Innovative Modeling Techniques and 3D Printing in Patients with Left Ventricular Assist Devices: A Bridge from Bench to Clinical Practice
Abstract
1. Introduction
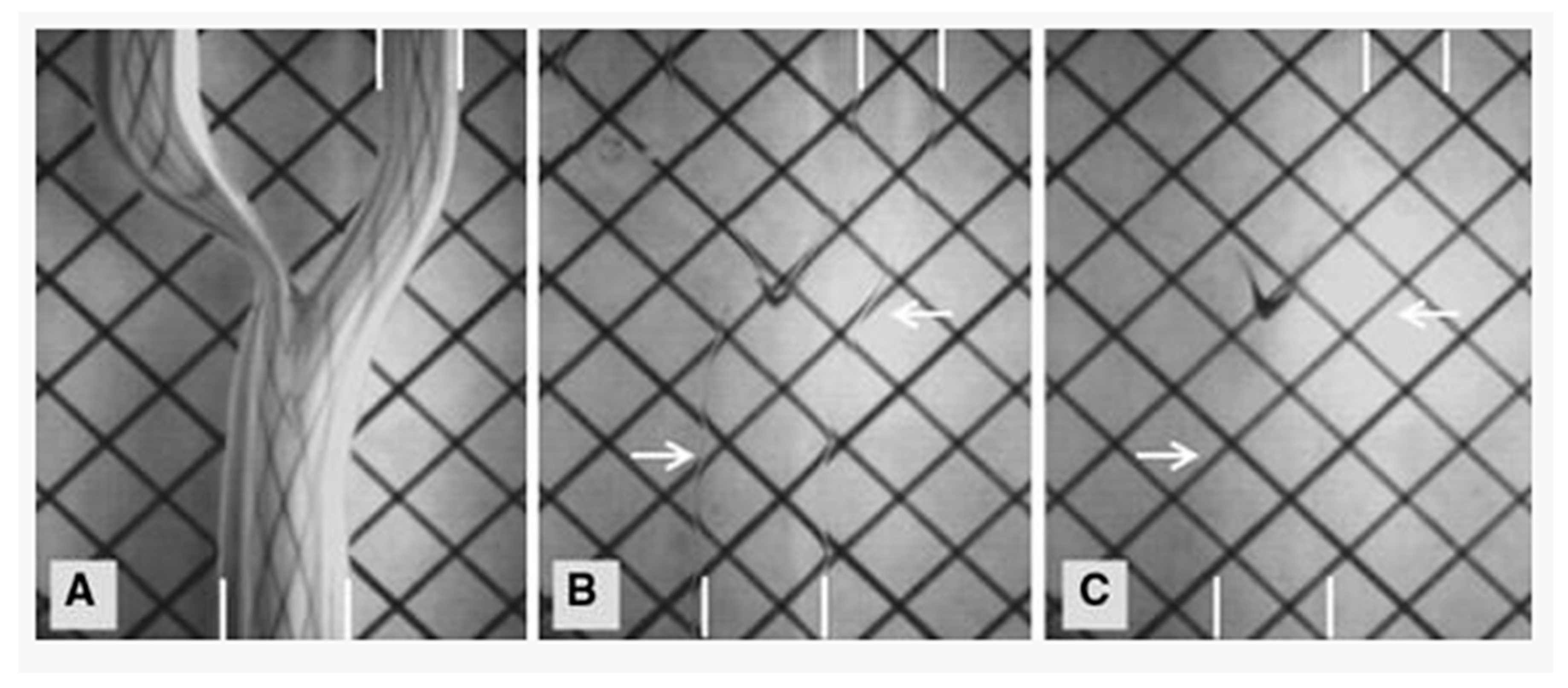
2. Computational Fluid Dynamics
3. Particle Image Velocimetry
4. 3D Printing
5. Discussion
Author Contributions
Conflicts of Interest
References
- Prinzing, A.; Herold, U.; Berkefeld, A.; Krane, M.; Lange, R.; Voss, B. Left ventricular assist devices-current state and perspectives. J. Thorac. Dis. 2016, 8, E660–E666. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.C.; Chen, C.W.; Chung, J.J.; Han, J.; Bermudez, C.A.; Wald, J.; Atluri, P. Is there a difference in bleeding after left ventricular assist device implant: Centrifugal versus axial? J. Cardiothorac. Surg. 2018, 13, 22. [Google Scholar] [CrossRef]
- Mehra, M.R.; Naka, Y.; Uriel, N.; Goldstein, D.J.; Cleveland, J.C., Jr.; Colombo, P.C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Jorde, U.P.; et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hennessy-Strahs, S.; Kwiatkowski, P.; Bermudez, C.A.; Acker, M.A.; Atluri, P.; McConnell, P.I.; Bartoli, C.R. Continuous-Flow LVAD Support Causes a Distinct Form of Intestinal Angiodysplasia. Circ. Res. 2017, 121, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Gurvits, G.E.; Fradkov, E. Bleeding with the artificial heart: Gastrointestinal hemorrhage in CF-LVAD patients. World J. Gastroenterol. 2017, 23, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Moazami, N.; Frontera, J.A. Stroke and Intracranial Hemorrhage in HeartMate II and HeartWare Left Ventricular Assist Devices: A Systematic Review. Neurocrit. Care 2017, 27, 17–25. [Google Scholar] [CrossRef]
- Blitz, A. Pump thrombosis—A riddle wrapped in a mystery inside an enigma. Ann. Cardiothorac. Surg. 2014, 3, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, M.; Ye, L.; Dong, Z. Computational fluid dynamics analysis and PIV validation of a bionic vortex flow pulsatile LVAD. Technol. Health Care 2015, 23 (Suppl. 2), S443–S451. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, B.; Yu, C. The hemodynamic effects of the LVAD outflow cannula location on the thrombi distribution in the aorta: A primary numerical study. Comput. Methods Programs Biomed. 2016, 133, 217–227. [Google Scholar] [CrossRef]
- Bourque, K.; Cotter, C.; Dague, C.; Harjes, D.; Dur, O.; Duhamel, J.; Spink, K.; Walsh, K.; Burke, E. Design Rationale and Preclinical Evaluation of the HeartMate 3 Left Ventricular Assist System for Hemocompatibility. ASAIO J. 2016, 62, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Medvitz, R.B.; Kreider, J.W.; Manning, K.B.; Fontaine, A.A.; Deutsch, S.; Paterson, E.G. Development and validation of a computational fluid dynamics methodology for simulation of pulsatile left ventricular assist devices. ASAIO J. 2007, 53, 122–131. [Google Scholar] [CrossRef]
- Medvitz, R.B.; Reddy, V.; Deutsch, S.; Manning, K.B.; Paterson, E.G. Validation of a CFD methodology for positive displacement LVAD analysis using PIV data. J. Biomech. Eng. 2009, 131, 111009. [Google Scholar] [CrossRef]
- Mazzitelli, R.; Boyle, F.; Murphy, E.; Renzulli, A.; Fragomeni, G. Numerical prediction of the effect of aortic Left Ventricular Assist Device outflow-graft anastomosis location. Biocybern. Biomed. Eng. 2016, 36, 327–343. [Google Scholar] [CrossRef]
- Itatani, K.; Miyazaki, S.; Furusawa, T.; Numata, S.; Yamazaki, S.; Morimoto, K.; Makino, R.; Morichi, H.; Nishino, T.; Yaku, H. New imaging tools in cardiovascular medicine: Computational fluid dynamics and 4D flow MRI. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K. Computational fluid dynamics in cardiovascular disease. Korean Circ. J. 2011, 41, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Tesche, C.; De Cecco, C.N.; Baumann, S.; Renker, M.; McLaurin, T.W.; Duguay, T.M.; Bayer, R.R., 2nd; Steinberg, D.H.; Grant, K.L.; Canstein, C.; et al. Coronary CT Angiography-derived Fractional Flow Reserve: Machine Learning Algorithm versus Computational Fluid Dynamics Modeling. Radiology 2018, 288, 64–72. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Roncon, L. Mathematics and transcatheter aortic valve implantation: Use of computational fluid dynamics and finite element analysis. Is this the future? Int. J. Cardiol. 2016, 207, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Karmonik, C.; Partovi, S.; Loebe, M.; Schmack, B.; Weymann, A.; Lumsden, A.B.; Karck, M.; Ruhparwar, A. Computational fluid dynamics in patients with continuous-flow left ventricular assist device support show hemodynamic alterations in the ascending aorta. J. Thorac. Cardiovasc. Surg. 2014, 147, 1326–1333.e1321. [Google Scholar] [CrossRef] [PubMed]
- Callington, A.; Long, Q.; Mohite, P.; Simon, A.; Mittal, T.K. Computational fluid dynamic study of hemodynamic effects on aortic root blood flow of systematically varied left ventricular assist device graft anastomosis design. J. Thorac. Cardiovasc. Surg. 2015, 150, 696–704. [Google Scholar] [CrossRef]
- Osorio, A.F.; Osorio, R.; Ceballos, A.; Tran, R.; Clark, W.; Divo, E.A.; Argueta-Morales, I.R.; Kassab, A.J.; DeCampli, W.M. Computational fluid dynamics analysis of surgical adjustment of left ventricular assist device implantation to minimise stroke risk. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 622–638. [Google Scholar] [CrossRef]
- Neidlin, M.; Corsini, C.; Sonntag, S.J.; Schulte-Eistrup, S.; Schmitz-Rode, T.; Steinseifer, U.; Pennati, G.; Kaufmann, T.A.S. Hemodynamic analysis of outflow grafting positions of a ventricular assist device using closed-loop multiscale CFD simulations: Preliminary results. J. Biomech. 2016, 49, 2718–2725. [Google Scholar] [CrossRef]
- Al-Azawy, M.G.; Turan, A.; Revell, A. Investigating the impact of non-Newtonian blood models within a heart pump. Int. J. Numer. Method Biomed. Eng. 2017, 33. [Google Scholar] [CrossRef]
- Jackson, N.S.; Stokes, J.; Sadler, M.; Heikal, M.R.; Faure, M.; Pommier, L. Correlation of the Combustion Characteristics of Spark Ignition Engines With the In-Cylinder Flow Field Characterised Using PIV in a Water Analogy Rig. SAE Trans. 1997, 106, 1766–1778. [Google Scholar]
- Hopkins, L.M.; Kelly, J.T.; Wexler, A.S.; Prasad, A.K. Particle image velocimetry measurements in complex geometries. Exp. Fluids 2000, 29, 91–95. [Google Scholar] [CrossRef]
- Yousif, M.Y.; Holdsworth, D.W.; Poepping, T.L. A blood-mimicking fluid for particle image velocimetry with silicone vascular models. Exp. Fluids 2011, 50, 769–774. [Google Scholar] [CrossRef]
- Zimpfer, D.; Strueber, M.; Aigner, P.; Schmitto, J.D.; Fiane, A.E.; Larbalestier, R.; Tsui, S.; Jansz, P.; Simon, A.; Schueler, S.; et al. Evaluation of the HeartWare ventricular assist device Lavare cycle in a particle image velocimetry model and in clinical practice. Eur. J. Cardiothorac. Surg. 2016, 50, 839–848. [Google Scholar] [CrossRef]
- Scardulla, F.; Bellavia, D.; D’Acquisto, L.; Raffa, G.M.; Pasta, S. Particle image velocimetry study of the celiac trunk hemodynamic induced by continuous-flow left ventricular assist device. Med. Eng. Phys. 2017, 47, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Samaroo, G.; Ling, I.; Dembitsky, W.; Adamson, R.; del Alamo, J.C.; May-Newman, K. Intraventricular flow patterns and stasis in the LVAD-assisted heart. J. Biomech. 2014, 47, 1485–1494. [Google Scholar] [CrossRef]
- Maragiannis, D.; Jackson, M.S.; Igo, S.R.; Schutt, R.C.; Connell, P.; Grande-Allen, J.; Barker, C.M.; Chang, S.M.; Reardon, M.J.; Zoghbi, W.A.; et al. Replicating Patient-Specific Severe Aortic Valve Stenosis With Functional 3D Modeling. Circ. Cardiovasc. Imaging 2015, 8, e003626. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc. Imaging 2017, 10, 171–184. [Google Scholar] [CrossRef]
- Schmauss, D.; Gerber, N.; Sodian, R. Three-dimensional printing of models for surgical planning in patients with primary cardiac tumors. J. Thorac. Cardiovasc. Surg. 2013, 145, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Al Jabbari, O.; Abu Saleh, W.K.; Patel, A.P.; Igo, S.R.; Reardon, M.J. Use of three-dimensional models to assist in the resection of malignant cardiac tumors. J. Card. Surg. 2016, 31, 581–583. [Google Scholar] [CrossRef]
- Farooqi, K.M.; Saeed, O.; Zaidi, A.; Sanz, J.; Nielsen, J.C.; Hsu, D.T.; Jorde, U.P. 3D Printing to Guide Ventricular Assist Device Placement in Adults With Congenital Heart Disease and Heart Failure. JACC Heart Fail. 2016, 4, 301–311. [Google Scholar] [CrossRef]
- Farooqi, K.M.; Cooper, C.; Chelliah, A.; Saeed, O.; Chai, P.J.; Jambawalikar, S.R.; Lipson, H.; Bacha, E.A.; Einstein, A.J.; Jorde, U.P. 3D Printing and Heart Failure: The Present and the Future. JACC Heart Fail. 2019, 7, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Karimov, J.H.; Steffen, R.J.; Byram, N.; Sunagawa, G.; Horvath, D.; Cruz, V.; Golding, L.A.; Fukamachi, K.; Moazami, N. Human Fitting Studies of Cleveland Clinic Continuous-Flow Total Artificial Heart. ASAIO J. 2015, 61, 424–428. [Google Scholar] [CrossRef]
- Saeed, D.; Ootaki, Y.; Noecker, A.; Weber, S.; Smith, W.A.; Duncan, B.W.; Fukamachi, K. The Cleveland Clinic PediPump: Virtual fitting studies in children using three-dimensional reconstructions of cardiac computed tomography scans. ASAIO J. 2008, 54, 133–137. [Google Scholar] [CrossRef]
- Vukicevic, M.; Maragiannis, D.; Jackson, M.; Little, S.H. Functional Evaluation of a Patient-specific 3D Printed Model of Aortic Regurgitation. Circulation 2015, 132, A18647. [Google Scholar]
- Gaudio, L.T.; Veltri, P.; Fragomeni, G. Modeling and application of aorta coarctation: Support system for pre-operative decision. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; pp. 2031–2032. [Google Scholar]
- Russ, M.; O’Hara, R.; Setlur Nagesh, S.V.; Mokin, M.; Jimenez, C.; Siddiqui, A.; Bednarek, D.; Rudin, S.; Ionita, C. Treatment Planning for Image-Guided Neuro-Vascular Interventions Using Patient-Specific 3D Printed Phantoms. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9417. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Naftel, D.C.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B. Seventh INTERMACS annual report: 15,000 patients and counting. J. Heart Lung Transplant. 2015, 34, 1495–1504. [Google Scholar] [CrossRef]
- Sommer, K.N.; Shepard, L.; Karkhanis, N.V.; Iyer, V.; Angel, E.; Wilson, M.F.; Rybicki, F.J.; Mitsouras, D.; Rudin, S.; Ionita, C.N. 3D Printed Cardiovascular Patient Specific Phantoms Used for Clinical Validation of a CT-derived FFR Diagnostic Software. Proc. SPIE Int. Soc. Opt. Eng. 2018, 10578. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaker, R.; Araujo-Gutierrez, R.; Marcos-Abdala, H.G.; Agrawal, T.; Fida, N.; Kassi, M. Innovative Modeling Techniques and 3D Printing in Patients with Left Ventricular Assist Devices: A Bridge from Bench to Clinical Practice. J. Clin. Med. 2019, 8, 635. https://doi.org/10.3390/jcm8050635
Thaker R, Araujo-Gutierrez R, Marcos-Abdala HG, Agrawal T, Fida N, Kassi M. Innovative Modeling Techniques and 3D Printing in Patients with Left Ventricular Assist Devices: A Bridge from Bench to Clinical Practice. Journal of Clinical Medicine. 2019; 8(5):635. https://doi.org/10.3390/jcm8050635
Chicago/Turabian StyleThaker, Rishi, Raquel Araujo-Gutierrez, Hernan G. Marcos-Abdala, Tanushree Agrawal, Nadia Fida, and Mahwash Kassi. 2019. "Innovative Modeling Techniques and 3D Printing in Patients with Left Ventricular Assist Devices: A Bridge from Bench to Clinical Practice" Journal of Clinical Medicine 8, no. 5: 635. https://doi.org/10.3390/jcm8050635
APA StyleThaker, R., Araujo-Gutierrez, R., Marcos-Abdala, H. G., Agrawal, T., Fida, N., & Kassi, M. (2019). Innovative Modeling Techniques and 3D Printing in Patients with Left Ventricular Assist Devices: A Bridge from Bench to Clinical Practice. Journal of Clinical Medicine, 8(5), 635. https://doi.org/10.3390/jcm8050635




