Effect of Ketorolac on the Prevention of Postoperative Catheter-Related Bladder Discomfort in Patients Undergoing Robot-Assisted Laparoscopic Radical Prostatectomy: A Randomized, Double-Blinded, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
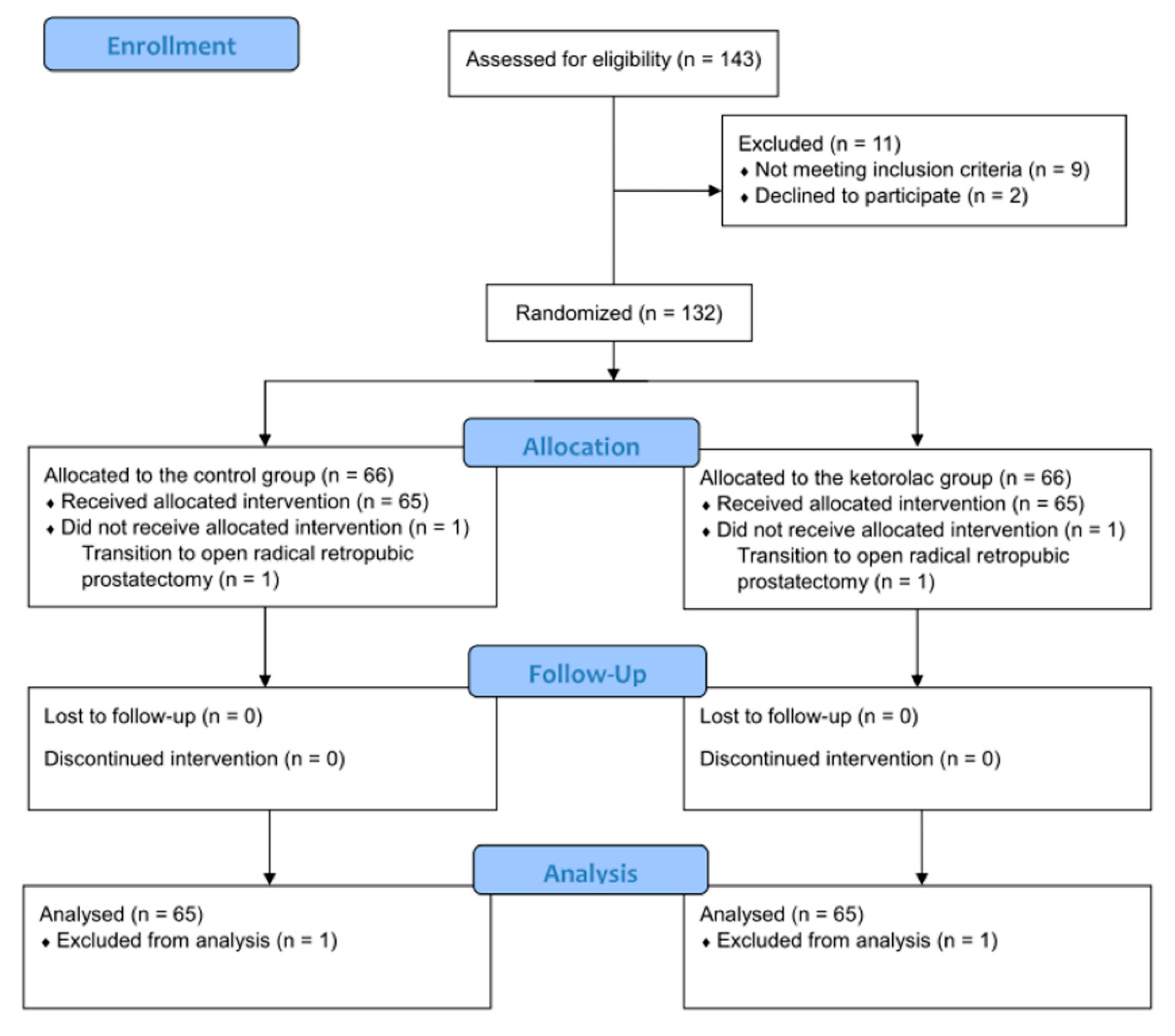
2.2. Randomization, Concealment, and Blinding
2.3. Anesthetic and Surgical Techniques
2.4. Assessments
2.5. Primary and Secondary Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| CRBD | catheter-related bladder discomfort |
| PACU | post-anesthesia care unit |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| RALP | robot-assisted laparoscopic radical prostatectomy |
| KDIGO | Kidney Disease: IMPROVING Global Outcomes |
| SD | standard deviation |
| IQR | interquartile range |
| BMI | body mass index |
| ASA PS | American Society of Anesthesiologists physical status |
| DM | diabetes mellitus |
| T category | tumor category |
References
- Hu, B.; Li, C.; Pan, M.; Zhong, M.; Cao, Y.; Zhang, N.; Yuan, H.; Duan, H. Strategies for the prevention of catheter-related bladder discomfort: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4859. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Hwang, J.; Lee, J.; Seo, J.; Park, H.; Oh, A.; Jeon, Y.; Do, S. Efficacy of butylscopolamine for the treatment of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Br. J. Anaesth. 2013, 111, 932–937. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Li, X.; Pu, C.; Yuan, H.; Tang, Y.; Li, J.; Wei, Q.; Han, P. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J. Endourol. 2015, 29, 640–649. [Google Scholar] [CrossRef]
- Agarwal, A.; Dhiraaj, S.; Singhal, V.; Kapoor, R.; Tandon, M. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Br. J. Anaesth. 2006, 96, 377–380. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder: Synopsis of the kidney disease: Improving global outcomes 2017 clinical practice guideline update. Ann. Intern. Med. 2018, 168, 422–430. [Google Scholar] [CrossRef]
- Kim, H.-C.; Lee, Y.-H.; Jeon, Y.-T.; Hwang, J.-W.; Lim, Y.-J.; Park, J.-E.; Park, H.-P. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumour resection: A double-blind randomised study. Eur. J. Anaesthesiol. 2015, 32, 596–601. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, J.; Bechu, S.M.; Yin, X.; Wan, Q.; Luo, L.; Zhu, Y.; Luo, J. Efficacy of pudendal nerve block for alleviation of catheter-related bladder discomfort in male patients undergoing lower urinary tract surgeries: A randomized, controlled, double-blind trial. Medicine 2017, 96, 8932. [Google Scholar]
- Li, C.; Liu, Z.; Yang, F. Predictors of catheter-related bladder discomfort after urological surgery. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 559–562. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012, 116, 248–273. [Google Scholar] [CrossRef]
- Polanco-Garcia, M.; Garcia-Lopez, J.; Fabregas, N.; Meissner, W.; Puig, M.M. Postoperative Pain Management in Spanish Hospitals: A Cohort Study Using the PAIN-OUT Registry. J. Pain Off. J. Am. Pain Soc. 2017, 18, 1237–1252. [Google Scholar] [CrossRef] [Green Version]
- Tijani, K.; Akanmu, N.; Olatosi, J.; Ojewola, R. Role of tolterodine in the management of postoperative catheter-related bladder discomfort: Findings in a Nigerian teaching hospital. Niger. J. Clin. Pract. 2017, 20, 484–488. [Google Scholar] [CrossRef]
- Tauzin-Fin, P.; Sesay, M.; Svartz, L.; Krol-Houdek, M.-C.; Maurette, P. Sublingual oxybutynin reduces postoperative pain related to indwelling bladder catheter after radical retropubic prostatectomy. Br. J. Anaesth. 2007, 99, 572–575. [Google Scholar] [CrossRef]
- Nam, K.; Seo, J.-H.; Ryu, J.-H.; Oh, A.-Y.; Lee, T.; Park, H.-P.; Jeon, Y.-T.; Hwang, J.-W. Randomized, clinical trial on the preventive effects of butylscopolamine on early postoperative catheter-related bladder discomfort. Surgery 2015, 157, 396–401. [Google Scholar] [CrossRef]
- Shariat Moharari, R.; Lajevardi, M.; Khajavi, M.; Najafi, A.; Shariat Moharari, G.; Etezadi, F. Effects of Intra-Operative Ketamine Administration on Postoperative Catheter-Related Bladder Discomfort: A Double-Blind Clinical Trial. Pain Pract. 2014, 14, 146–150. [Google Scholar] [CrossRef]
- Kim, H.-C.; Lim, S.-M.; Seo, H.; Park, H.-P. Effect of glycopyrrolate versus atropine coadministered with neostigmine for reversal of rocuronium on postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of bladder tumor: A prospective randomized study. J. Anesth. 2015, 29, 831–835. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Forrest, J.B.; Camu, F.; Greer, I.A.; Kehlet, H.; Abdalla, M.; Bonnet, F.; Ebrahim, S.; Escolar, G.; Jage, J.; Pocock, S.; et al. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. Br. J. Anaesth. 2002, 88, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Vadivelu, N.; Gowda, A.M.; Urman, R.D.; Jolly, S.; Kodumudi, V.; Maria, M.; Taylor, R.; Pergolizzi, J.V. Ketorolac Tromethamine—Routes and Clinical Implications. Pain Pract. 2015, 15, 175–193. [Google Scholar] [CrossRef]
- Wick, E.C.; Grant, M.C.; Wu, C.L. Postoperative Multimodal Analgesia Pain Management with Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017, 152, 691–697. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Agarwal, A.; Yadav, G.; Gupta, D.; Singh, P.; Singh, U. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: A prospective, randomized, double-blind study. Br. J. Anaesth. 2008, 101, 506–510. [Google Scholar] [CrossRef]
- King, C.R. Patterns of prostate cancer biopsy grading: Trends and clinical implications. Int. J. Cancer 2000, 90, 305–311. [Google Scholar] [CrossRef]
- Partin, A.W.; Kattan, M.W.; Subong, E.N.; Walsh, P.C.; Wojno, K.J.; Oesterling, J.E.; Scardino, P.T.; Pearson, J. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer: A multi-institutional update. JAMA 1997, 277, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Allsbrook, W.C., Jr.; Amin, M.B.; Egevad, L.L.; Committee, I.G. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. JCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Power, I.; Noble, D.W.; Douglas, E.; Spence, A.A. Comparison of i.m. ketorolac trometamol and morphine sulphate for pain relief after cholecystectomy. Br. J. Anaesth. 1990, 65, 448–455. [Google Scholar] [CrossRef]
- McPherson, M.L. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing; ASHP: Bethesda, MD, USA, 2009. [Google Scholar]
- Bhardwaj, P.; Yadav, R.K. Measuring pain in clinical trials: Pain scales, endpoints, and challenges. Int. J. Clin. Exp. Physiol. 2015, 2, 151. [Google Scholar] [CrossRef]
- Langman, M.J.; Jensen, D.M.; Watson, D.J.; Harper, S.E.; Zhao, P.-L.; Quan, H.; Bolognese, J.A.; Simon, T.J. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 1999, 282, 1929–1933. [Google Scholar] [CrossRef]
- Siddiqui, N.; Arzola, C.; Teresi, J.; Fox, G.; Guerina, L.; Friedman, Z. Predictors of desaturation in the postoperative anesthesia care unit: An observational study. J. Clin. Anesth. 2013, 25, 612–617. [Google Scholar] [CrossRef]
- Lepouse, C.; Lautner, C.; Liu, L.; Gomis, P.; Leon, A. Emergence delirium in adults in the post-anaesthesia care unit. Br. J. Anaesth. 2006, 96, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.C.; Park, H.P.; Lee, J.; Jeong, M.H.; Lee, K.H. Sevoflurane vs. propofol in post-operative catheter-related bladder discomfort: A prospective randomized study. Acta Anaesthesiol. Scand. 2017, 61, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Hong, W.-P.; Lim, Y.-J.; Park, H.-P. The effect of sevoflurane versus desflurane on postoperative catheter-related bladder discomfort in patients undergoing transurethral excision of a bladder tumour: A randomized controlled trial. Can. J. Anaesth. 2016, 63, 596–602. [Google Scholar] [CrossRef]
- Agarwal, A.; Dhiraaj, S.; Pawar, S.; Kapoor, R.; Gupta, D.; Singh, P.K. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Anesth. Analg. 2007, 105, 1454–1457. [Google Scholar] [CrossRef]
- Green, G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–60. [Google Scholar] [CrossRef]
- Mikhailidis, D.; Jeremy, J.; Dandona, P. Urinary bladder prostanoids—Their synthesis, function and possible role in the pathogenesis and treatment of disease. J. Urol. 1987, 137, 577–582. [Google Scholar] [CrossRef]
- De Groat, W. Anatomy and physiology of the lower urinary tract. Urol. Clin. N. Am. 1993, 20, 383. [Google Scholar]
- Maggi, C.; Santicioli, F.; Meli, A. Evidence for the involvement of a capsaicin-sensitive innervation in the afferent branch of micturition reflex in rats. Acta Physiol. Hung. 1987, 69, 425–435. [Google Scholar] [PubMed]
- Maggi, C.A.; Giuliani, S.; Conte, B.; Furio, M.; Santicioli, P.; Meli, P.; Gragnani, L.; Meli, A. Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur. J. Pharmacol. 1988, 145, 105–112. [Google Scholar] [CrossRef]
- Ergenoglu, P.; Akin, S.; Cok, O.Y.; Eker, E.; Kuzgunbay, B.; Turunc, T.; Aribogan, A. Effect of intraoperative paracetamol on catheter-related bladder discomfort: A prospective, randomized, double-blind study. Curr. Ther. Res. Clin. Exp. 2012, 73, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M. Acetaminophen and the US Acute Liver Failure Study Group: Lowering the risks of hepatic failure. Hepatology 2004, 40, 6–9. [Google Scholar] [CrossRef]
- Jozwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar]
- Claridge, L.C.; Eksteen, B.; Smith, A.; Shah, T.; Holt, A.P. Acute liver failure after administration of paracetamol at the maximum recommended daily dose in adults. BMJ 2010, 341, c6764. [Google Scholar] [CrossRef]
- Bray, G.P. Liver failure induced by paracetamol. BMJ 1993, 306, 157. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Moodie, H.; Arroyo, V.; Williams, R. Frequency of renal impairment in paracetamol overdose compared with other causes of acute liver damage. J. Clin. Pathol. 1977, 30, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Jaquenod, M.; Rönnhedh, C.; Cousins, M.J.; Eckstein, R.P.; Jordan, V.; Mather, L.E.; Power, I. Factors influencing ketorolac-associated perioperative renal dysfunction. Anesth. Analg. 1998, 86, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int. J. Clin. Pract. Suppl. 2013, 37–42. [Google Scholar] [CrossRef] [PubMed]


| Variables | Control Group (n = 65) | Ketorolac Group (n = 65) | p-Value |
|---|---|---|---|
| Age (years) | 66.7 ± 6.5 | 65.4 ± 7.0 | 0.282 |
| Sex (male/female) | 65/0 (100/0) | 65/0 (100/0) | 1.000 |
| BMI (kg/m2) | 24.5 (23.0–26.6) | 24.2 (22.4–25.8) | 0.329 |
| ASA PS | 0.440 | ||
| 1/2 | 2/63 (3.1/96.9) | 5/60 (7.7/92.3) | |
| DM | 9 (13.8) | 9 (13.8) | 1.000 |
| Hypertension | 27 (41.5) | 30 (46.2) | 0.596 |
| Gleason score (points) | 0.940 | ||
| ≤6 | 15 (23.1) | 16 (24.6) | |
| 7 | 33 (50.8) | 31 (47.7) | |
| >7 | 17 (26.2) | 18 (27.7) | |
| T category | 0.720 | ||
| T2 | 38 (58.5) | 40 (61.5) | |
| T3a | 24 (36.9) | 18 (27.7) | |
| T3b | 2 (3.1) | 6 (4.0) | |
| T4 | 1 (1.5) | 1 (1.5) | |
| Operation time (min) | 168.0 (156.5–186.5) | 167.0 (150.0–184.0) | 0.373 |
| Intraoperative fluid (mL) | 1100.0 (900.0–1325.0) | 1150.0 (800.0–1325.0) | 0.393 |
| Urinary catheter size (F) | 0.387 | ||
| 16/18/20 | 32 (49.2)/5 (7.7)/28 (43.1) | 30 (46.2)/10 (15.4)/25 (38.5) |
| CRBD | Control Group (n = 65) | Ketorolac Group (n = 65) | p-Value |
|---|---|---|---|
| Postoperative hour 0 | <0.001 | ||
| None | 12 (18.5) | 20 (30.8) | |
| Mild | 20 (30.8) | 31 (47.7) | |
| Moderate | 17 (26.2) | 14 (21.5) | |
| Severe | 16 (24.6) | 0 (0.0) | |
| Postoperative hour 1 | <0.001 | ||
| None | 5 (7.7) | 9 (13.8) | |
| Mild | 34 (52.3) | 51 (78.5) | |
| Moderate | 25 (38.5) | 5 (7.7) | |
| Severe | 1 (1.5) | 0 (0.0) | |
| Postoperative hour 2 | <0.001 | ||
| None | 0 (0.0) | 1 (1.5) | |
| Mild | 27 (41.5) | 57 (87.7) | |
| Moderate | 38 (58.5) | 7 (10.8) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Postoperative hour 6 | <0.001 | ||
| None | 0 (0.0) | 16 (24.6) | |
| Mild | 41 (63.1) | 41 (63.1) | |
| Moderate | 24 (36.9) | 8 (12.3) | |
| Severe | 0 (0.0) | 0 (0.0) |
| Variables | Control Group (n = 65) | Ketorolac Group (n = 65) | p-Value |
|---|---|---|---|
| Postoperative pain score | |||
| Postoperative hour 0 | 5.4 ± 1.1 | 4.8 ± 1.5 | 0.012 * |
| Postoperative hour 1 | 3.0 ± 1.3 | 2.5 ± 0.7 | 0.007 * |
| Postoperative hour 2 | 4.5 ± 1.5 | 4.4 ± 1.5 | 0.766 |
| Postoperative hour 6 | 2.9 ± 1.0 | 2.7 ± 0.9 | 0.132 |
| Opioid requirement during 24 h after surgery (μg) | 125.0 (87.5–175.0) | 100 (75.0–125.0) | <0.001 |
| Ketorolac-related complications | |||
| Acute kidney injury | 2 (3.7) | 1 (2.2) | >0.999 |
| Hemoglobin changes (mg/dL) | −1.7 ± 1.2 | −2.0 ± 1.1 | 0.148 |
| Gastrointestinal bleeding | 0 (0.0) | 0 (0.0) | 1.000 |
| Desaturation events | 1 (1.5) | 0 (0.0) | >0.999 |
| Patient satisfaction score | 4.0 (4.0–4.0) | 5.0 (4.0–6.0) | <0.001 |
| Hospitalization duration (days) | 7.0 (5.0–7.0) | 7.0 (5.0–7.0) | 0.722 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Hong, J.H.; Yu, J.; Kim, D.-H.; Koh, G.-H.; Lee, S.-A.; Hwang, J.-H.; Kong, Y.-G.; Kim, Y.-K. Effect of Ketorolac on the Prevention of Postoperative Catheter-Related Bladder Discomfort in Patients Undergoing Robot-Assisted Laparoscopic Radical Prostatectomy: A Randomized, Double-Blinded, Placebo-Controlled Study. J. Clin. Med. 2019, 8, 759. https://doi.org/10.3390/jcm8060759
Park J-Y, Hong JH, Yu J, Kim D-H, Koh G-H, Lee S-A, Hwang J-H, Kong Y-G, Kim Y-K. Effect of Ketorolac on the Prevention of Postoperative Catheter-Related Bladder Discomfort in Patients Undergoing Robot-Assisted Laparoscopic Radical Prostatectomy: A Randomized, Double-Blinded, Placebo-Controlled Study. Journal of Clinical Medicine. 2019; 8(6):759. https://doi.org/10.3390/jcm8060759
Chicago/Turabian StylePark, Jun-Young, Jun Hyuk Hong, Jihion Yu, Doo-Hwan Kim, Gi-Ho Koh, Sang-A Lee, Jai-Hyun Hwang, Yu-Gyeong Kong, and Young-Kug Kim. 2019. "Effect of Ketorolac on the Prevention of Postoperative Catheter-Related Bladder Discomfort in Patients Undergoing Robot-Assisted Laparoscopic Radical Prostatectomy: A Randomized, Double-Blinded, Placebo-Controlled Study" Journal of Clinical Medicine 8, no. 6: 759. https://doi.org/10.3390/jcm8060759






