Osteogenic Cell Behavior on Titanium Surfaces in Hard Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Study
2.2. Sample Preparation and Implant Surface Modification
2.3. Surface Characteristics
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Immunofluorescence Microscopy (IF) Analysis
2.6. Transmission Electron Microscopy (TEM) Analysis
2.7. TEM Sample Preparation by Focused Ion Beam (FIB)
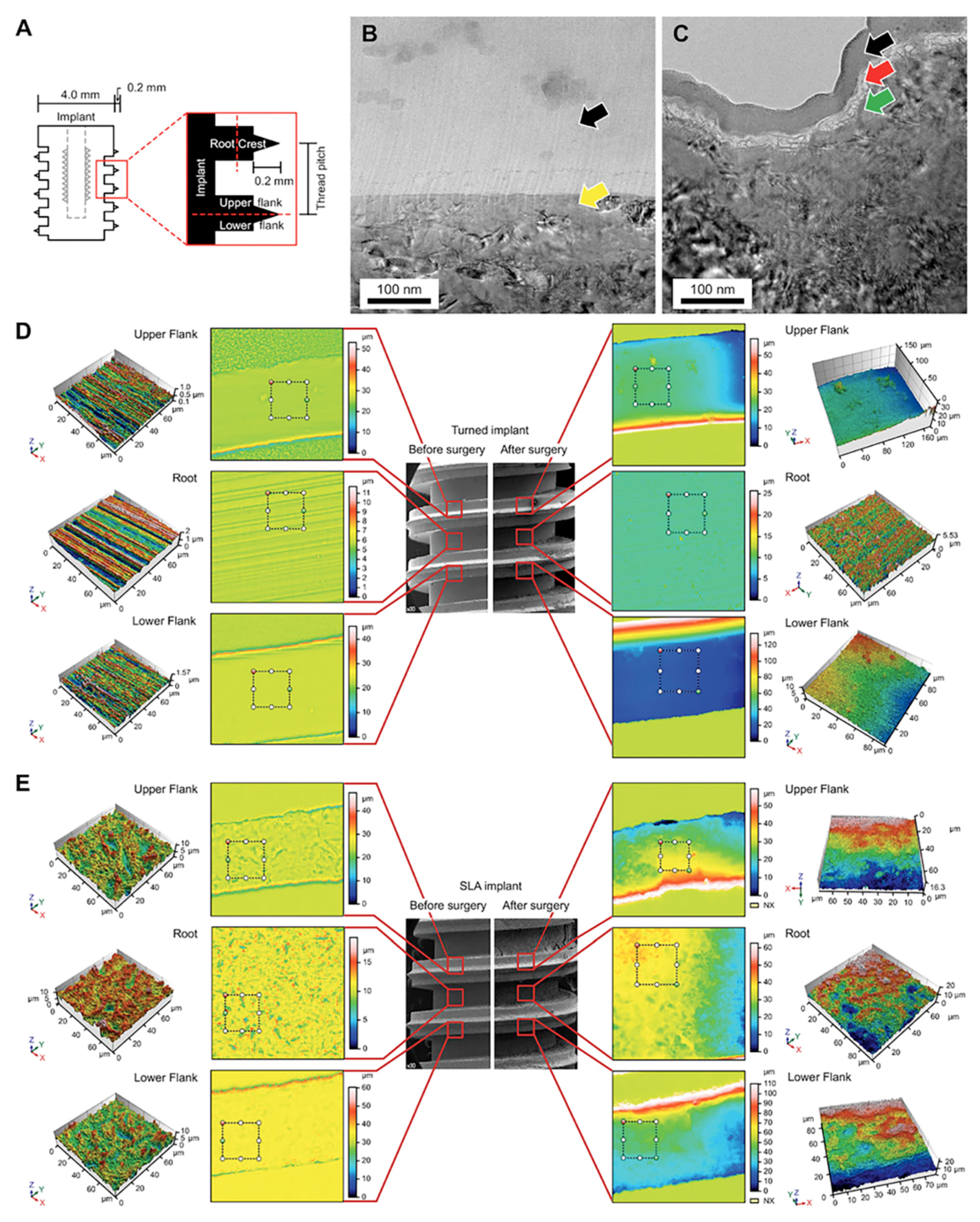
3. Results
3.1. Parts of the Implant
3.2. TEM Sample Preparation by Focused Ion Beam (FIB)
3.3. Confocal Laser Scanning Microscopy (CLSM) Analysis of the Implant
3.4. Scanning Electron Microscopy (SEM) Analysis of the Implant
3.5. Scanning Electron Microscopy (SEM) Analysis of the Surrounding Bone of the Retrieved Implant
3.6. Immunofluorescence Microscopy (IF) Analysis
3.7. Transmission Electron Microscopy (TEM) Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kohles, S.S.; Clark, M.B.; Brown, C.A.; Kenealy, J.N. Direct assessment of profilometric roughness variability from typical implant surface types. Int. J. Oral Maxillofac. Implants 2004, 19, 510–516. [Google Scholar]
- Yeo, I.S.; Han, J.S.; Yang, J.H. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, H.J.; Jang, J.U.; Yeo, I.S. Comparison between bioactive fluoride modified and bioinert anodically oxidized implant surfaces in early bone response using rabbit tibia model. Implant Dent. 2012, 21, 124–128. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, O.B.; Min, S.K.; Jung, S.Y.; Jang, D.H.; Kwon, T.K.; Min, B.M.; Yeo, I.S. The effect of the dltiddsywyri motif of the human laminin alpha2 chain on implant osseointegration. Biomaterials 2013, 34, 4027–4037. [Google Scholar] [CrossRef]
- Koh, J.W.; Kim, Y.S.; Yang, J.H.; Yeo, I.S. Effects of a calcium phosphate-coated and anodized titanium surface on early bone response. Int. J. Oral Maxillofac. Implants 2013, 28, 790–797. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, T.K.; Lee, H.J.; Min, S.K.; Yeo, I.S. Evaluation of early bone response to fluoride-modified and anodically oxidized titanium implants through continuous removal torque analysis. Implant Dent. 2012, 21, 427–432. [Google Scholar] [CrossRef]
- Yeo, I.S.; Min, S.K.; Kang, H.K.; Kwon, T.K.; Jung, S.Y.; Min, B.M. Identification of a bioactive core sequence from human laminin and its applicability to tissue engineering. Biomaterials 2015, 73, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11 (Suppl. 1), S123–S136. [Google Scholar] [PubMed]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants 2010, 25, 63–74. [Google Scholar] [PubMed]
- Choi, J.Y.; Jung, U.W.; Kim, C.S.; Jung, S.M.; Lee, I.S.; Choi, S.H. Influence of nanocoated calcium phosphate on two different types of implant surfaces in different bone environment: An animal study. Clin. Oral Implants Res. 2013, 24, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Molne, J.; Wennerberg, A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Eur. J. Oral Implantol. 2018, 11 (Suppl. 1), S37–S46. [Google Scholar]
- Albrektsson, T. On implant prosthodontics: One narrative, twelve voices-1. Int. J. Prosthodont. 2018, 31, s11–s14. [Google Scholar]
- Choi, J.Y.; Kang, S.H.; Kim, H.Y.; Yeo, I.L. Control variable implants improve interpretation of surface modification and implant design effects on early bone responses: An in vivo study. Int. J. Oral Maxillofac. Implants 2018, 33, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Kang, H.K.; Jang, D.H.; Jung, S.Y.; Kim, O.B.; Min, B.M.; Yeo, I.S. Titanium surface coating with a laminin-derived functional peptide promotes bone cell adhesion. Biomed. Res. Int. 2013, 2013, 638348. [Google Scholar] [CrossRef]
- Araújo-Gomes, N.; Romero-Gavilán, F.; Sánchez-Pérez, A.M.; Gurruchaga, M.; Azkargorta, M.; Elortza, F.; Martinez-Ibañez, M.; Iloro, I.; Suay, J.; Goñi, I. Characterization of serum proteins attached to distinct sol-gel hybrid surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1477–1485. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Bone immune response to materials, part i: Titanium, peek and copper in comparison to sham at 10 days in rabbit tibia. J. Clin. Med. 2018, 7, 526. [Google Scholar] [CrossRef]
- Choi, J.Y.; Sim, J.H.; Yeo, I.L. Characteristics of contact and distance osteogenesis around modified implant surfaces in rabbit tibiae. J. Periodontal Implant Sci. 2017, 47, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 172–184. [Google Scholar] [CrossRef]
- Yeo, I.S. Reality of dental implant surface modification: A short literature review. Open Biomed. Eng. J. 2014, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Davies, J.; Turner, S.; Sandy, J.R. Distraction osteogenesis—A review. Br. Dent. J. 1998, 185, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998, 11, 391–401. [Google Scholar]
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Kwon, T.K.; Kim, H.Y.; Yang, J.H.; Wikesjö, U.M.; Lee, J.; Koo, K.T.; Yeo, I.S. First-order mathematical correlation between damping and resonance frequency evaluating the bone-implant interface. Int. J. Oral Maxillofac. Implants 2016, 31, 1008–1015. [Google Scholar] [CrossRef]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar]
- Donath, K.; Laass, M.; Günzl, H.J. The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 131–137. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Gomes, N.C.; Ródenas, J.; Sánchez, A.; Azkargorta, M.; Iloro, I.; Elortza, F.; García Arnáez, I.; Gurruchaga, M.; Goñi, I.; et al. Proteome analysis of human serum proteins adsorbed onto different titanium surfaces used in dental implants. Biofouling 2017, 33, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Dodo, C.G.; Senna, P.M.; Custodio, W.; Paes Leme, A.F.; Del Bel Cury, A.A. Proteome analysis of the plasma protein layer adsorbed to a rough titanium surface. Biofouling 2013, 29, 549–557. [Google Scholar] [CrossRef]
- Bormann, K.H.; Gellrich, N.C.; Kniha, H.; Schild, S.; Weingart, D.; Gahlert, M. A prospective clinical study to evaluate the performance of zirconium dioxide dental implants in single-tooth edentulous area: 3-year follow-up. BMC Oral Health 2018, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (peek) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-Y.; Albrektsson, T.; Jeon, Y.-J.; Yeo, I.-S.L. Osteogenic Cell Behavior on Titanium Surfaces in Hard Tissue. J. Clin. Med. 2019, 8, 604. https://doi.org/10.3390/jcm8050604
Choi J-Y, Albrektsson T, Jeon Y-J, Yeo I-SL. Osteogenic Cell Behavior on Titanium Surfaces in Hard Tissue. Journal of Clinical Medicine. 2019; 8(5):604. https://doi.org/10.3390/jcm8050604
Chicago/Turabian StyleChoi, Jung-Yoo, Tomas Albrektsson, Young-Jun Jeon, and In-Sung Luke Yeo. 2019. "Osteogenic Cell Behavior on Titanium Surfaces in Hard Tissue" Journal of Clinical Medicine 8, no. 5: 604. https://doi.org/10.3390/jcm8050604
APA StyleChoi, J.-Y., Albrektsson, T., Jeon, Y.-J., & Yeo, I.-S. L. (2019). Osteogenic Cell Behavior on Titanium Surfaces in Hard Tissue. Journal of Clinical Medicine, 8(5), 604. https://doi.org/10.3390/jcm8050604






