Abstract
Petadolex®, a defined butterbur extract has clinically proven efficacy against migraine attacks. However, spontaneous reports indicate cases of herbal induced liver injury (HILI). While most HILI patients presented mild serum biochemistry changes (<3 ULN, dose range 50 to 225 mg/day; treatment duration 4–730 days) nine developed severe HILI (average time-to-onset 103 days, ALT-range 3–153; AST 2–104-fold ULN). HILI cases resolved after medication withdrawal though two patients required liver transplantation. Liver biopsies revealed an inconsistent injury pattern, i.e. necrosis, macrovesicular steatosis, inflammation, cholestasis, and bile duct proliferation. Causality assessment rated 3 cases likely, 13 possible, 8 unlikely and 24 as unclassifiable/unclassified. Note, 22 patients reported hepatotoxic co-medications especially during periods of pain. A no-observable-adverse-effect-level at 15-fold of the maximal clinical dose (3 mg/kg/day MCD) was established for rats. At >45 and 90-fold MCD bile duct hyperplasia was observed but could not be confirmed in an explorative minipig study at 218-fold MCD. Human hepatocyte studies at 49-fold Cmax serum petasins (=active ingredient) and therapeutic Ibuprofen, Paracetamol and Naratriptan concentrations evidenced liver transaminase and CYP-monooxygenase changes. Collectively, Petadolex® HILI cases are rare, idiosyncratic and frequently confounded by co-medications. A physician-supervised self-medication plan with herbs and pain relief medication is needed to minimize risk for HILI.
1. Introduction
Herbal and Dietary Supplements (HDS) are a multi-billion dollar enterprise and are considered to be safe by the public. However, the U.S. Drug-Induced-Liver Injury-Network reported HDS related liver injury to be on the rise accounting for about 20% of all DILI cases [1]. There is unmet need to better diagnose herb induced liver injury (HILI) and to provide guidance for its clinical management.
We report the case of Petadolex®, a defined Petasites hybridus (butterbur) extract used as migraine preventive treatment in adults. The extract is primarily composed of sesquiterpene esters, mainly of the petasin and the furanopetasin chemotype [2] and constitutes a mixture of petasin, isopetasin, neopetasin as well as furanoeremophilanes and eremophilanlactones. In the following this mixture is referred to as ‘petasin’. Petadolex® is specified to ≥15% petasin, i.e. the active ingredient of butterbur and was introduced in Germany in 1972 and in the US in 1998. Its clinical efficacy in migraine therapy was evidenced in placebo-controlled double-blinded clinical investigations. A significant reduction in the frequency of migraine attacks was reported [3,4] particularly after four months of treatment [5,6]. In 2012, the American Academy of Neurology concluded butterbur to be effective for migraine prevention [7]. However, post marketing surveillance in Europe revealed HILI cases, which prompted concern over its safe use. Note, butterbur contains pyrrolizidine alkaloids (PAs) and once metabolically activated [8] can damage sinusoidal endothelium resulting in sinusoidal obstruction syndrome (SOS) [9,10].
Our study aimed to (1) evaluate the causality of spontaneously reported hepatobiliary ADRs involving Petadolex® (2) to compare liver biopsy findings of suspected HILI cases with injury patterns observed in rats and minipigs after repeated oral dosing and (3) to assess hepatotoxicity in primary human hepatocyte (PHH) cultures treated with Petadolex® and combinations of NSAIDs, Paracetamol and/or triptanes commonly used in the treatment of migraine attacks.
2. Materials and Methods
2.1. Petasites Hybridus Extracts
Certified product 056180 (Extract A, 37% petasin), 096120 (Extract B, <1% petasin) and 056180/096120 (Extract C = Petadolex®, 19.1% petasin) were supplied by Dr. Koch, Weber & Weber International GmbH & Co. KG, Inning/Ammersee, Germany. PHH studies were performed with Paracetamol (CAS: 103-90-2, Sigma-Aldrich, Germany), Ibuprofen (Alpha-Methyl-4-(isobutyl)phenylacetic acid, CAS: 31121-93-4, Sigma-Aldrich, Germany) and Naratriptan (N-methyl-2-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl] ethanesulfonamide hydrochlorid, CAS: 143388-64-1, GlaxoSmithKline, Germany). DMSO was used as vehicle (final concentration in cell culture <0.5%).
2.2. Pharmacovigilance—Periodic Safety Updated Reports (PSUR)
Periodic safety update reports (PSUR) were provided by Weber & Weber International with the aim to assess causality independently. A total of 60 hepatobiliary ADRs were reported and included 42 female, 16 male and two unspecified cases. Information regarding the 48 spontaneously reported HILI cases is given in Table 1. Furthermore, Table 2 compiles 12 cases recorded during clinical trials.

Table 1.
Forty-eight spontaneous hepatobiliary ADR case reports in association with Petadolex® intake.

Table 2.
Twelve non-serious hepatobiliary ADR case reports recorded during clinical trials with 397 patients on Petadolex®. Information on 12 hepatobiliary events recorded during clinical studies and suspected to be related to exposure with Petasites hybridus extracts.
Patients provided informed consent prior to the study entry and details of the clinical trials are given in [3,4,5,6]. The HILI cases were evaluated according to the WHO-UMC system by an expert panel consisting of specialists in internal medicine/neurology, board certified pathologist and clinical pharmacologist and toxicologist. There was agreement among the reviewers in the grading of cases. Independently, a subset of Petadolex® suspecting severe HILI cases was evaluated by hepatologists/gastroenterologists using the RUCAM method. The findings were reported in a journal which is not indexed in Medline [11].
2.3. Toxicity Studies in Animals
The results of an acute and repeated oral dose good laboratory practice (GLP) compliant toxicology study in rats were previously published [12]. The studies were carried out at Harlan Bioservices for Science GmbH, Walsrode, Germany, in accordance with the EEC Council Directives 65/65 and 73/318 and subsequent amendments detailed in the Rules Governing Medicinal Products in the European Union, the ICH Guideline S4 for Toxicity Testing (Proceedings of the First International Conference on Harmonization 1991), EEC Directive 92/69/EEC (31 July 1992), Annex V, Test B1 (20 December 1992), and the Organisation for Economic Co-operation and Development Guideline 401.
In the six-month chronic toxicity study the doses were >15-, 45 and 90-fold of the maximum clinical dose (MCD), i.e. 3 mg/kg/day. Note, the histopathology and serum biochemistries of animals four weeks after treatment termination (“recovery animals”) were not reported so far.
Additionally, an explorative study with n = 6 Göttingen minipigs weighing approximate 25 kg was performed at the LPT research facility in Hamburg, Germany. The study was not GLP compliant but followed the principles of Good Laboratory Practice Regulations of the EC as well as the OECD Principles of Good Laboratory Practice’ Document Nos. 1, 8 and 13 ENV/MC/CHEM (98) 17, ENV/JM/MONO (99) 24 and ENV/JM/MONO (2002) 9, respectively.
All experimental protocols were approved by the Lower Saxony State Office for Consumer Protection and Food Safety and the Office for Health and Consumer protection of the city of Hamburg, Germany.
Two different butterbur extracts defined by their low (0.84%) and rich petasin content (16.1%) were evaluated. Dose levels of 60, 180, 360 and 720 mg/kg body weight were administered by oral gavage for three consecutive days. No signs of toxicity were observed. Subsequently, a 28-day repeated oral dose study was completed at 218-fold MCD.
2.4. Studies with Primary Human Hepatocyte Cultures
The use of human liver resection material was approved by the Ethical Committee of Hannover Medical School; patients had given informed consent. Supplementary Table S1 provides basic patient characteristics. Isolation and culture of human hepatocyte cultures was done as previously reported [12]. All experiments were performed in accordance with relevant guidelines and regulations.
2.5. Treatment of Hepatocyte Cultures
Hepatocyte cultures were treated with extracts B and C at 60 ng/mL and 15 µg/mL and extract A at 10 µg/mL for 72 h. Due to differences in petasin content, the concentration range tested in hepatocyte cultures was 11.5–3700 ng/mL petasin. Specifically, extract C contains 19.1% petasin and assays performed at 15 µg/mL are equal to 2865 ng/mL petasin, i.e. 15,000 ng/mL × 19.1% = 2865 ng/mL petasin. Given that petasin is the major active ingredient of butterbur, the petasin concentrations can also be related to human PK data. For instance, an oral dose of either 116 mg or 232 mg butterbur extract Ze339 given to 24 healthy volunteers yielded Cmax petasin serum concentrations of 25.5 ± 14.8 and 58.1 ± 26.7 ng/mL, respectively [13]. Correspondingly, the 15 µg/mL extract C tested in hepatocyte cultures is equal to >114 or >49-fold Cmax petasin concentrations based on a dose of 116 mg or 232 mg butterbur extract. At the 15 µg/mL extract C concentration, a 30% reduction in cell viability was determined in the MTT assay after daily dosing for 72 h. To assess possible interactions, hepatocyte cultures were treated with combinations of Petadolex® and therapeutic Cmax Paracetamol (20 μg/mL), Ibuprofen (30 μg/mL) or Naratriptan (10 ng/mL) concentrations.
2.6. Assays to Determine Cell Viability and Metabolic Competence
Cell viability and mitochondrial toxicity was determined by the MTT assay [12]; urea, ALT, AST and GGT were measured on a Cobas FARA clinical chemistry analyser (Roche, Germany). CYP450 monooxygenase activity was assayed with testosterone as a substrate; the metabolites were quantified by HPLC-UV [12].
2.7. Statistical Analyses
Statistical significance testing was performed with the GraphPad Prism software version 6.05 (GraphPad Software, San Diego, CA, USA). The Shapiro–Wilk test for normality was applied to experimental data. Depending on the distribution of the data, the Student’s t-test or the non-parametric Wilcoxon Rank test was employed. The Fisher exact test was used to determine significant differences in dosage related bile duct proliferations and its reversibility in animals after treatment cessation.
3. Results
3.1. Pharmacovigilance of HILI Cases
Until 2015, a total of 60 hepatobiliary ADRs were reported and included 42 female, 16 male and two unspecified cases. Given the higher liability for migraine in women, the observed gender bias is in line with expectations [14]. Information regarding the 48 spontaneously reported HILI cases is given in Table 1. Furthermore, Table 2 compiles 12 cases recorded during clinical trials with 397 patients on Petadolex®. Importantly, none of the cases reported from clinical trials were serious and involved very minor changes in serum biochemistries (range ALT 0.6 to 1.2; AST 0.3 to 1.3 of ULN, Table 2).
The majority of spontaneously reported HILI cases (Table 1) presented ≤3-fold ULN serum biochemistry changes (ALT, AST and occasionally GGT, urea and antibody titres). Causality assessment revealed 3 cases as likely, 13 as possible, 8 as unlikely and 24 as unclassifiable/unclassified. However, nine females and one male patient presented marked changes in serum biochemistries (ALT 3-150-fold ULN; AST 2-104-fold) with two patients requiring liver transplantation. More detailed information of severe HILI cases is given in Table 3 and for three cases a relationship between exposure to Petadolex® and hepatobiliary ADR was questioned by the expert panel.

Table 3.
A summary of 10 severe HILI cases in association with Petadolex® intake.
Specifically, case #23 occurred 14 days after surgery and blood transfusion; total Petadolex® intake was 3 to 6 days. Based on the long-lasting medication experience with Petadolex® this would be an unprecedented case and may possibly arise from anaesthesia or blood transfusion. Moreover, patient #21 received tamoxifen for the treatment of breast cancer; an increased risk for NASH has been reported for this drug [15]. Patient #17 displayed marked serum transaminase elevations with extensive weight loss and a medical history of thoracic pain episodes prior to treatment with Petadolex®. Additionally, patients #12, #39 and #30 were positive for antinuclear autoantibodies to conceivable indicate an autoimmune hepatitis that may have been unmasked by Petadolex® treatment. Notwithstanding, histopathology of case #12 was judged atypical for autoimmune hepatitis and case #39 showed a chronic hepatic necro-inflammatory disorder, which is consistent with the morphological feature of an autoimmune hepatitis. This patient presented a medical history of severe hepatitis prior to Petadolex® treatment 10 years ago. A second episode of hepatitis was associated with jaundice and arthralgia and occurred two months after tonsillectomy. Hypersensitivity resulting from anaesthesia was suspected as a cause for hepatitis. The patient went into encephalopathy grade III and received a liver transplant within 1 week of hospital admission. Two cases were judged unlikely; case #48 was diagnosed with choledocholithiasis. The patient presented an isolated >46-fold bilirubin elevation due to bile flow obstruction with no change in liver transaminases. Moreover, case #9 was considered unlikely as hepatitis developed six weeks after Petadolex® treatment cessation.
To search for possible dose and/or time-to-onset relationships, scatter blots of serum biochemistries of suspected HILI cases were prepared. Cases were divided into cohorts of ≤3 and >3-fold ULN serum biochemistry changes. Except for ALT and the parameter time-to-onset at ≤3 ULN, no clear relationship could be ascertained (Supplementary Figure S1). Therefore, the cases are considered to be primarily idiosyncratic nature.
3.2. Co-Medications as A Possible Cause for Hepatobiliary ADRs
Among the suspected Petadolex® HILI cases, 22 patients reported co-medication with drugs known to cause liver injury, such as pain relief and migraine prevention therapeutics, including amitriptyline and metoprolol. In particular NSAIDs are associated with risk of hepatic injury [16]. Of the severe HILI cases, 50% had additional migraine medication (n = 3 NSAIDs, n = 2 zolmitriptan as well as an unspecified herbal product) that may have contributed or even elicited liver injury. Patients #9 and #12 received contraceptives known to potentially cause cholestasis [17]. For another nine cases, co-medication with triptans was reported, which have been limited in their use due to modulation of CYP1A2 or MAOA activity [18,19]. In addition, eight patients reported Paracetamol alone or combinations of NSAIDs for pain relief, particularly during acute migraine attacks.
3.3. Histopathology Findings of Severe HILI Cases
Liver biopsy findings for eight severe HILI cases were available. An inconsistent pattern of mixed type injury was observed. Major findings were necrosis, macro-vesicular steatosis, cholestasis and fibrosis. Cases #7, #12, #23, #30 and #35 presented inflammation and inflammatory infiltrates as major finding; notwithstanding case #23 and #39 displayed bile duct proliferation.
3.4. Results from A Chronic Toxicity Study in Rats
Absolute and relative organ weights were dose-related increased at mid and high doses after six months of treatment. Histopathology revealed bile duct hyperplasia at 45 and bile duct dilatation at 90-fold of MCD with male rats being more frequently affected.
The bile duct proliferation was corroborated by mild increases in plasma bilirubin and GGT. There was no clear pattern in the temporary alterations of serum biochemistries during the six-month chronic toxicity study with very slight but statistically insignificant changes of AST and ALT (Supplementary Table S2). These were reduced to approximately 90% of control values at the end of the study, while bilirubin was insignificantly increased by 4% in females and 85% in male rats, but with considerable variability amongst individual animals. Supplementary Table S2 summarizes clinical biochemistry data and major histopathology findings. Unlike severe HILI cases (see above), no evidence for necrosis, cholestasis or mixed type toxic liver injury was observed. However, mild lipidosis was noted in vehicle-treated control and high dose-treated animals. The vehicle Miglyol 812 is a medium change triglyceride and lipids derived from it may elicit serum biochemistry abnormalities and histocytosis, as reported by other investigators [20].
3.5. Liver Pathology Findings four Weeks after Treatment Cessation of Rats
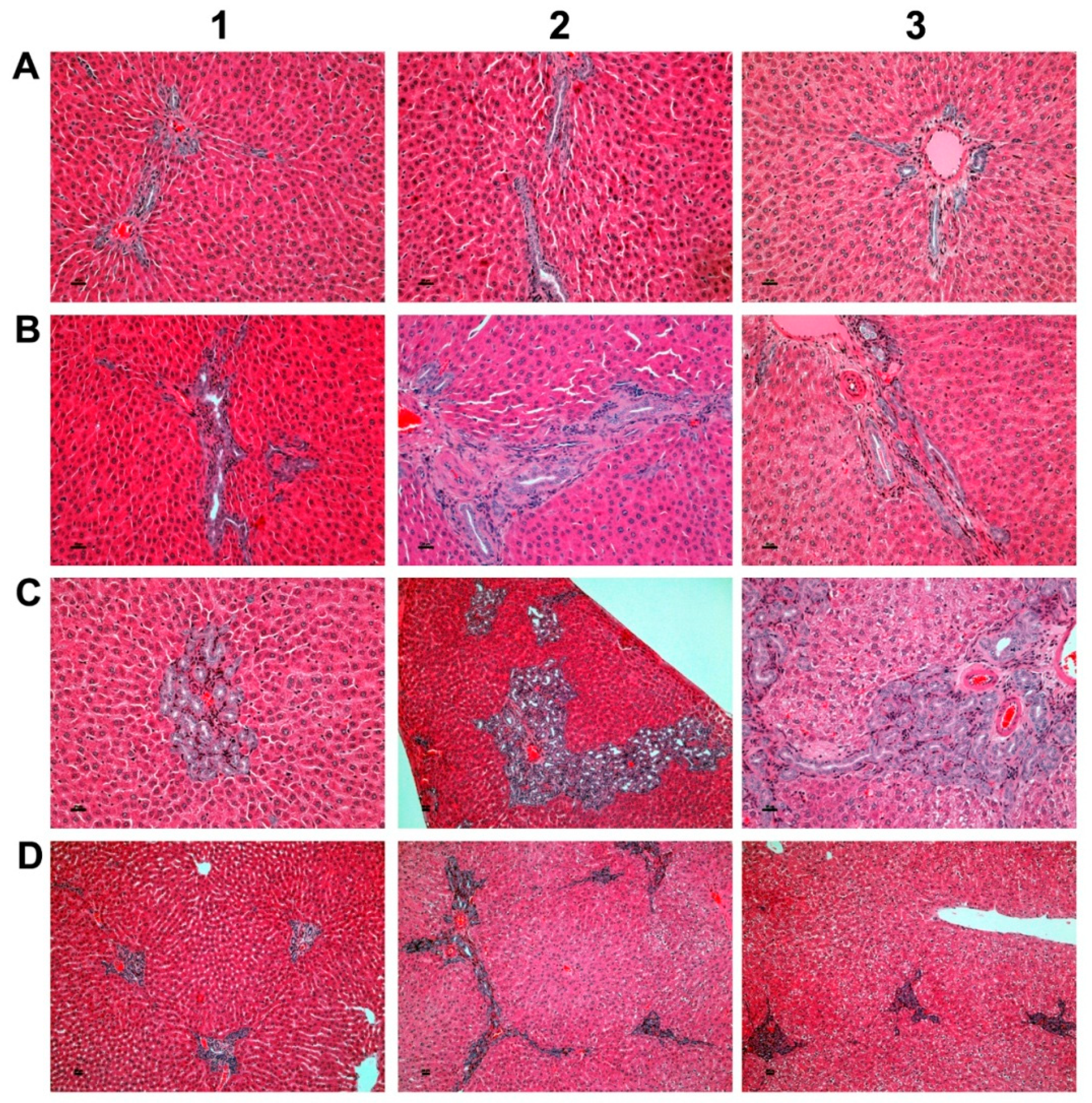
As part of the chronic toxicity study, 40 rats per dose group were treated at >15-, 45 and 90-fold of the maximum clinical dose (MCD, i.e. 3 mg/kg/day) for six months. Figure 1 depicts examples of bile duct proliferations which were observed in the mid and high dose groups.
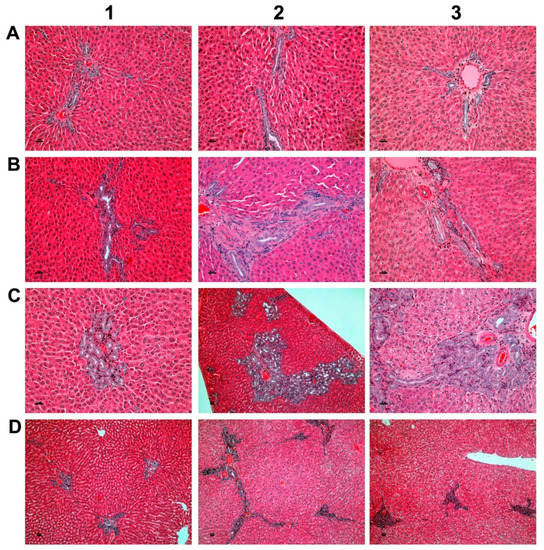
Figure 1.
Bile duct hyperplasia after repeated oral dosing of rats with a Petasites hybridus extract (Petadolex) for six months. (A). HE staining of liver sections from control animals that were treated with the vehicle Migylol 812 for six months (A1, A2). Shown in A3 is a control recovery animal which also received vehicle treatment for six months, but was subsequently given a normal laboratory diet for four weeks. No lesions were observed. (B) Animals treated with the Petasites hybridus extract at 45-fold of maximum clinical doses. HE staining of liver sections revealed mild to moderate bile duct proliferations. (C) Animals treated with the Petasites hybridus extract at 90-fold of maximum clinical doses. HE staining of liver sections revealed moderate to marked bile duct proliferations. (D) Recovery animals were treated for six months with the Petasites hybridus extract at 90-fold of maximum clinical doses. Subsequently the recovery animals were given a normal laboratory diet for four weeks. Reversibility of bile duct proliferations was observed but did not resolve within the four weeks of the recovery period. All images are a magnification of 125× and were taken with a Nikon Ni-E microscope, Japan. Image capture was done with the Nikon NIS basic research microscopic imaging software version 4.3. The images were converted into tiff files and compiled in Adobe Photoshop version CS5.
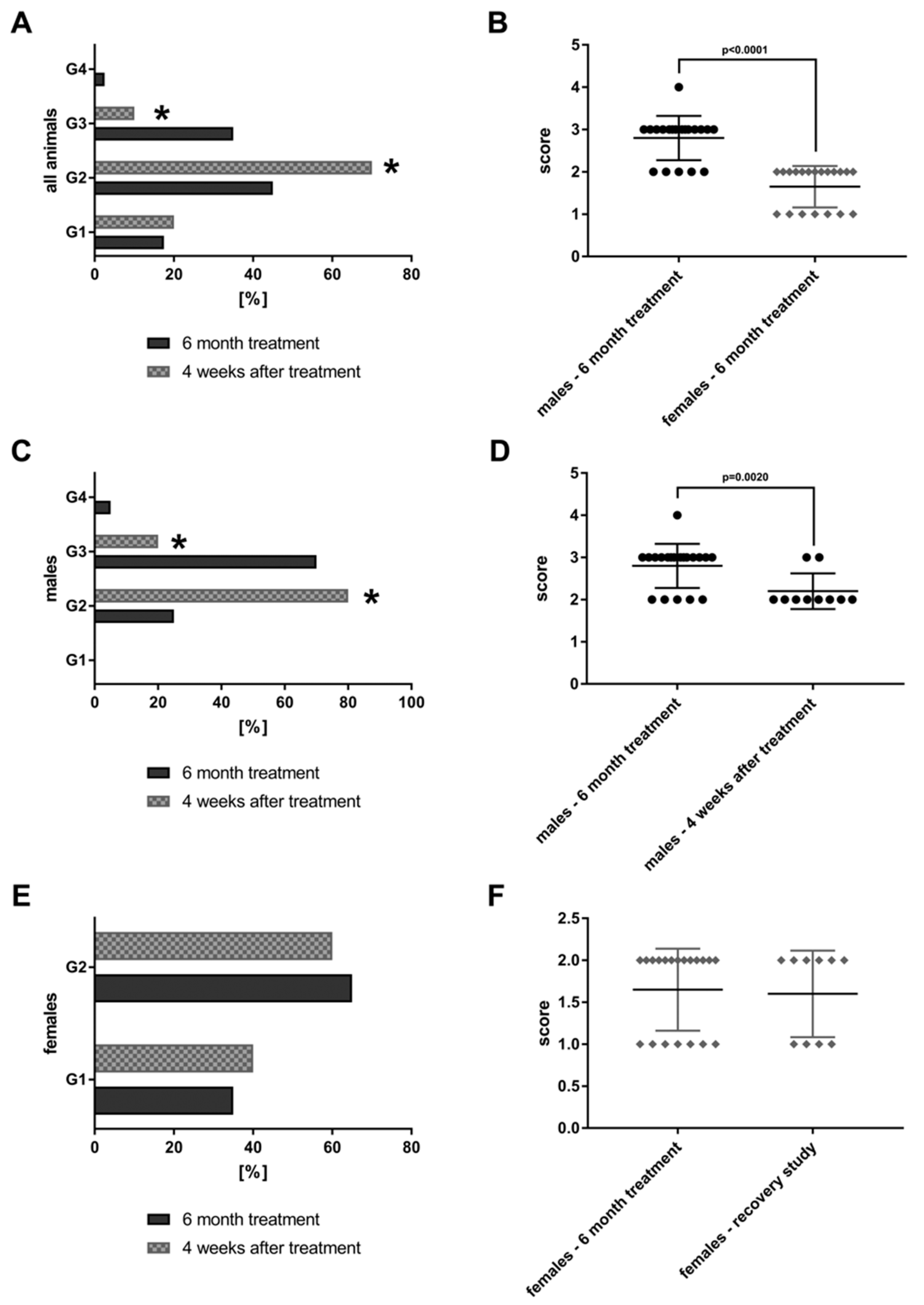
The findings are compared to 20 animals that were treated at 90-fold MCD for six months but were allowed to recover from the treatment for a period of four weeks (“recovery animals”). Subsequently, the lesions were graded by a board certified veterinary pathologist in compliance with international rules of good laboratory practice (GLP). The lesions were also evaluated independently by a board certified human pathologist. The grading of lesions ranges from minimal (G1), mild (G2), moderate (G3) to marked (G4). The grades of 40 high dose animals and 20 recovery animals are shown in Figure 2.
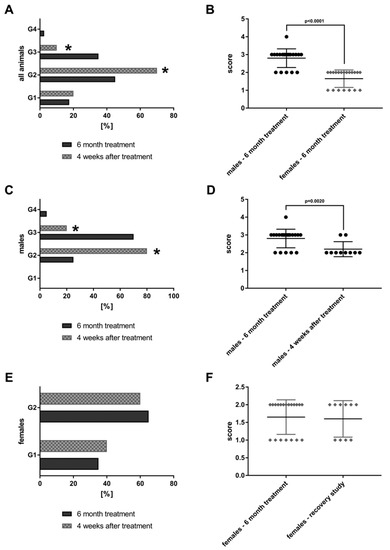
Figure 2.
Bile duct proliferations after repeated oral dosing of rats with a Petasites hybridus extract (Petadolex) for six months and a recovery period of four weeks. Bile duct proliferations were dosage related with both sex. The grading of lesions ranges from minimal (G1), mild (G2), moderate (G3) to marked (G4). (A) Grading of bile duct proliferations of 40 high dose animals (dark column) and 20 recovery animals (grey patterned) treated for 6 month. Using the Fischer exact test, a statistically significant reduction from moderate (G3) to mild (G2) was calculated for animals which were allowed to recover from the treatment for four weeks (p = 0.0001). (B) The scores for male female animals after repeated treatment for six months are compared. Males are more sensitive to the treatment effects as determined by the Wilcoxon-Mann-Whitney-Test. (C) Grading of bile duct proliferations of 20 high dose males (dark column) and 10 male recovery animals (grey patterned) treated for six months. Using the Fischer exact test, a statistically significant reduction from moderate (G3) to mild (G2) was calculated for animals that were allowed to recover from the treatment for four weeks (p = 0.0161). (D) The scores for male animals after repeated treatment for six months and male recovery animals are compared. The grades of lesion differed significantly as determined by the Wilcoxon-Mann-Whitney-Test. (E) Grading of bile duct proliferations of 20 high dose females (dark column) and 10 female recovery animals (grey patterned) treated for six months. There was no difference between the groups, albeit the lesions are less pronounced when compared to males. (F) The scores for female animals after repeated treatment for six months and female recovery animals. There was no difference between the groups.
Both sex bile duct proliferations were dosage related and tended to be reversible, at least in part. A statistically significant reduction from moderate (G3) to mild (G2) was determined using the Fischer exact test (p = 0.0001). Furthermore, the average score for bile duct proliferations differed significantly by gender (Figure 2B) with males presenting higher grades. Figure 2C,D show the grading for male animals only. Once again, a significant reduction from G3 to G2 is observed (p = 0.0161) after treatment cessation; the average scores are given in Figure 2D. Conversely there was no difference in the grading of bile duct proliferations of females when recovery animals are considered. However, the grades in females are significantly lower as compared to males (Figure 2B).
Importantly, the bile duct proliferations occurred in the absence of significant serum biochemistry changes. Next to proliferation of the existing bile duct epithelium, metaplastic changes of periportal hepatocytes were observed to assist in bile drainage at excessive treatment doses.
3.6. Explorative Studies with Minipigs
Minipigs were treated by daily oral gavage for 28 days with a butterbur extract, either low (<1%) or rich in petasin (>16%) at 218-fold of MCD. No morphological changes in the liver were observed with any of the extracts; however, a two-fold increase in indirect bilirubin and an approximately 70% reduction in bile acid serum concentrations were noted. Other serum biochemistries, i.e., aPTT, ALT, AST, GGT and 5′-nucleotidase were unchanged after repeated oral dosing for 28 days.
3.7. In vitro Hepatotoxicity Assays
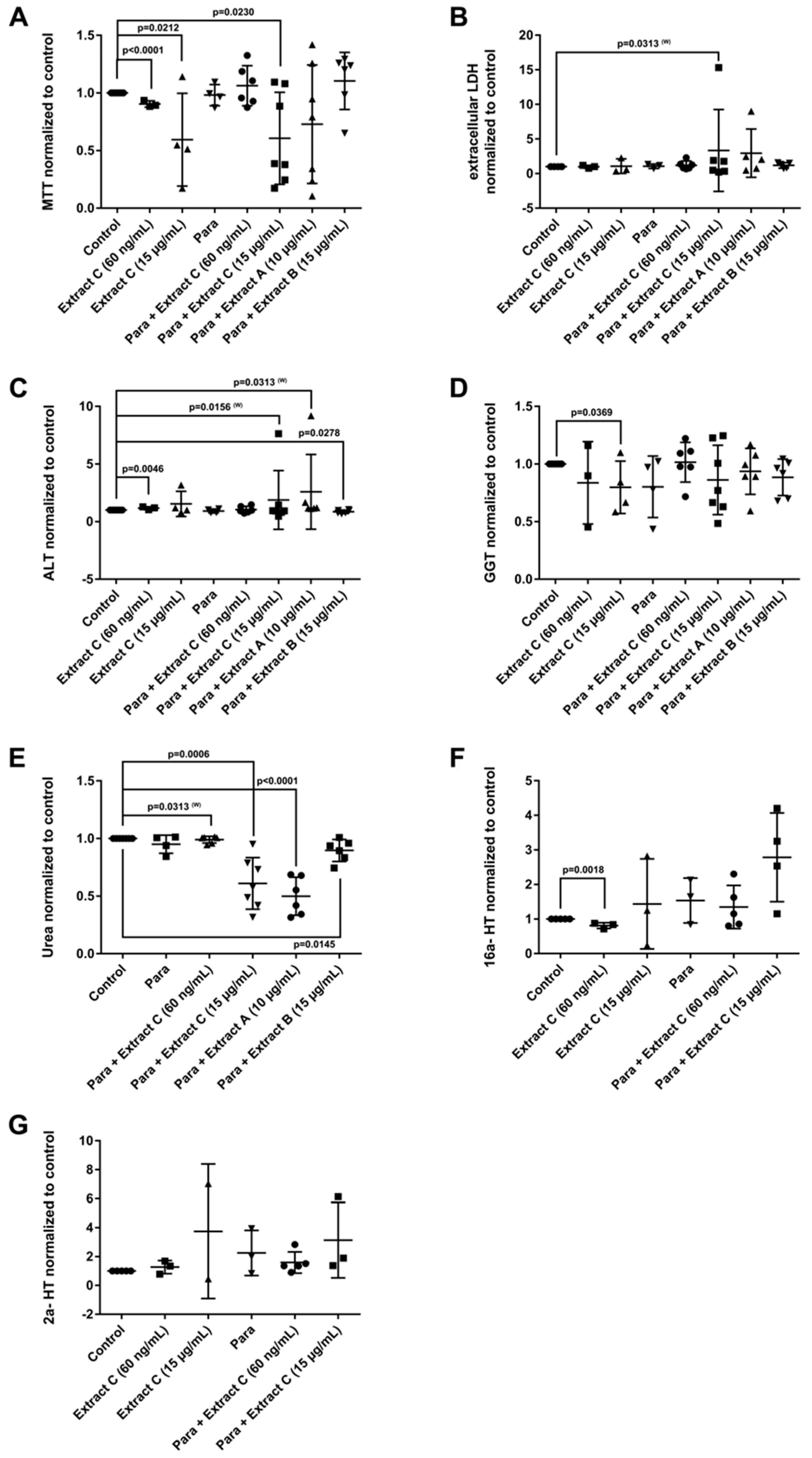
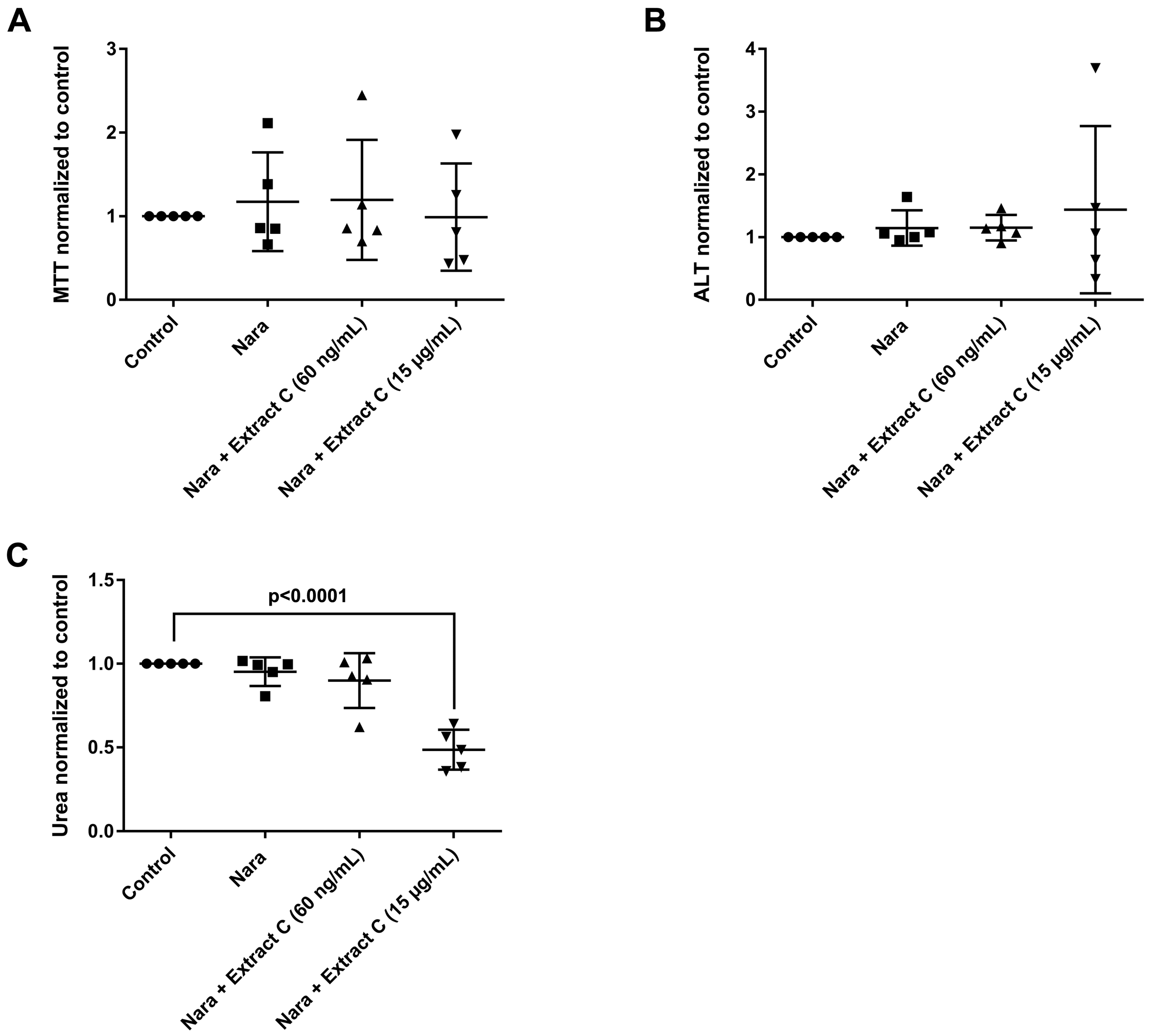
Liver function tests (LFTs) were evaluated in cultures of primary human hepatocytes (PHH) that were treated with different butterbur extracts (extract A-C) and medications commonly used for migraine attacks. This enabled an assessment of possible drug-herb interactions (Figure 3, Figure 4 and Figure 5). Given that therapeutic Petadolex® concentrations are defined by the petasin content, the data are also expressed relative to therapeutic Cmax petasin concentrations. Furthermore, hepatocyte CYP monooxygenase activities were evaluated with testosterone as a substrate. No hepatotoxicity was observed at therapeutic Cmax Petadolex® or Paracetamol concentrations, as determined by the MTT, LDH, ALT, GGT and urea assay (Figure 3A–E).
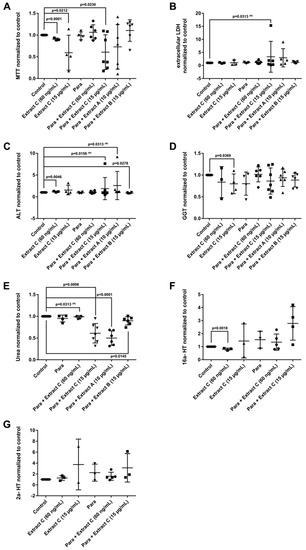
Figure 3.
Effects of different Petasites hybridus extracts and Paracetamol on clinical liver function tests in cultures of human hepatocytes after daily treatment for 72 h. (A) MTT mitochondrial reductase activity. (B) LDH leakage/extracellular activity. (C) Alanine aminotransferase activity. (D) glutamyltransferase activity. (E) Urea production. (F,G) Testosterone 16 and 2 hydroxylation activity. The data of individual donors are given normalised to the DMSO vehicle control. The Petasites hybridus extracts A, B and C are specified to 37%, <1% and 19.1% petasin, respectively. Extract C is the marketed product Petadolex® and human hepatocyte cultures were treated at therapeutic Cmax (=60 ng/mL) and >49-fold therapeutic petasin Cmax (=15 µg/mL) concentrations. Hepatocyte cultures were also treated with Paracetamol at therapeutic Cmax (=Para; 20 µg/mL) concentrations and in combinations with different Petasites hybridus extracts. The data were analysed with the GraphPad Prism software version 6.05.
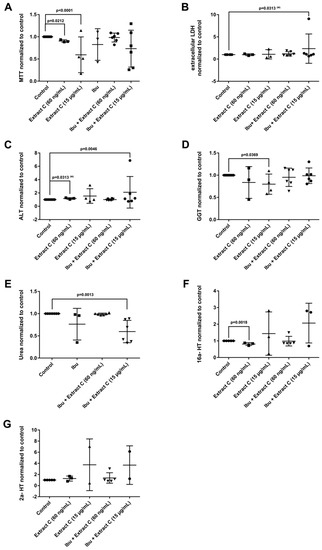
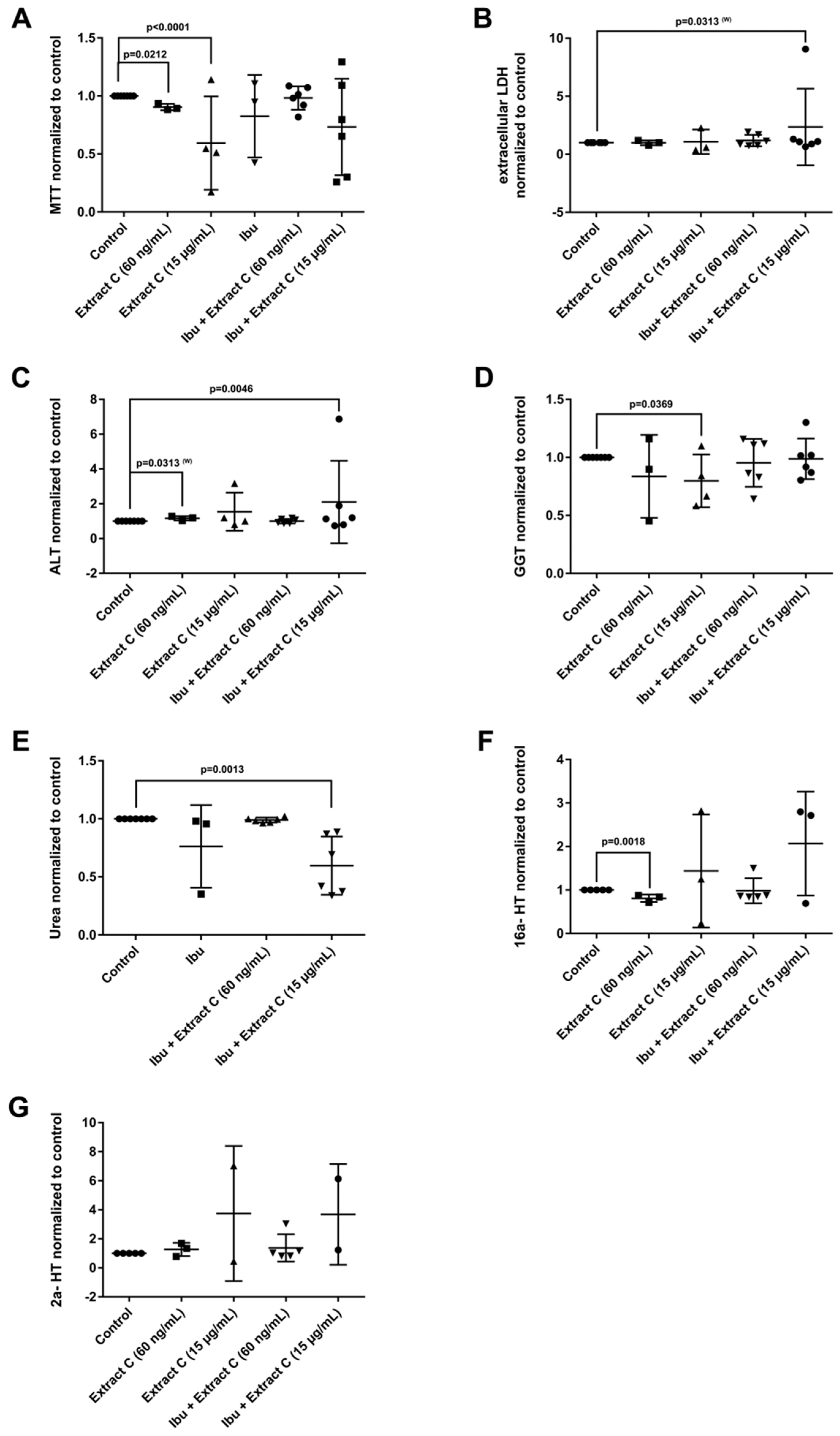
Figure 4.
Effects of different Petasites hybridus extracts and Ibuprofen on clinical liver function tests in cultures of human hepatocytes after daily treatment for 72 h. (A) MTT mitochondrial reductase activity. (B) LDH activity. (C) Alanine aminotransferase activity. (D) Glutamyltransferase activity. (E) Urea production. (F,G) Testosterone 16 and 2 hydroxylation activity. The data of individual donors are given normalised to the DMSO vehicle control. The Petasites hybridus extracts A, B and C are specified to 37%, <1% and 19.1% petasin, respectively. Extract C is the marketed product Petadolex® and human hepatocyte cultures were treated at therapeutic Cmax (= 60 ng/mL) and >49-fold therapeutic petasin Cmax (=15 µg/mL) concentrations. Hepatocyte cultures were also treated with Paracetamol at therapeutic Cmax (=Para; 20 µg/mL) concentrations and in combinations with different Petasites hybridus extracts. The data were analysed with the GraphPad Prism software version 6.05.
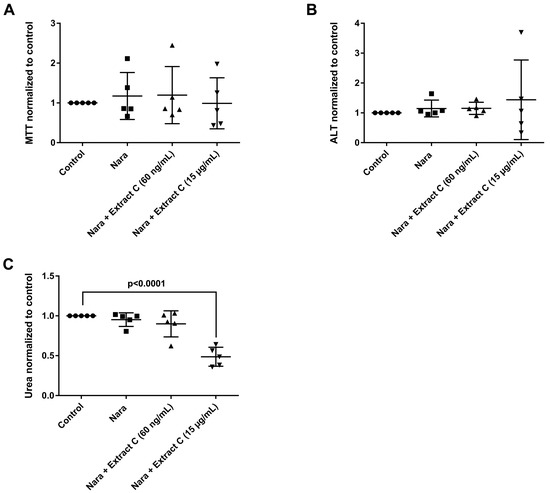
Figure 5.
Effects of different Petasites hybridus extracts and Naratriptan on clinical liver function tests in cultures of human hepatocytes after daily treatment for 72 h. (A) MTT mitochondrial reductase activity. (B) Alanine aminotransferase activity. (C) Urea production. The data of individual donors are given normalised to the DMSO vehicle control. The Petasites hybridus extracts A, B and C are specified to 37%, <1% and 19.1% petasin, respectively. Extract C is the marketed product Petadolex® and human hepatocyte cultures were treated at therapeutic Cmax (=60 ng/mL) and >49-fold therapeutic petasin Cmax (=15 µg/mL) concentrations. Hepatocyte cultures were also treated with Paracetamol at therapeutic Cmax (=Para; 20 µg/mL) concentrations and in combinations with different Petasites hybridus extracts. The data were analysed with the GraphPad Prism software version 6.05.
The combined Petadolex® and Paracetamol treatment did not cause hepatotoxicity at therapeutic Cmax concentrations. Similarly, treatment of PHH at therapeutic Cmax Petadolex® concentrations did not influence CYP monooxygenase activity using testosterone as a substrate (Figure 3F,G). However, at >49-fold therapeutic Cmax petasin (=active ingredient) concentrations, marked reduction in MTT activity among four donors was noted to indicate burdened mitochondrial metabolism. One donor also presented three-fold increases in ALT. Equally, the combined high dose Petadolex® and Paracetamol treatment caused marked reductions in MTT activity and urea detoxification (Figure 3A,E) as well as ≤9- and 7-fold increases in ALT. Furthermore, LDH was increased up to 14- and 7-fold, respectively, among individual donors exposed to extract A and C (Figure 3B,C). Except for one donor (MTT-assay), extract B did not elicit significant changes in LFTs. Note GGT-activities were too variable to permit meaningful interpretation of the data. Depicted in Figure 3F,G are up to 3- and 7-fold increases in 2α- and 16α-testosterone hydroxylation after treatment of PHH at >49-fold therapeutic Cmax petasin concentrations. The increased production of these metabolites indicates induced CYP2C8, CYP2C9, CYP2C19 and CYP3A4 monooxygenase activities. A similar 4- and 6-fold increase in CYP-monooxygenase activities was observed at high dose Petadolex® (15 µg/mL), but therapeutic Paracetamol concentrations. Therapeutic Cmax Paracetamol concentrations alone also elicited induction of 2α-testosterone hydroxylation.
The combined Petadolex® and Ibuprofen treatment of PHH was well tolerated at therapeutic Cmax concentrations. The results for cell viability (MTT and LDH assay), ALT, GGT, urea and CYP monooxygenase activities are given in Figure 4.
At >49-fold Cmax petasin concentrations, marked reductions in MTT activities were observed. With one donor, the combined treatment with Ibuprofen caused 8- and 7-fold increases in LDH and ALT activities (Figure 4B,C). Individual donors responded with an increased activity in 2α and 16α- testosterone hydroxylation; however, urea detoxification was significantly reduced at 15 µg/mL Petadolex® and therapeutic Ibuprofen concentrations.
Likewise, treatment with Petadolex® and Naratriptan did not impair mitochondrial MTT activity at therapeutic Cmax concentrations. However, MTT activities were markedly reduced with two donors at >49-fold Cmax petasin and Naratriptan therapeutic concentrations. One donor presented marked reduction in mitochondrial MTT activity and a small increase in ALT as well as reduced urea measurements at therapeutic Naratriptan concentrations (Figure 5A) to indicate hepatotoxicity (Figure 5). At >49-fold Cmax petasin and therapeutic Naratriptan concentrations, MTT activity and urea production was reduced (Figure 5A,C); here, one donor presented a nearly four-fold increase in ALT activity (Figure 5B).
4. Discussion
Petadolex® has been manufactured for over four decades and was granted market authorization in 1978. It was introduced into the US market in 1998. Unlike many HDS, it was extensively evaluated for safety and efficacy. In clinical studies, the spasmolytic properties and effectiveness in the prevention of migraine attacks was proven [5,6,21]. Therefore, butterbur extracts were recommended for migraine prevention [7]; however, reports of severe HILI cases (8 in Germany, 1 in UK and 1 in Austria) prompted concern regarding its safe use.
4.1. PAs and the Sinusoidal Obstruction Syndrome
Pyrrolizidine alkaloids (PAs) are ingredients commonly found in foods, teas, and medicinal herbs [22,23]. Given the >400 PA species in addition to N-oxides and other derivatives, a comprehensive analysis of PAs is difficult [24]. Butterbur also contains PAs; however, these can be removed to trace amounts during the manufacturing process.
What is the link between PAs and the sinusoidal obstruction syndrome? PAs are metabolically activated by hepatic CYP monooxygenase and sinusoidal endothelium to produce reactive electrophiles capable of forming adducts with cellular macromolecules and DNA [23,25]. Particularly, exposure to higher concentrations of metabolically activated PAs causes SOS and impaired blood flow. SOS is characterized by endothelial cell damage, proliferation and activation of stellate cells, enhanced collagen deposition around central veins, as well as infiltration of activated macrophages within the lumen and the wall of central veins [26]. Endothelial cell injury is considered to be an initiating step in the manifestation of SOS [27]. Clinically, the disease is characterized by hyperbilirubinemia, hepatomegaly, and fluid retention [28].
Several reports on the outcome of human exposure to pyrrolizidine alkaloids describe the risk of SOS. Even childhood liver cirrhosis was reported as a consequence of exposure to pyrrolizidine alkaloids [29]. Notwithstanding SOS might be reversible as reported for an infant exposed to a PA-mixture for 15 months [30]. Case reports associated with PA toxicity include Senecio tea, Gynura root and comfrey [31,32,33].
Remarkably, none of the suspected Petadolex® HILI cases were diagnosed with SOS, but presented an inconsistent injury pattern to possibly suggest polypharmacy as a culprit. Similarly, chronic rat toxicity studies did not evidence SOS; however, bile duct proliferation and its dilatation in the absence of significant liver transaminase elevations after dosing for six months [12]. Unlikely clinical cases with a female predilection the preclinical studies with rats suggest males to be more sensitive to excessive dosages. Moreover, the grading of lesions indicated some reversibility of bile duct proliferation after treatment cessation, while explorative studies with minipigs failed to demonstrate morphological changes of the liver at 218-fold MCD. The mechanism of bile duct proliferation following biliary obstruction was the subject of an earlier report [34]. Apparently, increased biliary pressure is an initiating factor. Although hepatocytes and cholangiocytes originate from the same stem cell, the plasticity of hepatocytes and the regulations thereof differ substantially between humans and rodents [35,36].
4.2. In vitro Hepatotoxicity Testing Provide Mechanistic Clues for Bile Duct Proliferations Observed in Rat Toxicity Studies
At therapeutic Cmax, Petadolex® did not cause hepatocyte toxicity, either given alone or in combination with commonly used migraine medications. However, at supra-therapeutic concentrations, some donors presented changes in LFT and CYP monooxygenase activity after repeated treatment of PHH for 72 h. Given that extract B is low in petasin (<1%) and this extract did not elicit LFT changes, we speculate that excessive petasin concentrations can be harmful to hepatocytes.
We previously reported regulation of bile acid transporters and solute carriers after treatment of PHH with different butterbur extracts [12]. It is tempting to postulate a relationship between PHH cell culture findings and bile duct proliferations seen in the rat chronic toxicity study. Specifically, ABCB1 was significantly upregulated in PHH and a similar upregulation is observed after bile duct ligation of rats [37]. ABCB1 has broad substrate specificity; its expression is largely dependent on the activity of the Farnesoid X (FXR) bile acid receptor [38]. Conversely, ABCB4 (Mdr2/MDR3) was repressed. This canalicular membrane transporter plays an essential role in the biliary excretion of phospholipids. Furthermore, the sodium/taurocholate co-transporting polypeptide (SLC10A1) was repressed at >49-fold therapeutic Cmax petasin concentrations and functions in bile acid uptake to protect hepatocytes against toxic intracellular bile acid accumulation. Altogether, 18 solute carriers were regulated in PHH and the observed bile duct proliferations seen at 45 and 90-fold MCD might be regarded as adaptive responses to impaired bile transport.
4.3. Pharmacovigilance and the Complexity of Drug Use in Migraine Therapy
Migraine is a complex disease and during attacks patients frequently rely on pain relief medications (NSAIDs, Paracetamol). Equally, the management of migraine pain is multifaceted and next to pain relief medications is a wide range of drugs for prevention therapies, including ß-blockers, Topiramate, Ibuprofen, Amitriptyline and others as summarized by the AHS/AAN [39]. Consequently, it is of no surprise that about half of the suspected Petadolex® HILI cases reported co-medication with drugs known for their liver liabilities. Such co-medication may aggravate risk for HILI [16]. Specifically, an analysis of 54,583 reports of the French Pharmacovigilance database suggested the risk for severe hepatic injury and acute renal failure to be six- to sevenfold higher when two NSAIDs or more were taken [16]. Co-medications in suspected Petadolex® cases involved antibiotics, neuroleptics, antiviral drugs and hormones. Their known liver liabilities may contribute to risk for liver injury [40,41]. In view of the complex pharmacotherapies in some migraine patients, suspected HILI cases may well be the result of co-medication with NSAIDs at least in three out of eight cases, as suggested by the pathologist. Typically, the incidence of hepatobiliary ADRs for NSAIDs is in the range of 2 per 10,000 [31,42]. One study reported an incidence for prescribed drugs of 19.1 cases per 100,000 inhabitants [43]. A Korean study estimated an incidence of HILI at 0.6%; all cases were of the hepatocellular injury type and confined to females [44]. A similar incidence was reported for the Japanese herbal medicine Kampo [45]. To the best of our knowledge no HILI case in association with Petadolex® intake has been reported in the US until today.
4.4. Regulatory Issues
The risk for HILI and its possible mechanisms was the subject of an independent review [46]. Likewise, the need for developing better scientific methods to link ingredients of HDS to risk for HILI has been emphasized [47]. Note, in 1988, the manufacturer of Petadolex® changed the extraction solvent from methylene chloride to CO2 to improve the removal of pyrrolizidine alkaloids. In 2002, the German regulatory authority BfArM evaluated this modification in the extraction protocol and concluded that this change would alter the composition of active ingredient. Correspondingly, the product is not the same as the original one that received market authorization and was therefore removed from the market. Likewise, in 2011, the UK Medicines and Healthcare products Regulatory Agency (MHRA) declined an application of the manufacturer for a new traditional license on safety concerns related to the distribution of Petadolex® (alias Migravoid) by retail stores as migraine preventive medication.
4.5. Study Limitations
The following caveats need to be considered. First, under-reporting of adverse drug reactions is a significant problem. Therefore, an estimation of the incidence of hepatobiliary ADRs remains vague. Second, the reported ADRs frequently do not provide comprehensive information on the patient’s medical history, laboratory parameters and a complete listing of concurrent medications. In fact, nearly half of the hepatobiliary ADRs could not be assessed adequately for causality and therefore were judged unclassifiable. A recent review article on the safety of a special butterbur extract also highlighted this problem [48]. Third, it is difficult to perform valid RUCAM assessments only from PSUR data for the reasons described above. Note the causality assessment reported in Table 3 differs from a recently published RUCAM evaluation of severe Petadolex® suspected HILI cases [11]. In the present study, five severe HILI cases were judged as possible, while the published RUCAM assessment considered these as unlikely. This highlights potential pitfalls of RUCAM assessments based on limited PSUR data and reinforces the importance of expert opinion as was summarized in the seminal review of Lewis [49].
In conclusion, herbal remedies offer alternative treatments for migraine therapy. The risk for HILI can be minimized by monitoring liver transaminases, especially in patients with polypharmacy and hypersensitivity to the active ingredient or members of the Asteraceae family or any of the excipients. Individuals with a history of pseudoallergic drug reactions, previous liver diseases, alcohol abuse, and familial non-haemolytic hyperbilirubinanaemia (Gilbert’s syndrome) should be carefully monitored, and the concomitant use of St. John’s wort is not recommended.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/5/652/s1, Figure S1: Serum biochemistry changes (ALT, AST, GGT) in regards to dose and time to onset of HILI, Table S1: Information on individual human donors, which donated liver tissue for the generation of human hepatocyte cultures, Table S2: Clinical biochemistry data and major histopathology findings after repeated oral dosing of rats with a Petasites hybridus extract for six months.
Author Contributions
Conceptualization, J.B.; Formal analysis, N.A. and J.B.; Funding acquisition, J.B.; Investigation, N.A.; Project administration, J.B.; Resources, J.B.; Supervision, J.B.; Validation, J.B.; Visualization, N.A. and J.B.; Writing—original draft, N.A. and J.B.; Writing—review & editing, J.B.
Funding
This work was in part funded by the Lower Saxony Ministry of Culture and Sciences and the Volkswagen Foundation, Germany to JB. Grant number: 25A.5-7251-99-3/00. The sponsor had no role in the design, execution, interpretation or writing of the study. We also acknowledge support by the German Research Foundation (DFG) and the Open Access Publication Fund of Hannover Medical School (MHH).
Acknowledgments
We would like to thank the members of the expert panel H.C. Diener (Department of Neurology, University Essen), S. Evers (Department of Neurology, University Hospital Münster) and R. Spanel (board certified pathologist, Institute of Pathology, Viersen) for the scientific discussion as well as A. Roskowetz, D. Schellbach, U. Sänger and I. Voepel for their excellent technical support.
Conflicts of Interest
Several mechanistic safety studies were performed at the Fraunhofer Institute of Toxicology and Experimental Medicine, Germany and were funded by the manufacturer of Petadolex, Weber & Weber GmbH & Co. KG, Germany in 2008–2010. J.B. served as a scientific advisor to Weber & Weber and received a consultancy fee. The sponsor had no role in the design, execution, interpretation or writing of the study.
References
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L.; Davern, T.; Fontana, R.J.; Grant, L.; Reddy, K.R.; Seeff, L.B.; Serrano, J.; Sherker, A.H.; et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R.; Langer, T.; Franz, C. An approach to the inheritance of the sesquiterpene chemotypes within Petasites hybridus. Planta Med. 2006, 72, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Rahlfs, V.W.; Danesch, U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur. Neurol. 2004, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Schmidramsl, H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Int. J. Clin. Pharmacol. Ther. 2000, 38, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Gobel, H.; Einhaupl, K.M.; Wilks, K.; Mauskop, A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology 2004, 63, 2240–2244. [Google Scholar] [CrossRef] [PubMed]
- Pothmann, R.; Danesch, U. Migraine prevention in children and adolescents: Results of an open study with a special butterbur root extract. Headache 2005, 45, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.; Silberstein, S.D.; Freitag, F.; Dodick, D.W.; Argoff, C.; Ashman, E.; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012, 78, 1346–1353. [Google Scholar]
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic activation of pyrrolizidine alkaloids: Insights into the structural and enzymatic basis. Chem. Res. Toxicol. 2014, 27, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Cohen, L.; Opris, M.; Nanau, R.M.; Hyunjin, J. Hepatotoxicity of Pyrrolizidine alkaloids. J. Pharm. Pharm. Sci. 2015, 18, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Q.; Crawford, J.M. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J. Clin. Exp. Hepatol. 2014, 4, 332–346. [Google Scholar] [CrossRef]
- Teschke, R.; Eickhoff, A.; Schulze, J.; Wolff, A.; Frenzel, C.; Melchart, D. Petadolex®, a herbal extract for migraine prophylaxis with spontaneous case reports of disputed liver injury: Robust causality evaluation by RUCAM, the Roussel Uclaf Causality Assessment Method. EJPMR 2016, 3, 154–177. [Google Scholar]
- Anderson, N.; Meier, T.; Borlak, J. Toxicogenomics applied to cultures of human hepatocytes enabled an identification of novel petasites hybridus extracts for the treatment of migraine with improved hepatobiliary safety. Toxicol. Sci. 2009, 112, 507–520. [Google Scholar] [CrossRef]
- Käufeler, R.; Thomet, O.A.R.; Simon, H.U.; Meier, B.; Brattström, A. Phytopharmaka VI. In Der Pestwurzextrakt Ze 339—Wirkprinzipien and klinische Pharmakologie; Rietbrock, N., Donath, E., Loew, D., Roots, I., Schulz, V., Eds.; Steinkopff Verlag: Heidelberg, Germany, 2001; pp. 237–246. [Google Scholar]
- Stewart, W.F.; Wood, C.; Reed, M.L.; Roy, J.; Lipton, R.B.; AMPP Advisory Group. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008, 28, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Saphner, T.; Triest-Robertson, S.; Li, H.; Holzman, P. The association of nonalcoholic steatohepatitis and tamoxifen in patients with breast cancer. Cancer 2009, 115, 3189–3195. [Google Scholar] [CrossRef]
- Clinard, F.; Sgro, C.; Bardou, M.; Hillon, P.; Dumas, M.; Kreft-Jais, C.; Escousse, A.; Bonithon-Kopp, C. Association between concomitant use of several systemic NSAIDs and an excess risk of adverse drug reaction. A case/non-case study from the French Pharmacovigilance system database. Eur. J. Clin. Pharmacol. 2004, 60, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Stieger, B.; Fattinger, K.; Madon, J.; Kullak-Ublick, G.A.; Meier, P.J. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology 2000, 118, 422–430. [Google Scholar] [CrossRef]
- Tepper, S.J.; Millson, D. Safety profile of the triptans. Expert Opin. Drug Saf. 2003, 2, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.J.; McKillop, D.; Butters, C.J. Determination of the human cytochrome P450 isoforms involved in the metabolism of zolmitriptan. Xenobiotica 1999, 29, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Antman, M.; Phillips, J.; Khan, K.N.; Furst, S.M. Effects of miglyol 812 on rats after 4 weeks of gavage as compared with methylcellulose/tween 80. Drug Chem. Toxicol. 2005, 28, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.; Schmidramsl, H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern. Med. Rev. 2001, 6, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar] [PubMed]
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.; Seawright, A.A. Pyrrolizidine alkaloids in human diet. Mutat. Res. 1999, 443, 53–67. [Google Scholar] [CrossRef]
- Kempf, M.; Reinhard, A.; Beuerle, T. Pyrrolizidine alkaloids (PAs) in honey and pollen-legal regulation of PA levels in food and animal feed required. Mol. Nutr. Food Res. 2010, 54, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.W.; Fu, P.P. Formation of DHP-derived DNA adducts in vivo from dietary supplements and Chinese herbal plant extracts containing carcinogenic pyrrolizidine alkaloids. Toxicol. Ind. Health 2006, 22, 321–327. [Google Scholar] [CrossRef]
- Tang, W.; Ziring, D.; Gershman, G.; French, S. Role of macrophages and stellate cells in the pathogenesis of veno-occlusive disease: An electron microscopic case study. Exp. Mol. Pathol. 2003, 75, 201–209. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Shulman, H.M.; McDonald, G.B. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin. Liver Dis. 2002, 22, 27–42. [Google Scholar] [CrossRef]
- Senzolo, M.; Germani, G.; Cholongitas, E.; Burra, P.; Burroughs, A.K. Veno occlusive disease: Update on clinical management. World J. Gastroenterol. 2007, 13, 3918–3924. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Sperl, W.; Stuppner, H.; Gassner, I.; Judmaier, W.; Dietze, O.; Vogel, W. Reversible hepatic veno-occlusive disease in an infant after consumption of pyrrolizidine-containing herbal tea. Eur. J. Pediatr. 1995, 154, 112–116. [Google Scholar] [CrossRef]
- Zuckerman, M.; Steenkamp, V.; Stewart, M.J. Hepatic veno-occlusive disease as a result of a traditional remedy: Confirmation of toxic pyrrolizidine alkaloids as the cause, using an in vitro technique. J. Clin. Pathol. 2002, 55, 676–679. [Google Scholar] [CrossRef]
- Dai, N.; Yu, Y.C.; Ren, T.H.; Wu, J.G.; Jiang, Y.; Shen, L.G.; Zhang, J. Gynura root induces hepatic veno-occlusive disease: A case report and review of the literature. World J. Gastroenterol. 2007, 13, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Rode, D. Comfrey toxicity revisited. Trends Pharmacol. Sci. 2002, 23, 497–499. [Google Scholar] [CrossRef]
- Slott, P.A.; Liu, M.H.; Tavoloni, N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology 1990, 99, 466–477. [Google Scholar] [CrossRef]
- Yovchev, M.I.; Grozdanov, P.N.; Zhou, H.; Racherla, H.; Guha, C.; Dabeva, M.D. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 2008, 47, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Kordes, C.; Häussinger, D. Hepatic stem cell niches. J. Clin. Invest. 2013, 123, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Arrese, M.; Soroka, C.J.; Ananthanarayanan, M.; Koeppel, T.A.; Schlosser, S.F.; Suchy, F.J.; Keppler, D.; Boyer, J.L. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology 1997, 113, 255–264. [Google Scholar] [CrossRef]
- Halilbasic, E.; Claudel, T.; Trauner, M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 2013, 58, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Loder, E.; Burch, R.; Rizzoli, P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: A summary and comparison with other recent clinical practice guidelines. Headache 2012, 52, 930–945. [Google Scholar] [CrossRef]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. The prevalence and etiology of elevated aminotransferase levels in the United States. Am. J. Gastroenterol. 2003, 98, 960–967. [Google Scholar] [CrossRef]
- Navarro, V.J.; Senior, J.R. Drug-related hepatotoxicity. N. Engl. J. Med. 2006, 354, 731–739. [Google Scholar] [CrossRef]
- Teoh, N.C.; Farrell, G.C. Hepatotoxicity associated with non-steroidal anti-inflammatory drugs. Clin. Liver Dis. 2003, 7, 401–413. [Google Scholar] [CrossRef]
- Bjornsson, E.S.; Bergmann, O.M.; Bjornsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef]
- Cho, J.H.; Oh, D.S.; Hong, S.H.; Ko, H.; Lee, N.H.; Park, S.E.; Han, C.W.; Kim, S.M.; Kim, Y.C.; Kim, K.S.; et al. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch. Toxicol. 2017, 91, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Mantani, N.; Kogure, T.; Sakai, S.; Goto, H.; Shibahara, N.; Kita, T.; Shimada, Y.; Terasawa, K. Incidence and clinical features of liver injury related to Kampo (Japanese herbal) medicine in 2496 cases between 1979 and 1999: Problems of the lymphocyte transformation test as a diagnostic method. Phytomedicine 2002, 9, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Patsenker, E.; Schuppan, D. Herbal hepatotoxicity. J. Hepatol. 2005, 43, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Avigan, M.I.; Mozersky, R.P.; Seeff, L.B. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int. J. Mol. Sci. 2016, 17, 331. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Freitag, F.G.; Danesch, U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Rep. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Lewis, J.H. Causality assessment: Which is best-expert opinion or RUCAM? Clin. Liver Dis. 2014, 4, 4–8. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).