Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study
Abstract
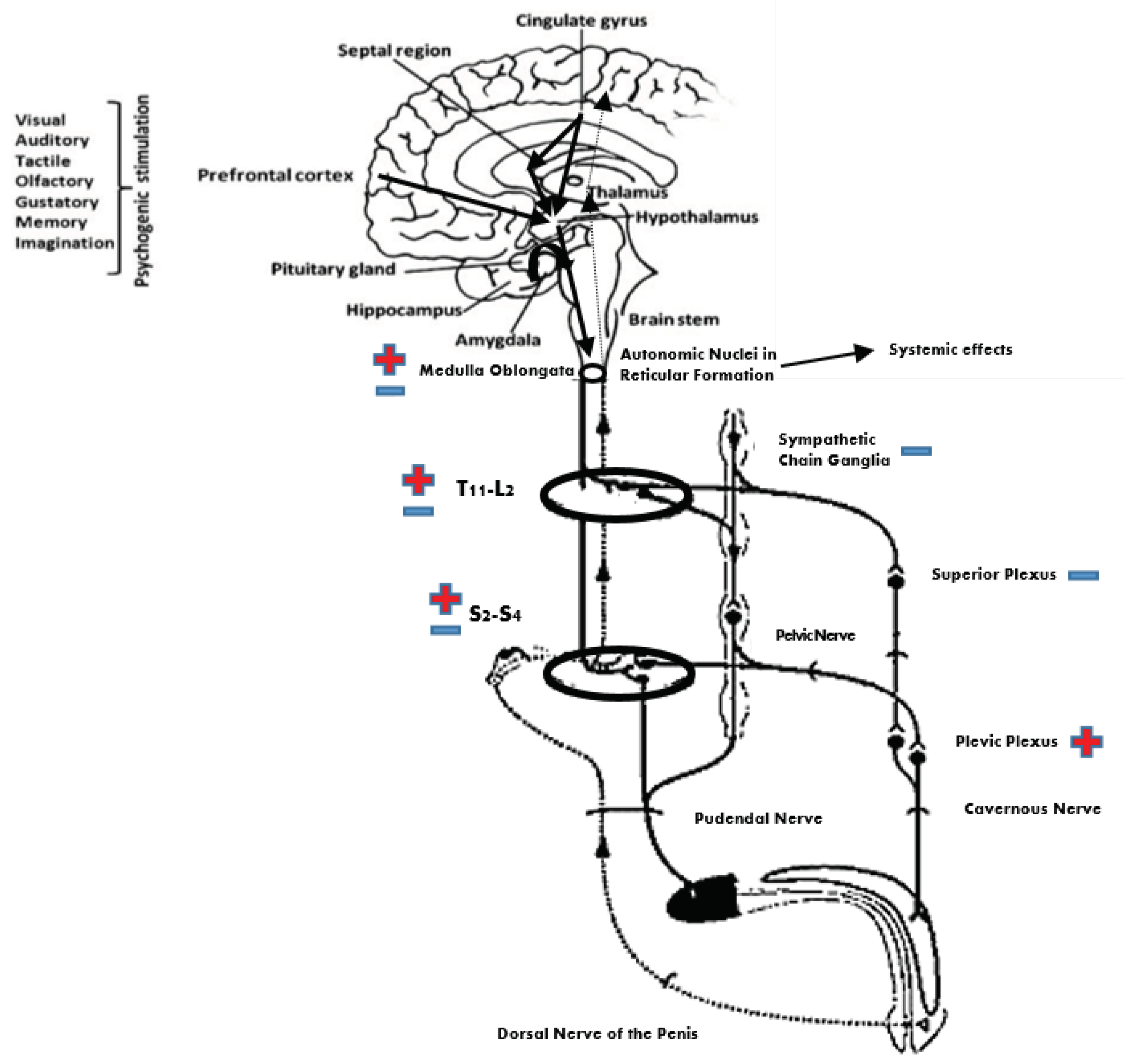
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Intervention
2.3. Outcome Measures
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
4. Discussion
5. Limitations and Conclusions
Author Contributions
Conflicts of Interest
References
- Mahoney, C.; Smith, A.; Marshall, A.; Reid, F. Pelvic floor dysfunction and sensory impairment: Current evidence. Neurourol. Urodyn. 2016, 36, 550–556. [Google Scholar] [CrossRef]
- Ramos, A.S.; Samsó, J.V. Specific aspects of erectile dysfunction in spinal cord injury. Int. J. Impot. Res. 2004, 16, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Everaert, K.; Waard, W.I.; Hoof, T.V.; Kiekens, C.; Mulliez, T.; Dherde, C. Neuroanatomy and neurophysiology related to sexual dysfunction in male neurogenic patients with lesions to the spinal cord or peripheral nerves. Spinal Cord 2010, 48, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabro, R.S.; Polimeni, G.; Bramanti, P. Current and Future Therapies of Erectile Dysfunction in Neurological Disorders. Recent Pat. CNS Drug Discov. 2011, 6, 48–64. [Google Scholar] [CrossRef]
- Aikman, K.; Oliffe, J.L.; Kelly, M.T.; Mccuaig, F. Sexual Health in Men with Traumatic Spinal Cord Injuries: A Review and Recommendations for Primary Health-Care Providers. Am. J. Mens Health 2018, 12, 2044–2054. [Google Scholar] [CrossRef]
- Previnaire, J.G.; Soler, J.M.; Alexander, M.S.; Courtois, F.; Elliott, S.; Mclain, A. Prediction of sexual function following spinal cord injury: A case series. Spinal Cord Ser. Cases 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Over, R. Psychophysiological assessment of male sexual arousal following spinal cord injury. Arch Sex Behav. 1990, 19, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Calabro, R.S.; Polimeni, G.; Ciurleo, R.; Casella, C.; Bramanti, P. Neurogenic Ejaculatory Disorders: Focus on Current and Future Treatments. Recent Pat. CNS Drug Discov. 2011, 6, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Daleo, G.; Sessa, E.; Leo, A.; Cola, M.C.; Bramanti, P. Sexual Dysfunction Induced by Intrathecal Baclofen Administration: Is This the Price to Pay for Severe Spasticity Management? J. Sex. Med. 2014, 11, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Derry, F.; Hultling, C.; Seftel, A.D.; Sipski, M.L. Efficacy and safety of sildenafil citrate (Viagra®) in men with erectile dysfunction and spinal cord injury: A review. Urology 2002, 60, 49–57. [Google Scholar] [CrossRef]
- Lieber, R.L.; Steinman, S.; Barash, I.A.; Chambers, H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve 2004, 29, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Slot, O.; Drewes, A.; Andreasen, A.; Olsson, A. Erectile and ejaculatory function of males with spinal cord injury. Int. Disabil. Stud. 1989, 11, 75–77. [Google Scholar] [CrossRef]
- Anderson, K.D.; Borisoff, J.F.; Johnson, R.D.; Stiens, S.A.; Elliott, S.L. Long-term effects of spinal cord injury on sexual function in men: implications for neuroplasticity. Spinal Cord 2007, 45, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Courtois, F.; Elliott, S.; Tepper, M. Improving Sexual Satisfaction in Persons with Spinal Cord Injuries: Collective Wisdom. Top Spinal Cord Inj. Rehabil. 2017, 23, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naro, A.; Leo, A.; Russo, M.; Casella, C.; Buda, A.; Crespantini, A.; Calabrò, R.S. Breakthroughs in the spasticity management: Are non-pharmacological treatments the future? J. Clin. Neurosci. 2017, 39, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Poenaru, D.; Cinteza, D.; Petrusca, I.; Cioc, L.; Dumitrascu, D. Local Application of Vibration in Motor Rehabilitation - Scientific and Practical Considerations. Maedica (Buchar) 2016, 11, 227–231. [Google Scholar] [PubMed]
- Chang, E.; Ghosh, N.; Yanni, D.; Lee, S.; Alexandru, D.; Mozaffar, T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit. Rev. Physic. Rehabil. Med. 2013, 25, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936. [Google Scholar] [CrossRef]
- Lauper, M.; Kuhn, A.; Gerber, R.; Luginbühl, H.; Radlinger, L. Pelvic Floor Stimulation: What are the good vibrations? Neurourol. Urodyn. 2008, 28, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Farzinmehr, A.; Moezy, A.; Koohpayehzadeh, J.; Kashanian, M. A Comparative Study of Whole Body Vibration Training and Pelvic Floor Muscle Training on Women’s Stress Urinary Incontinence: Three-Month Follow-Up. J. Family Reprod. Health 2015, 9, 147–154. [Google Scholar]
- Crevenna, R.; Cenik, F.; Margreiter, M.; Marhold, M.; Komanadj, T.S.; Keilani, M. Whole body vibration therapy on a treatment bed as additional means to treat postprostatectomy urinary incontinence. Wien. Med. Wochenschr. 2016, 167, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Brackett, N. Penile vibratory stimulation for men with spinal cord injury. Video. Hum. Reprod. Update 1999, 5, 551–552. [Google Scholar] [CrossRef] [Green Version]
- Guess, M.K.; Connell, K.A.; Chudnoff, S.; Adekoya, O.; Richmond, C.; Nixon, K.E.; Melman, A. The Effects of a Genital Vibratory Stimulation Device on Sexual Function and Genital Sensation. Female Pelvic Med. Reconstr. Surg. 2017, 23, 256–262. [Google Scholar] [CrossRef]
- Laessøe, L.; Nielsen, J.B.; Biering-Sørensen, F.; Sønksen, J. Antispastic effect of penile vibration in men with spinal cord lesion. Arch. Phys. Med. Rehabil. 2004, 85, 919–924. [Google Scholar]
- Tajkarimi, K.; Burnett, A. Viberect® device use by men with erectile dysfunction: Safety, ease of use, tolerability, and satisfaction survey. J. Sex. Med. 2011, 8, 441–444. [Google Scholar]
- Tantawy, S.A.; Elgohary, H.M.; Abdelbasset, W.K.; Kamel, D.M. Effect of 4 weeks of whole-body vibration training in treating stress urinary incontinence after prostate cancer surgery: A randomised controlled trial. Physiotherapy 2018. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Paiva, L.L.; Ramos, J.G.; Ferla, L. Vibratory perineal stimulation for the treatment of female stress urinary incontinence: A systematic review. Int. Urogynecol. J. 2018, 29, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Rosen, R.C.; Cappelleri, J.C.; Gendrano, N., 3rd. The International Index of Erectile Function (IIEF): A state-of-the-science review. Int. J. Impot. Res. 2002, 14, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Cappelleri, J.C.; Rosen, R.C.; Smith, M.D.; Mishra, A.; Osterloh, I.H. Diagnostic evaluation of the erectile function domain of the international index of erectile function. Urology 1999, 54, 346–351. [Google Scholar] [CrossRef]
- Previnaire, J.G. The importance of the bulbocavernosus reflex. Spinal Cord Ser Cases 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Podnar, S. Neurophysiologic studies of the penilo-cavernosus reflex: normative data. Neurourol. Urodyn. 2007, 26, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.; Jost, W.H.; Osterhage, J.; Derouet, H.; Schimrigk, K. Penile and perianal pudendal nerve somatosensory evoked potentials in the diagnosis of erectile dysfunction. Int. J. Impot. Res. 2001, 13, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorey, G.; Speakman, M.; Feneley, R.; Swinkels, A.; Dunn, C.; Ewings, P. Randomised controlled trial of pelvic floor muscle exercises and manometric biofeedback for erectile dysfunction. Br. J. Gen. Pract. 2004, 54, 819–825. [Google Scholar] [PubMed]
- Cohen, D.; Gonzalez, J.; Goldstein, I. The Role of Pelvic Floor Muscles in Male Sexual Dysfunction and Pelvic Pain. Sex. Med. Rev. 2016, 4, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lavoisier, P.; Roy, P.; Dantony, E.; Watrelot, A.; Ruggeri, J.; Dumoulin, S. Pelvic-floor muscle rehabilitation in erectile dysfunction and premature ejaculation. Physical Therapy 2014, 94, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Petersen, N.; Crone, C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain 1995, 118, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Mileva, K.N.; Bowtell, J.L.; Kossev, A.R. Effects of low-frequency whole-body vibration on motor-evoked potentials in healthy men. Exper. Physiol. 2008, 94, 103–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocchi, L.; Suppa, A.; Leodori, G.; Celletti, C.; Camerota, F.; Rothwell, J.; Berardelli, A. Plasticity Induced in the Human Spinal Cord by Focal Muscle Vibration. Front. Neurol. 2019, 9, 1170. [Google Scholar] [CrossRef]
- Morita, H.; Crone, C.; Christenhuis, D.; Petersen, N.T.; Nielsen, J.B. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 2001, 124, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of spasticity: implications for neurorehabilitation. BioMed Res. Internat. 2014, 2014, 354906. [Google Scholar] [CrossRef]
- Saggini, R.; Bellomo, R.G.; Cosenza, L. Vibration in Neurorehabilitation: a narrative review. Med. Res. Arch. 2017, 5, 11. [Google Scholar]
- Rosenkranz, K.; Rothwell, J.C. Differential effect of muscle vibration on intra-cortical inhibitory circuits in humans. J. Physiol. 2003, 551, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Sawatzky, B. Effects of vibration on spasticity in individuals with spinal cord injury: A scoping systematic review. Am. J. Physic. Med. Rehabil. 2014, 93, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Kossev, A.; Siggelkow, S.; Schubert, M.; Wohlfarth, K.; Dengler, R. Muscle vibration: Different effects on transcranial magnetic and electrical stimulation. Muscle Nerve 1999, 22, 946–948. [Google Scholar] [CrossRef]
- Valls-Sole, J.; Alvarez, R.; Tolosa, E.S. Vibration-induced presynaptic inhibition of the soleus H reflex is temporarily reduced by cortical magnetic stimulation in human subjects. Neurosci. Lett. 1994, 170, 149–152. [Google Scholar] [CrossRef]
- Siggelkow, S.; Kossev, A.; Schubert, M.; Kappels, H.H.; Wolf, W.; Dengler, R. Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle Nerve 1999, 22, 1544–1558. [Google Scholar] [CrossRef]
- Saval, A.; Chiodo, A.E. Sexual dysfunction associated with intrathecal baclofen use: A report of two cases. J. Spin. Cord Med. 2008, 31, 103–105. [Google Scholar] [CrossRef]
- Gitlin, M. Sexual dysfunction with psychotropic drugs. Expert Opin. Pharmacother. 2003, 4, 2259–2269. [Google Scholar] [CrossRef]



| Age (y) | dd (m) | SCI Level | ASIA Level | BCR | eBCR | Cremasteric Reflex | Sensory Preservation | PSEP | MAS | VAS | IIEF | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| amp (μV) | lat (ms) | lat (ms) | amp (μV) | Total | A | B | |||||||||
| 65 | 8 | T12–L1 | D | no | 5 | 34 | present/asymmetric | yes | 48 | 3 | 2 | 2 | 16 | 2 | 1 |
| 38 | 10 | L1–L2 | D | weak | 6 | 35 | present/asymmetric | no | / | 0 | 1 | 0 | 18 | 6 | 2 |
| 62 | 12 | T9–D12 | C | no | 6 | 34 | absent | no | 44 | 1 | 1 | 2 | 17 * | 5 | 2 |
| 26 | 14 | T10–L1 | C | yes | 11 | 34 | present/asymmetric | yes | 41 | 3 | 2 | 2 | 18 | 8 | 3 |
| 45 | 6 | L1–L2 | D | no | 6 | 36 | present/asymmetric | yes | 43 | 4 | 2 | 3 | 13 * | 9 | 0 |
| 48 | 10 | L1–L3 | C | no | 8 | 33 | absent | no | 39 | 2 | 1 | 0 | 16 * | 9 | 1 |
| 45 | 4 | L1–L3 | C | weak | 11 | 36 | present/asymmetric | no | 38 | 1 | 1 | 2 | 14 | 12 | 2 |
| 22 | 13 | T12–L2 | C | no | 4 | 34 | absent | no | / | 0 | 1 | 1 | 14 | 5 | 3 |
| 39 | 3 | T12–L1 | D | no | 5 | 34 | present/asymmetric | yes | 36 | 3 | 3 | 3 | 11 | 6 | 3 |
| 48 | 12 | T8–D10 | C | weak | 8 | 34 | absent | no | 42 | 1 | 1 | 0 | 18 * | 7 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, R.S.; Naro, A.; Pullia, M.; Porcari, B.; Torrisi, M.; La Rosa, G.; Manuli, A.; Billeri, L.; Bramanti, P.; Quattrini, F. Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study. J. Clin. Med. 2019, 8, 658. https://doi.org/10.3390/jcm8050658
Calabrò RS, Naro A, Pullia M, Porcari B, Torrisi M, La Rosa G, Manuli A, Billeri L, Bramanti P, Quattrini F. Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study. Journal of Clinical Medicine. 2019; 8(5):658. https://doi.org/10.3390/jcm8050658
Chicago/Turabian StyleCalabrò, Rocco Salvatore, Antonino Naro, Massimo Pullia, Bruno Porcari, Michele Torrisi, Gianluca La Rosa, Alfredo Manuli, Luana Billeri, Placido Bramanti, and Fabrizio Quattrini. 2019. "Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study" Journal of Clinical Medicine 8, no. 5: 658. https://doi.org/10.3390/jcm8050658
APA StyleCalabrò, R. S., Naro, A., Pullia, M., Porcari, B., Torrisi, M., La Rosa, G., Manuli, A., Billeri, L., Bramanti, P., & Quattrini, F. (2019). Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study. Journal of Clinical Medicine, 8(5), 658. https://doi.org/10.3390/jcm8050658






