Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
2.5. Subgroup Analyses
3. Results
3.1. Characteristics of the Identified Studies
3.2. Influenza Vaccine Characteristics and Vaccination Strategy
3.3. Vaccination Immunogenicity in the Identified Studies
3.4. Double Simultaneous Dose Influenza Vaccine
3.5. Booster-Dose Influenza Vaccine
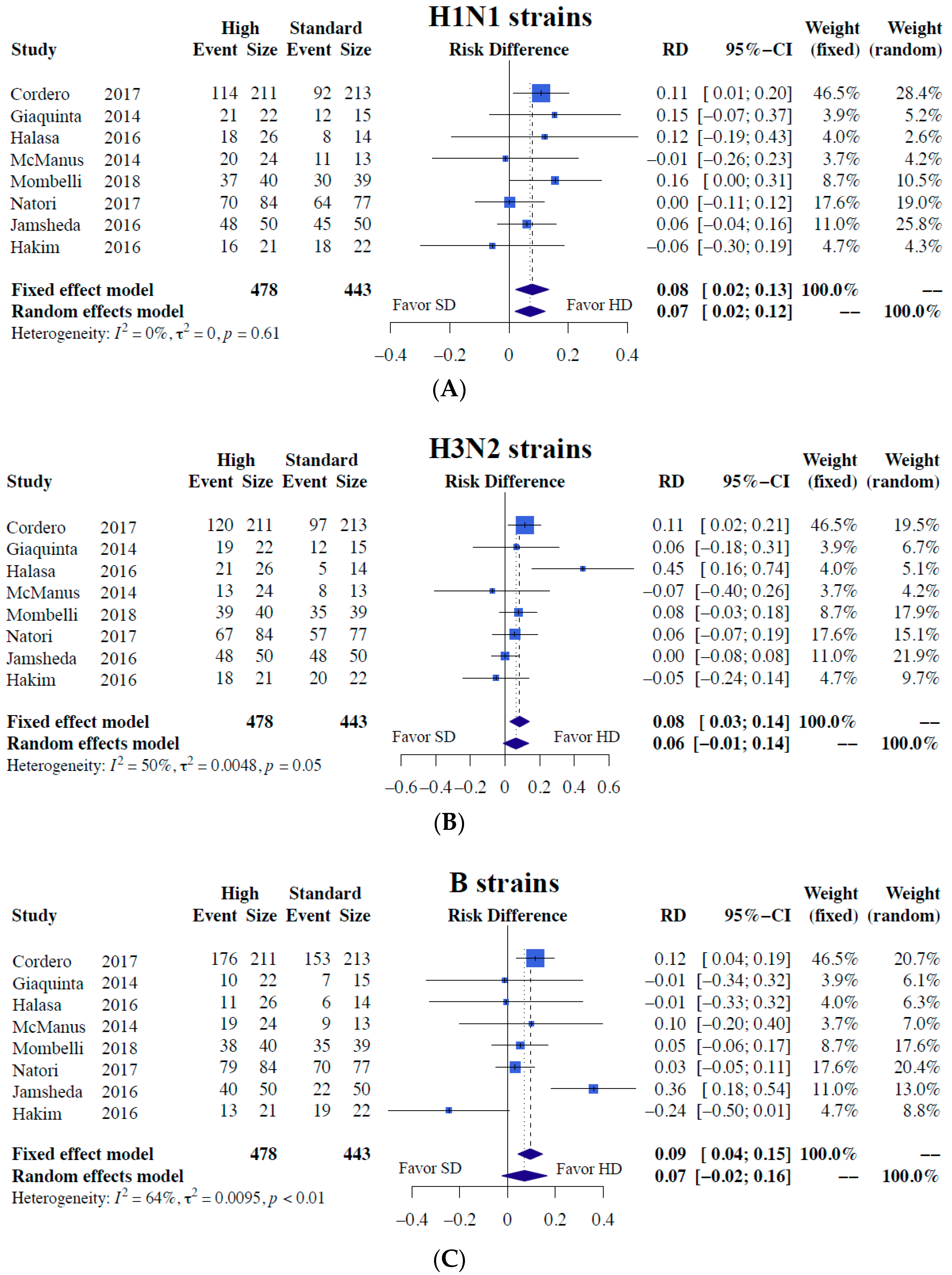
3.6. Meta-Analysis of Vaccine Efficacy
3.6.1. Immunogenicity
3.6.2. Subgroup Analyses of Immunogenicity
3.7. Safety
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 November 2018).
- Kumar, D.; Michaels, M.G.; Morris, M.I.; Green, M.; Avery, R.K.; Liu, C.; Danziger-Isakov, L.; Stosor, V.; Estabrook, M.; Gantt, S.; et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: A multicentre cohort study. Lancet Infect. Dis. 2010, 10, 521–526. [Google Scholar] [CrossRef]
- Helantera, I.; Anttila, V.J.; Lappalainen, M.; Lempinen, M.; Isoniemi, H. Outbreak of Influenza A(H1N1) in a Kidney Transplant Unit-Protective Effect of Vaccination. Am. J. Transplant. 2015, 15, 2470–2474. [Google Scholar] [CrossRef]
- Bosaeed, M.; Kumar, D. Seasonal influenza vaccine in immunocompromised persons. Hum. Vaccin. Immunother. 2018, 14, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–2018 Influenza Season. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1–20. [Google Scholar]
- Kumar, D.; Campbell, P.; Hoschler, K.; Hidalgo, L.; Al-Dabbagh, M.; Wilson, L.; Humar, A. Randomized Controlled Trial of Adjuvanted Versus Nonadjuvanted Influenza Vaccine in Kidney Transplant Recipients. Transplantation 2016, 100, 662–669. [Google Scholar] [CrossRef]
- Natori, Y.; Shiotsuka, M.; Slomovic, J.; Hoschler, K.; Ferreira, V.; Ashton, P.; Rotstein, C.; Lilly, L.; Schiff, J.; Singer, L.; et al. A Double-Blind, Randomized Trial of High-Dose vs Standard-Dose Influenza Vaccine in Adult Solid-Organ Transplant Recipients. Clin. Infect. Dis. 2018, 66, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Loulergue, P.; Goldwasser, F.; Alexandre, J.; Mir, O.; Ropert, S. Low influenza vaccination rate among patients receiving chemotherapy for cancer. Ann. Oncol. 2008, 19, 1658–1659. [Google Scholar]
- Miller, R.M.; Rohde, K.A.; Tingle, M.T.A.; Moran, J.J.M.; Hayney, M.S. Antibody responses to influenza vaccine in pre- and post-lung transplant patients. Clin. Transplant. 2016, 30, 606–612. [Google Scholar] [CrossRef]
- Cordero, E.; Roca-Oporto, C.; Bulnes-Ramos, A.; Aydillo, T.; Gavalda, J.; Moreno, A.; Torre-Cisneros, J.; Montejo, J.M.; Fortun, J.; Munoz, P.; et al. Two Doses of Inactivated Influenza Vaccine Improve Immune Response in Solid Organ Transplant Recipients: Results of TRANSGRIPE 1-2, a Randomized Controlled Clinical Trial. Clin. Infect. Dis. 2017, 64, 829–838. [Google Scholar] [CrossRef]
- GiaQuinta, S.; Michaels, M.G.; McCullers, J.A.; Wang, L.; Fonnesbeck, C.; O’Shea, A.; Green, M.; Halasa, N.B. Randomized, double-blind comparison of standard-dose vs. high-dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatr. Transplant. 2015, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Hakim, H.; Allison, K.J.; Van de Velde, L.A.; Tang, L.; Sun, Y.; Flynn, P.M.; McCullers, J.A. Immunogenicity and safety of high-dose trivalent inactivated influenza vaccine compared to standard-dose vaccine in children and young adults with cancer or HIV infection. Vaccine 2016, 34, 3141–3148. [Google Scholar] [CrossRef]
- Halasa, N.B.; Savani, B.N.; Asokan, I.; Kassim, A.; Simons, R.; Summers, C.; Bourgeois, J.; Clifton, C.; Vaughan, L.A.; Lucid, C.; et al. Randomized Double-Blind Study of the Safety and Immunogenicity of Standard-Dose Trivalent Inactivated Influenza Vaccine versus High-Dose Trivalent Inactivated Influenza Vaccine in Adult Hematopoietic Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2016, 22, 528–535. [Google Scholar] [CrossRef]
- Jamshed, S.; Walsh, E.E.; Dimitroff, L.J.; Santelli, J.S.; Falsey, A.R. Improved immunogenicity of high-dose influenza vaccine compared to standard-dose influenza vaccine in adult oncology patients younger than 65 years receiving chemotherapy: A pilot randomized clinical trial. Vaccine 2016, 34, 630–635. [Google Scholar] [CrossRef]
- McManus, M.; Frangoul, H.; McCullers, J.A.; Wang, L.; O’Shea, A.; Halasa, N. Safety of high dose trivalent inactivated influenza vaccine in pediatric patients with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2014, 61, 815–820. [Google Scholar] [CrossRef]
- Mombelli, M.; Rettby, N.; Perreau, M.; Pascual, M.; Pantaleo, G.; Manuel, O. Immunogenicity and safety of double versus standard dose of the seasonal influenza vaccine in solid-organ transplant recipients: A randomized controlled trial. Vaccine 2018, 36, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Winokur, P.; Brady, R.; Belshe, R.; Chen, W.H.; Cate, T.R.; Sigurdardottir, B.; Hoeper, A.; Graham, I.L.; Edelman, R.; et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007, 25, 7656–7663. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; van der Klaauw, A.A.; Pereira, A.M.; Biermasz, N.R.; Honkoop, P.J.; Roelfsema, F.; Smit, J.W.; Romijn, J.A. Quality of life is decreased after treatment for nonfunctioning pituitary macroadenoma. J. Clin. Endocrinol. Metab. 2006, 91, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- GS., H.J. Cochrane handbook for systematic reviews of interventions version 5.1.0. Available online: http://handbook.cochrane.org/ (accessed on 28 March 2019).
- Roberts, C.; Torgerson, D.J. Understanding controlled trials: Baseline imbalance in randomised controlled trials. BMJ 1999, 319, 185. [Google Scholar] [CrossRef]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef]
- Onnestam, L.; Berinder, K.; Burman, P.; Dahlqvist, P.; Engstrom, B.E.; Wahlberg, J.; Nystrom, H.F. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J. Clin. Endocrinol. Metab. 2013, 98, 626–635. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49. [Google Scholar] [CrossRef]
- O’Sullivan, E.P.; Woods, C.; Glynn, N.; Behan, L.A.; Crowley, R.; O’Kelly, P.; Smith, D.; Thompson, C.J.; Agha, A. The natural history of surgically treated but radiotherapy-naive nonfunctioning pituitary adenomas. Clin. Endocrinol. 2009, 71, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Treanor, J.J.; Tornieporth, N.; Capellan, J.; Gorse, G.J. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J. Infect. Dis. 2009, 200, 172–180. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Kumar, D.; Blumberg, E.A.; Danziger-Isakov, L.; Kotton, C.N.; Halasa, N.B.; Ison, M.G.; Avery, R.K.; Green, M.; Allen, U.D.; Edwards, K.M.; et al. Influenza vaccination in the organ transplant recipient: Review and summary recommendations. Am. J. Transplant. 2011, 11, 2020–2030. [Google Scholar] [CrossRef]
- Englund, J.; Feuchtinger, T.; Ljungman, P. Viral infections in immunocompromised patients. Biol. Blood Marrow Transplant. 2011, 17, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Janoff, E.N. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect. Dis. 2009, 9, 493–504. [Google Scholar] [CrossRef]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; Nguyen-Van-Tam, J.S. Influenza vaccination for immunocompromised patients: Systematic review and meta-analysis by etiology. J. Infect. Dis. 2012, 206, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; Zanuzdana, A.; Agboado, G.; Orton, E.; Bechard-Evans, L.; Morgan, G.; Stevenson, C.; et al. Influenza vaccination for immunocompromised patients: Systematic review and meta-analysis from a public health policy perspective. PLoS ONE 2011, 6, e29249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention and control of influenza pandemics and annual epidemics. Resolution of the World Health Assembly (WHA 56.19). 28 May 2003. Available online: https://www.who.int/immunization/sage/1_WHA56_19_Prevention_and_control_of_influenza_pandemics.pdf?ua=1 (accessed on 28 March 2019).
- Gravenstein, S.; Davidson, H.E.; Taljaard, M.; Ogarek, J.; Gozalo, P.; Han, L.; Mor, V. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: A cluster-randomised trial. Lancet Infect. Dis. 2017, 5, 738–746. [Google Scholar] [CrossRef]
- Gravenstein, S.; Davidson, H.E.; Han, L.F.; Ogarek, J.A.; Dahal, R.; Gozalo, P.L.; Taljaard, M.; Mor, V. Feasibility of a cluster-randomized influenza vaccination trial in U.S. nursing homes: Lessons learned. Hum. Vaccin. Immunother. 2018, 14, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Kim, J.; Krishnan, A.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: A systematic review and meta-analysis. Expert Rev. Vaccines 2018, 17, 435–443. [Google Scholar] [CrossRef]
- Larson, D.A.; Flickinger, J.C.; Loeffler, J.S. The radiobiology of radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 557–561. [Google Scholar] [CrossRef]
- Shay, D.K.; Chillarige, Y.; Kelman, J.; Forshee, R.A.; Foppa, I.M.; Wernecke, M.; Lu, Y.; Ferdinands, J.M.; Iyengar, A.; Fry, A.M.; et al. Comparative Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among US Medicare Beneficiaries in Preventing Postinfluenza Deaths During 2012–2013 and 2013–2014. J. Infect. Dis. 2017, 215, 510–517. [Google Scholar] [CrossRef]


| Author Year | Design (Country) | Duration (Strains *) | Population | Case (Mean Age) | Vaccine Doseage | Time to Vaccine | Follow up (Quality *) |
|---|---|---|---|---|---|---|---|
| Giaquinta 2015 [11] (NCT01525004) | DB, Phase I RCT, (US) | 2011–2012 (A/H3N2) | Pediatric SOTR (kidney, liver, heart, lungs, intestine) | 38 (12.8) | HD (60μg HA) | Post-transplant for 6 months | 4 weeks (7) |
| McManus 2014 [15] (NCT01216332) | DB, Phase I RCT, (US) | 2010–2011 (A/H3N2) 2011–2012 (A/H3N2) | Pediatric patients with Acute lymphocytic leukemia | 50 (8.5) | HD (60μg HA) | Under C/T for 1 month | 4 weeks (6) |
| Halasa 2016 [13] (NCT01215734) | DB, Phase I, RCT (US) | 2010–201 1(A/H3N2) 2011–2012 (A/H3N2) | Adult Stem Cell Hematopoietic Transplant Recipients | 44 (50.0) | HD (60μg HA) | Post-transplant for 6 months | 4 weeks (7) |
| Hakim 2016 [12] (NCT01205581) | OP, Phase II, RCT (US) | 2010–2011 (A/H3N2) 2011–2012 (A/H3N2) | Children with Cancer | 44 (11.3) | HD (60μg HA) | Under C/T or received C/T in the past 3 months | 3 weeks (5) |
| Jamshed 2016 [14] (NCT01666782) | DB, Phase II, RCT (US) | 2012–2013 (A/H3N2) 2013–2014 (A/H1N1) | Adult with Cancer | 105 (53.4) | HD (60μg HA) | First day of chemotherapy | 4 weeks (6) |
| Cordero 2017 [10] (EudraCT 2011-003243-21) | OP, Phase III, RCT (Spanish) | 2012–2013 (A/H3N2) | Adult SOTR (kidney, liver, heart, lungs) | 499 (55.9) | Booster (30μg HA) | Post-transplant for 1 month | 10 weeks (5) |
| Natori 2018 [7] (NCT03139565) | DB, Phase III, RCT (Canada) | 2016–2017 (A/H3N2) | Adult SOTR (kidney, liver, heart, lung and pancreas) | 161 (57) | HD (60μg HA) | Post-transplant for 3 months | 4 weeks (6) |
| Mombelli 2018 [16] (NCT02746783) | OP, Phase II, RCT (Switzerland) | 2014–2015 (A/H3N2) | Adult SOTR (kidney, liver) | 79 (58.6) | DD (30μg HA) | Post-transplant for 3 months | 4 weeks (5) |
| Outcome Assessment | H1N1 | H3N2 | B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number Trials (Patients) | Risk Difference (95% CI) | I2 (%) p-Value | Number Trials (Patients) | Risk Ratio (95% CI) | I2 (%) p-Value | Number Trials (Patients) | Risk Ratio (95% CI) | I2 (%) p-Value | |
| SeroConversion | 8 (921) | 0.1278 (0.0347; 0.2208) | 48.7% 0.0071 * | 8 (921) | 0.1000 (-0.0140; 0.2141) | 65.7% 0.0856 | 8 (921) | −0.0004 (−0.0999; 0.0991) | 63.7% 0.9938 |
| Double does (30 µg) | 2 (503) | 0.0483 (−0.0195; 0.1161) | 0.0% 0.1625 | 2 (503) | 0.0327 (−0.0374; 0.1028) | 0.0% 0.3603 | 2 (503) | 0.0424 (−0.0301; 0.1149) | 0.0% 0.2514 |
| High does (60 µg) | 6 (418) | 0.1801 (0.0651; 0.2952) | 31.8% 0.0021 * | 6 (418) | 0.1300 (−0.0349; 0.2950) | 66.9% 0.1223 | 6 (418) | −0.0225 (−0.1865; 0.1416) | 73.6% 0.7883 |
| Mean Age > 18 | 5 (804) | 0.1236 (0.0310; 0.2162) | 44.4% 0.0088 * | 5 (804) | 0.1164 (0.0088; 0.2240) | 56.3% 0.0340 * | 5 (804) | −0.0043 (−0.1406; 0.1319) | 76.3% 0.9503 |
| Mean Age < 18 | 3 (117) | 0.1094 (−0.1916; 0.4105) | 68.6% 0.4760 | 3 (117) | 0.0548 (−0.3253; 0.4349) | 82.0% 0.7776 | 3 (117) | 0.0182 (−0.1292; 0.1657) | 20.4% 0.8083 |
| Chemotherapy | 3 (180) | 0.1386 (−0.1294; 0.4066) | 72.3% 0.3107 | 3 (180) | 0.0044 (−0.2737; 0.2825) | 74.3% 0.9754 | 3 (180) | 0.1098 (0.0080; 0.2115) | 0.0% 0.0344 * |
| Post−transplant | 5 (739) | 0.0920 (0.0226; 0.1615) | 11.0% 0.0093 * | 5 (739) | 0.1392 (0.0021; 0.2763) | 67.9% 0.0466 * | 5 (739) | −0.0608 (−0.1972; 0.0756) | 70.2% 0.3821 |
| SeroProtection | 8 (921) | 0.0713 (0.0209; 0.1217) | 0.0% 0.0056 * | 8 (921) | 0.0638 (−0.0092; 0.1368) | 50.2% 0.0868 | 8 (921) | 0.0709 (−0.0230; 0.1647) | 64.1% 0.1388 |
| Double does (30 µg) | 2 (503) | 0.1212 (0.0404; 0.2020) | 0.0% 0.0033 * | 2 (503) | 0.0976 (0.0268; 0.1684) | 0.0% 0.0068 * | 2 (503) | 0.0961 (0.0309; 0.1613) | 0.0% 0.0038 * |
| High does (60 µg) | 6 (418) | 0.0395 (−0.0250; 0.1040) | 0.0% 0.2303 | 6 (418) | 0.0505 (−0.0571; 0.1581) | 53.9% 0.3579 | 6 (418) | 0.0514 (−0.1139; 0.2168) | 72.2% 0.5420 |
| Mean Age > 18 | 5 (804) | 0.0767 (0.0224; 0.1309) | 0.0% 0.0056 * | 5 (804) | 0.0903 (−0.0025; 0.1832) | 68.3% 0.0565 | 5 (804) | 0.1094 (0.0090; 0.2099) | 69.8% 0.0327 * |
| Mean Age < 18 | 3 (117) | 0.0374 (−0.0987; 0.1735) | 0.0% 0.5898 | 3 (117) | −0.0200 (−0.1580; 0.1180) | 0.0% 0.7767 | 3 (117) | −0.0677 (−0.2800; 0.1445) | 37.5% 0.5317 |
| Chemotherapy | 3 (180) | 0.0365 (−0.0496; 0.1227) | 0.0% 0.4061 | 3 (180) | −0.0101 (−0.0798; 0.0596) | 0.0% 0.7759 | 3 (180) | 0.0788 (−0.2991; 0.4568) | 86.6% 0.6827 |
| Post-transplant | 5 (739) | 0.0894 (0.0272; 0.1515) | 0.0% 0.0048 * | 5 (739) | 0.1084 (0.0272; 0.1897) | 36.9% 0.0089 * | 5 (739) | 0.0672 (0.0175; 0.1170) | 0.0% 0.0080 * |
| Outcome Assessment | Number of Trials (Patients) | Risk Ratio (95% CI) Fixed-Effect Estimate | Risk Ratio (95% CI) Random-Effect | p-Value Random-Effect | Heterogeneity I2 (%) |
|---|---|---|---|---|---|
| Adverse Events, all | 8 (1007) | 0.8879 (0.8072; 0.9766) | 1.0211 (0.6770; 1.5401) | 0.9208 | 93.7% |
| Mild | 6 (792) | 0.8094 (0.7120; 0.9201) | 0.8698 (0.5722; 1.3221) | 0.5137 | 88.7% |
| Moderate | 6 (792) | 0.9003 (0.7546; 1.0741) | 0.9626 (0.5165; 1.7940) | 0.9044 | 91.5% |
| Severe | 6 (792) | 1.0360 (0.7095; 1.5129) | 1.1472 (0.4112; 3.2007) | 0.7931 | 75.6% |
| Serious | 5 (656) | 0.8787 (0.5823; 1.3259) | 0.8228 (0.5514; 1.2276) | 0.3394 | 0.0% |
| Rejection | 3 (787) | 1.3454 (0.4205; 4.3054) | 1.3795 (0.3858; 4.9330) | 0.6207 | 0.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, J.-J.; Lin, C.; Ho, C.-L.; Chen, P.-H.; Lee, C.-H. Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials. J. Clin. Med. 2019, 8, 590. https://doi.org/10.3390/jcm8050590
Lai J-J, Lin C, Ho C-L, Chen P-H, Lee C-H. Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials. Journal of Clinical Medicine. 2019; 8(5):590. https://doi.org/10.3390/jcm8050590
Chicago/Turabian StyleLai, Jiun-Ji, Chin Lin, Ching-Liang Ho, Po-Huang Chen, and Cho-Hao Lee. 2019. "Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials" Journal of Clinical Medicine 8, no. 5: 590. https://doi.org/10.3390/jcm8050590
APA StyleLai, J.-J., Lin, C., Ho, C.-L., Chen, P.-H., & Lee, C.-H. (2019). Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials. Journal of Clinical Medicine, 8(5), 590. https://doi.org/10.3390/jcm8050590







