles and Practice of Mechanical Ventilation, 3rd ed.; McGraw Hill Professional: New York, NY, USA, 2012; p. 87. [Google Scholar]
- Aubier, M.; Murciano, D.; Fournier, M.; Milic-Emili, J.; Pariente, R.; Derenne, J.P. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1980, 122, 191–199. [Google Scholar] [CrossRef]
- Aubier, M.; Murciano, D.; Milic-Emili, J.; Touaty, E.; Daghfous, J.; Pariente, R.; Derenne, J.P. Effects of the administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am. Rev. Respir. Dis. 1980, 122, 747–754. [Google Scholar] [CrossRef]
- Sassoon, C.S.; Hassell, K.T.; Mahutte, C.K. Hyperoxic-induced hypercapnia in stable chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1987, 135, 907–911. [Google Scholar] [CrossRef]
- Dick, C.R.; Liu, Z.; Sassoon, C.S.; Berry, R.B.; Mahutte, C.K. O2-induced change in ventilation and ventilatory drive in COPD. Am. J. Respir. Crit. Care Med. 1997, 155, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Abdo, W.F.; Heunks, L.M. Oxygen-induced hypercapnia in COPD: Myths and facts. Crit. Care 2012, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.D.; Freiberg, D.B.; Regnis, J.A.; Young, I.H. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 161, 1524–1529. [Google Scholar] [CrossRef]
- Luft, U.C.; Mostyn, E.M.; Loeppky, J.A.; Venters, M.D. Contribution of the Haldane effect to the rise of arterial Pco2 in hypoxic patients breathing oxygen. Crit. Care Med. 1981, 9, 32–37. [Google Scholar] [CrossRef]
- Mountain, R.D.; Heffner, J.E.; Brackett, N.C.; Sahn, S.A. Acid-base disturbances in acute asthma. Chest 1990, 98, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.O.; Azam, H.M.; DeBari, V.A.; Blamoun, A.I.; Moammar, M.Q.; Khan, M.A. Non-anion gap acidosis in asthma: Clinical and laboratory features and outcomes for hospitalized patients. Ann. Clin. Lab. Sci. 2008, 38, 228–234. [Google Scholar]
- Rabbat, A.; Laaban, J.P.; Boussairi, A.; Rochemaure, J. Hyperlactatemia during acute severe asthma. Intensive Care Med. 1998, 24, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Meert, K.L.; McCaulley, L.; Sarnaik, A.P. Mechanism of lactic acidosis in children with acute severe asthma. Pediatr. Crit. Care Med. 2012, 13, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, A.; Adrogué, H.; De Obrutsky, C.W.; Marchisio, M.L.; Herrera, M. Metabolic acidosis in status asthmaticus. Respiration 1976, 33, 85–94. [Google Scholar] [CrossRef]
- Okrent, D.G.; Tessler, S.; Twersky, R.A.; Tashkin, D.P. Metabolic acidosis not due to lactic acidosis in patients with severe acute asthma. Crit. Care Med. 1987, 15, 1098–1101. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Secondary responses to altered acid-base status: The rules of engagement. J. Am. Soc. Nephrol. 2010, 21, 920–923. [Google Scholar] [CrossRef]
- Madias, N.E.; Adrogué, H.J. Cross-talk between two organs: How the kidney responds to disruption of acid-base balance by the lung. Nephron Physiol. 2003, 93, 61–66. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Differential diagnosis of non-gap metabolic acidosis: Value of a systematic approach. Clin. J. Am. Soc. Nephrol. 2012, 7, 671–679. [Google Scholar] [CrossRef]
- Gennari, F.J.; Goldstein, M.B.; Schwartz, W.B. The nature of the renal adaptation to chronic hypocapnia. J. Clin. Investig. 1972, 51, 1722–1730. [Google Scholar] [CrossRef]
- Krapf, R.; Beeler, I.; Hertner, D.; Hulter, H.N. Chronic respiratory alkalosis. The effect of sustained hyperventilation on renal regulation of acid-base equilibrium. N. Engl. J. Med. 1991, 324, 1394–1401. [Google Scholar] [CrossRef]
- Zouboules, S.M.; Lafave, H.C.; O’Halloran, K.D.; Brutsaert, T.D.; Nysten, H.E.; Nysten, C.E.; Steinback, C.D.; Sherpa, M.T.; Day, T.A. Renal reactivity: Acid-base compensation during incremental ascent to high altitude. J. Physiol. 2018, 596, 6191–6203. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A. Independent and dependent variables of acid-base control. Respir. Physiol. 1978, 33, 9–26. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamazaki, J. Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn. J. Pharmacol. 2001, 85, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Bulley, S.; Jaggar, J.H. Cl− channels in smooth muscle cells. Pflugers Arch. 2014, 466, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, H.L. Epithelial sodium and chloride channels and asthma. Chin. Med. J. (Engl.) 2015, 128, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Berend, K. Diagnostic use of base excess in acid-base disorders. N. Engl. J. Med. 2018, 378, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, W.B.; Relman, A.S. A critique of the parameters used in the evaluation of acid-base disorders. “Whole-blood buffer base” and “standard bicarbonate” compared with blood pH and plasma bicarbonate concentration. N. Engl. J. Med. 1963, 268, 1382–1388. [Google Scholar] [CrossRef]
- Severinghaus, J.W. Siggaard-andersen and the “great trans-atlantic acid-base debate”. Scand. J. Clin. Lab. Investig. Suppl. 1993, 214, 99–104. [Google Scholar]
- Neyra, J.A.; Canepa-Escaro, F.; Li, X.; Manllo, J.; Adams-Huet, B.; Yee, J.; Yessayan, L.; Acute Kidney Injury in Critical Illness Study Group. Association of hyperchloremia with hospital mortality in critically Ill septic patients. Crit. Care Med. 2015, 43, 1938–1944. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Cheng, Z.; Qian, Q. Chloride alterations in hospitalized patients: Prevalence and outcome significance. PLoS ONE 2017, 12, e0174430. [Google Scholar] [CrossRef]
- Huang, K.; Hu, Y.; Wu, Y.; Ji, Z.; Wang, S.; Lin, Z.; Pan, S. Hyperchloremia is associated with poorer outcome in critically Ill stroke patients. Front. Neurol. 2018, 9, 485. [Google Scholar] [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced crystalloids versus saline in critically Ill adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
- Krajewski, M.L.; Raghunathan, K.; Paluszkiewicz, S.M.; Schermer, C.R.; Shaw, A.D. Meta-analysis of high-versus low-chloride content in perioperative and critical care fluid resuscitation. Br. J. Surg. 2015, 102, 24–36. [Google Scholar] [CrossRef]
- Laffey, J.G.; Kavanagh, B.P. Hypocapnia. N. Engl. J. Med. 2002, 347, 43–53. [Google Scholar] [CrossRef]
- Rudolf, M.; Riordan, J.F.; Grant, B.J.; Maberly, D.J.; Saunders, K.B. Arterial blood gas tensions in acute severe asthma. Eur. J. Clin. Investig. 1980, 10, 55–62. [Google Scholar] [CrossRef]
- Buda, A.J.; Pinsky, M.R.; Ingels, N.B., Jr.; Daughters, G.T., 2nd; Stinson, E.B.; Alderman, E.L. Effect of intrathoracic pressure on left ventricular performance. N. Engl. J. Med. 1979, 301, 453–459. [Google Scholar] [CrossRef]
- Jardin, F.; Farcot, J.C.; Boisante, L.; Prost, J.F.; Gueret, P.; Bourdarias, J.P. Mechanism of paradoxic pulse in bronchial asthma. Circulation 1982, 66, 887–894. [Google Scholar] [CrossRef]
- Grassino, A.; Macklem, P.T. Respiratory muscle fatigue and ventilatory failure. Annu. Rev. Med. 1984, 35, 625–647. [Google Scholar] [CrossRef]
- Freedman, S.; Cooke, N.T.; Moxham, J. Production of lactic acid by respiratory muscles. Thorax 1983, 38, 50–54. [Google Scholar] [CrossRef]
- Brady, H.R.; Ryan, F.; Cunningham, J.; Tormey, W.; Ryan, M.P.; O’Neill, S. Hypophosphatemia complicating bronchodilator therapy for acute severe asthma. Arch. Intern. Med. 1989, 149, 2367–2368. [Google Scholar] [CrossRef]
- Pesta, D.H.; Tsirigotis, D.N.; Befroy, D.E.; Caballero, D.; Jurczak, M.J.; Rahimi, Y.; Cline, G.W.; Dufour, S.; Birkenfeld, A.L.; Rothman, D.L.; et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 2016, 30, 3378–3387. [Google Scholar] [CrossRef] [PubMed]
- Aubier, M.; Murciano, D.; Lecocguic, Y.; Viires, N.; Jacquens, Y.; Squara, P.; Pariente, R. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N. Engl. J. Med. 1985, 313, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, G.F. Hyperphosphatemia, hypophosphatemia, and the oxy-hemoglobin dissociation curve. J. Surg. Res. 1973, 14, 367–372. [Google Scholar] [CrossRef]
- Henrion, J.; Minette, P.; Colin, L.; Schapira, M.; Delannoy, A.; Heller, F.R. Hypoxic hepatitis caused by acute exacerbation of chronic respiratory failure: A case-controlled, hemodynamic study of 17 consecutive cases. Hepatology 1999, 29, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.M.; Book, W.; Spivey, J.R. Liver disease related to the heart. Transplant. Rev. (Orlando) 2015, 29, 33–37. [Google Scholar] [CrossRef]
- Waseem, N.; Chen, P.H. Hypoxic hepatitis: A review and clinical update. J. Clin. Transl. Hepatol. 2016, 4, 263–268. [Google Scholar] [PubMed]
- Sterling, S.A.; Puskarich, M.A.; Jones, A.E. The effect of liver disease on lactate normalization in severe sepsis and septic shock: A cohort study. Clin. Exp. Emerg. Med. 2015, 2, 197–202. [Google Scholar] [CrossRef]
- De Jonghe, B.; Cheval, C.; Misset, B.; Timsit, J.F.; Garrouste, M.; Montuclard, L.; Carlet, J. Relationship between blood lactate and early hepatic dysfunction in acute circulatory failure. J. Crit. Care 1999, 14, 7–11. [Google Scholar] [CrossRef]
- Gillani, S.; Cao, J.; Suzuki, T.; Hak, D.J. The effect of ischemia reperfusion injury on skeletal muscle. Injury 2012, 43, 670–675. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar]
- Ind, P.W.; Causon, R.C.; Brown, M.J.; Barnes, P.J. Circulating catecholamines in acute asthma. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 267–269. [Google Scholar] [CrossRef]
- Papiris, S.A.; Manali, E.D.; Kolilekas, L.; Triantafillidou, C.; Tsangaris, I. Acute severe asthma: New approaches to assessment and treatment. Drugs 2009, 69, 2363–2391. [Google Scholar] [CrossRef]
- Stratakos, G.; Kalomenidis, J.; Routsi, C.; Papiris, S.; Roussos, C. Transient lactic acidosis as a side effect of inhaled salbutamol. Chest 2002, 122, 385–386. [Google Scholar] [CrossRef]
- Barth, E.; Albuszies, G.; Baumgart, K.; Matejovic, M.; Wachter, U.; Vogt, J.; Radermacher, P.; Calzia, E. Glucose metabolism and catecholamines. Crit. Care Med. 2007, 35, S508–S518. [Google Scholar] [CrossRef] [PubMed]
- Reverte, M.; Moratinos, J. Effects of salbutamol and BRL 37344 on diastolic arterial blood pressure, plasma glucose and plasma lactate in rabbits. Fundam. Clin. Pharmacol. 1994, 8, 417–424. [Google Scholar] [CrossRef]
- Haffner, C.A.; Kendall, M.J. Metabolic effects of beta 2-agonists. J. Clin. Pharm. Ther. 1992, 17, 155–164. [Google Scholar] [CrossRef]
- Batenburg, J.J.; Olson, M.S. Regulation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. J. Biol. Chem. 1976, 251, 1364–1370. [Google Scholar] [PubMed]
- Kovacevic, A.; Schwahn, B.; Schuster, A. Hyperlactic acidosis as metabolic side-effect of albuterol and theophylline in acute severe asthma. Klin. Padiatr. 2010, 222, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Sturney, S.; Suntharalingam, J. Treating acute asthma-salbutamol may not always be the right answer. Clin. Med. (Lond.) 2012, 12, 181–182. [Google Scholar] [CrossRef]
- Stiles, G.L.; Caron, M.G.; Lefkowitz, R.J. Beta-adrenergic receptors: Biochemical mechanisms of physiological regulation. Physiol. Rev. 1984, 64, 661–743. [Google Scholar] [CrossRef] [PubMed]
- Dodda, V.R.; Spiro, P. Can albuterol be blamed for lactic acidosis? Respir. Care 2012, 57, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Vernon, C.; Letourneau, J.L. Lactic acidosis: Recognition, kinetics, and associated prognosis. Crit. Care Clin. 2010, 26, 55–83. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.; Wulf, M.E. Lactic acidosis in sepsis: A commentary. Intensive Care Med. 1996, 22, 6–16. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Study Population | Methods | Significant Findings |
|---|---|---|---|---|
| Osborne C.A. et al., 2000 [6] | Case-Control Study | 23 asymptomatic asthmatics, 17 healthy subjects | Measured various stable state parameters | PaCO2 and PETCO2 lower in asymptomatic asthmatics |
| Van den Elshout et al., 1991 [7] | Case- Control Study | 30 asthmatics, 17 healthy subjects | Induction of hypercapnia and hypocapnia | Hypocapnia induced increases in airway resistance in asthmatic patients |
| Raimondi et al., 2013 [8] | Case series | 314 patients admitted for ASA | ABGs, electrolytes and spirometry results documented | Hypocapnia was prominent in less severe asthma exacerbations |
| Study | Study Design | Study Population | Methods | Significant Findings |
|---|---|---|---|---|
| Mountain et al., 1988 [14] | Retrospective | 61 patients with hypercapnic ASA, 168 with nonhypercapnic ASA | Various outcomes documented | Hypercapnic patients had more severe airway obstruction, symptoms |
| Lee K.H. et al., 1997 [15] | Retrospective | 48 patients with 49 admissions to the ICU due to ASA | Various outcomes documented | Respiratory acidosis linked to higher mortality |
| Raimondi et al., 2013 [8] | Case series | 314 patients admitted for ASA | ABGs, electrolytes and spirometry results documented | Inverse correlation between FEV1 and respiratory acidosis. Inability to perform spirometry linked to high pCO2 |
| Cham et al., 2002 [16] | Prospective observational | 127 patients with severe exacerbation of asthma and COPD in the ED | Acute respiratory acidosis documented and linked to clinical presentation | Drowsiness linked to sevenfold likelihood of respiratory acidosis. Flushing and intercostal retractions good predictors of respiratory acidosis |
| Study | Study Design | Study Population | Methods | Significant Findings |
|---|---|---|---|---|
| Mountain, R.D. et al., 1990 [31] | Retrospective | 229 acute asthma episodes in 170 patients (Hospital Admissions) | Clinical features and arterial blood gases examined | Simple or mixed metabolic acidosis in 28% of the episodes. |
| Rashid, A.O. et al., 2008 [32] | Retrospective | 109 patients hospitalized for asthma exacerbations | Acid-base, electrolyte status and outcomes | 10.1% AG acidosis, 29.4% NAG acidosis. NAG acidosis patients had significantly higher intubation rates |
| Rabbat, A. et al., 1998 [33] | Prospective | 29 non-intubated patients admitted to the ICU for ASA | Serial lactate measurements during treatment, correlation with outcomes | Hyperlactatemia a common finding on admission (59%) or during treatment (100%). No prognostic value, no correlation with PaCO2 or PEF |
| Meert, K.L. et al., 2012 [34] | Prospective observational | 105 children with ASA admitted to a PICU | Blood lactate measurements followed by lactate/pyruvate ration measurements | Primarily type B lactic acidosis (associated with normal oxygen delivery). Presumed to be due to β-adrenergic stimulation |
| Raimondi et al., 2013 [8] | Case Series | 314 patients admitted for ASA | ABGs, electrolytes and spirometry results documented | Most cases of metabolic acidosis attributed to chronic hypocapnia. Hyperlactatemia attributed mostly to adrenergic stimulation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiadis, I.; Alevrakis, E.; Ampelioti, S.; Vagionas, D.; Rovina, N.; Koutsoukou, A. Acid-Base Disturbances in Patients with Asthma: A Literature Review and Comments on Their Pathophysiology. J. Clin. Med. 2019, 8, 563. https://doi.org/10.3390/jcm8040563
Vasileiadis I, Alevrakis E, Ampelioti S, Vagionas D, Rovina N, Koutsoukou A. Acid-Base Disturbances in Patients with Asthma: A Literature Review and Comments on Their Pathophysiology. Journal of Clinical Medicine. 2019; 8(4):563. https://doi.org/10.3390/jcm8040563
Chicago/Turabian StyleVasileiadis, Ioannis, Emmanouil Alevrakis, Sevasti Ampelioti, Dimitrios Vagionas, Nikoletta Rovina, and Antonia Koutsoukou. 2019. "Acid-Base Disturbances in Patients with Asthma: A Literature Review and Comments on Their Pathophysiology" Journal of Clinical Medicine 8, no. 4: 563. https://doi.org/10.3390/jcm8040563
APA StyleVasileiadis, I., Alevrakis, E., Ampelioti, S., Vagionas, D., Rovina, N., & Koutsoukou, A. (2019). Acid-Base Disturbances in Patients with Asthma: A Literature Review and Comments on Their Pathophysiology. Journal of Clinical Medicine, 8(4), 563. https://doi.org/10.3390/jcm8040563





