MaioRegen Osteochondral Substitute for the Treatment of Knee Defects: A Systematic Review of the Literature
Abstract
1. Introduction
- superficial layer (100% type I collagen): smooth surface, reproducing the articular surface.
- intermediate layer (60% type I collagen and 40% hydroxyapatite): tidemark-like layer.
- lower layer (30% type I collagen and 70% hydroxyapatite): reproducing the composition of the subchondral bone [13].
2. Experimental Section
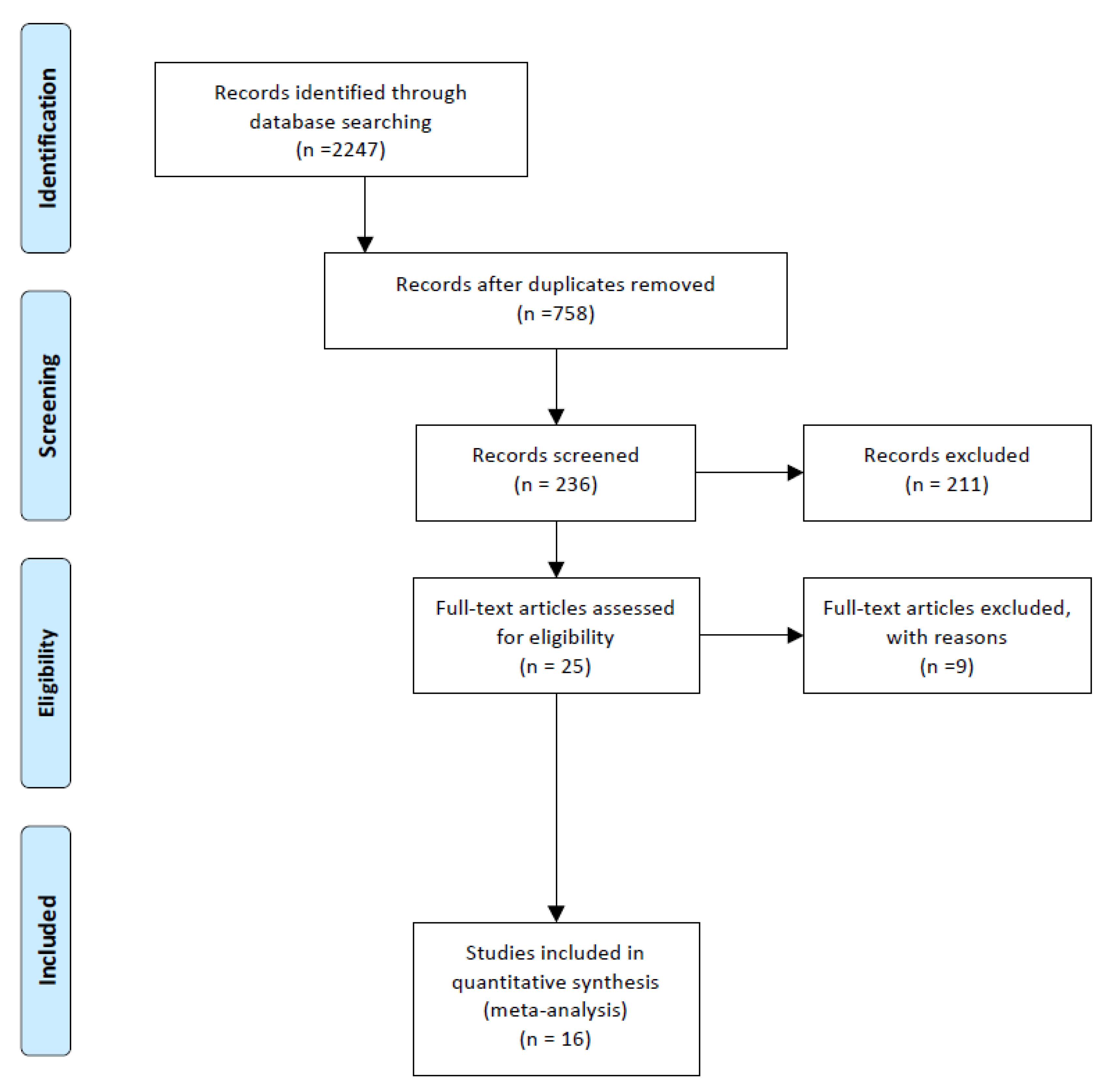
2.1. Data Search Protocol
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction, Synthesis, and Analysis
3. Results
3.1. Demographic Results
3.2. Clinical Outcome in Early Postoperative Period (12 Months)
3.3. Clinical Outcome at Intermediate Follow-Up (24 Months)
3.4. Clinical Outcome in Long Term Follow-Up (>24 Months)
3.5. Radiological Evaluation
3.6. Histological Evaluation
3.7. Minor Complications
3.8. Major Complications
3.9. Failures
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salzmann, G.M.; Niemeyer, P.; Hochrein, A.; Stoddart, M.J.; Angele, P. Articular Cartilage Repair of the Knee in Children and Adolescents. Orthop. J. Sports Med. 2018, 6. [Google Scholar] [CrossRef]
- DiBartola, A.C.; Everhart, J.S.; Magnussen, R.A.; Carey, J.L.; Brophy, R.H.; Schmitt, L.C.; Flanigan, D.C. Correlation between histological outcome and surgical cartilage repair technique in the knee: A meta-analysis. Knee 2016, 23, 344–349. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, R.; Ragone, V.; Ursino, N. What future in the treatment of osteochondral knee defects? Ann. Transl. Med. 2018, 6 (Suppl. 2), S100. [Google Scholar] [CrossRef]
- Curl, W.W.; Krome, J.; Gordon, E.S.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy 1997, 13, 456–460. [Google Scholar] [CrossRef]
- Widuchowski, W.; Widuchowski, J.; Trzaska, T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 2007, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Gomoll, A.H.; Canseco, J.A.; Far, J.; Lind, M.; Hui, J. Cartilage repair in the degenerative ageing Knee. Acta Orthop. 2016, 87, 26–38. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, R.; Giacco, F.; Ragone, V.; Ursino, N. Arthroscopic treatment of osteochondral knee defects with resorbable biphasic synthetic scaffold: Clinical and radiological results and long-term survival analysis. Int. Orthop. 2018. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Schönhuber, H.; Viganò, M.; Bait, C.; Quaglia, A.; Thiebat, G.; Volpi, P. Autologous Matrix-Induced Chondrogenesis (AMIC) and AMIC Enhanced by Autologous Concentrated Bone Marrow Aspirate (BMAC) Allow for Stable Clinical and Functional Improvements at up to 9 Years Follow-Up: Results from a Randomized Controlled Study. J. Clin. Med. 2019, 8, 392. [Google Scholar] [CrossRef]
- Elmalı, N.; Tandoğan, R.; Demirel, M.; Bozkurt, M.; Beyzadeoglu, T. Cartilage repair strategies in the knee: A survey of Turkish surgeons. Acta Orthop. Traumatol. Turc. 2016, 50, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Bowland, P.; Ingham, E.; Jennings, L.; Fisher, J. Review of the biomechanics and biotribology of osteochondral grafts used for surgical interventions in the Knee. Proc. Inst. Mech. Eng. H 2015, 229, 879–888. [Google Scholar] [CrossRef]
- Longley, R.; Ferreira, A.M.; Gentile, P. Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration. Int. J. Mol. Sci. 2018, 19, 1755. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Ragni, E.; Cucchiarini, M.; van Bergen, C.J.A.; Hunziker, E.B.; Chubinskaya, S. Cells, soluble factors and matrix harmonically play the concert of allograft integration. Knee Surg. Sports Traumatol. Arthrosc. 2018. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Altadonna, G.; Marcacci, M. Novel nano-composite multi-layered biomaterial for the treatment of multifocal degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 1312–1315. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.G.; Wilson, S.M.; Swiontkowski, M.F. Updating the assignment of levels of evidence. J. Bone Joint Surg. Am. 2015, 97, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Delcogliano, M.; de Caro, F.; Scaravella, E.; Ziveri, G.; De Biase, C.F.; Marotta, D.; Marenghi, P.; Delcogliano, A. Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the Knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Brix, M.; Kaipel, M.; Kellner, R.; Schreiner, M.; Apprich, S.; Boszotta, H.; Windhager, R.; Domayer, S.; Trattnig, S. Successful osteoconduction but limited cartilage tissue quality following osteochondral repair by a cell-free multilayered nano-composite scaffold at the Knee. Int. Orthop. 2016, 40, 625–632. [Google Scholar] [CrossRef]
- Berruto, M.; Ferrua, P.; Uboldi, F.; Pasqualotto, S.; Ferrara, F.; Carimati, G.; Usellini, E.; Delcogliano, M. Can a biomimetic osteochondral scaffold be a reliable alternative to prosthetic surgery in treating late-stage SPONK? Knee 2016, 23, 936–941. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Venieri, G.; Perdisa, F.; Marcacci, M. Tibial plateau lesions. Surface reconstruction with a biomimetic osteochondral scaffold: Results at 2 years of follow-up. Injury 2014, 45, S121–S125. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Kon, E.; Perdisa, F.; Sessa, A.; Filardo, G.; Neri, M.P.; Bragonzoni, L.; Marcacci, M. Surgical treatment of early knee osteoarthritis with a cell-free osteochondral scaffold: Results at 24 months of follow-up. Injury 2015, 46, S33–S38. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Perdisa, F.; Di Matteo, B.; Di Martino, A.; Iacono, F.; Zaffagnini, S.; Balboni, F.; Vaccari, V.; Marcacci, M. Osteochondral scaffold reconstruction for complex knee lesions: A comparative evaluation. Knee 2013, 20, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Berruto, M.; Delcogliano, M.; de Caro, F.; Carimati, G.; Uboldi, F.; Ferrua, P.; Ziveri, G.; De Biase, C.F. Treatment of Large Knee Osteochondral Lesions with a Biomimetic Scaffold: Results of a Multicenter Study of 49 Patients at 2-Year Follow-up. Am. J. Sports Med. 2014, 42, 1607–1617. [Google Scholar] [CrossRef]
- Perdisa, F.; Filardo, G.; Sessa, A.; Busacca, M.; Zaffagnini, S.; Marcacci, M.; Kon, E. One-Step Treatment for Patellar Cartilage Defects With a Cell-Free Osteochondral Scaffold: A Prospective Clinical and MRI Evaluation. Am. J. Sports Med. 2017, 45, 1581–1588. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Di Martino, A.; Busacca, M.; Altadonna, G.; Marcacci, M. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: Clinical and imaging evaluation at 2-year follow-up. Am. J. Sports Med. 2013, 41, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am. J. Sports Med. 2014, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Delcogliano, M.; Filardo, G.; Busacca, M.; Di Martino, A.; Marcacci, M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: A pilot clinical trial. Am. J. Sports Med. 2011, 39, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Perdisa, F.; Di Martino, A.; Busacca, M.; Balboni, F.; Sessa, A.; Marcacci, M. A one-step treatment for chondral and osteochondral knee defects: Clinical results of a biomimetic scaffold implantation at 2 years of follow-up. J. Mater. Sci. Mater. Med. 2014, 25, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Zaffagnini, S.; Kon, E.; Marcheggiani Muccioli, G.M.; Di Martino, A.; Di Matteo, B.; Bonanzinga, T.; Iacono, F.; Filardo, G. Unicompartmental osteoarthritis: An integrated biomechanical and biological approach as alternative to metal resurfacing. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D.T.; Kaelin, R.; Rasch, H.; Arnold, M.P.; Hirschmann, M.T. Good clinical results but moderate osseointegration and defect filling of a cell-free multi-layered nano-composite scaffold for treatment of osteochondral lesions of the Knee. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1273–1280. [Google Scholar] [CrossRef]
- Perdisa, F.; Kon, E.; Sessa, A.; Andriolo, L.; Busacca, M.; Marcacci, M.; Filardo, G. Treatment of Knee Osteochondritis Dissecans with a Cell-Free Biomimetic Osteochondral Scaffold: Clinical and Imaging Findings at Midterm Follow-up. Am. J. Sports Med. 2018, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, P.; Dhollander, A.; Almqvist, K.F.; Verdonk, R.; Victor, J. Treatment of osteochondral lesions in the knee using a cell-free scaffold. Bone Joint J. 2015, 97, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Roffi, A.; Andriolo, L.; Marcacci, M. New trends for knee cartilage regeneration: From cell-free scaffolds to mesenchymal stem cells. Curr. Rev. Musculoskelet. Med. 2012, 5, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Melton, J.T.; Wilson, A.J.; Chapman-Sheath, P.; Cossey, A.J. TruFit CB bone plug: Chondral repair, scaffold design, surgical technique and early experiences. Expert Rev. Med. Devices 2010, 7, 333–341. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Robinson, D.; Eisman, J.A.; Levy, A.; Zaslav, K.; Shani, J.; Altschuler, N. Osteochondral regeneration using a novel aragonite-hyaluronate bi-phasic scaffold in a goat model. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1452–1464. [Google Scholar] [CrossRef]
- Williams, R.J.; Gamradt, S.C. Articular cartilage repair using a resorbable matrix scaffold. Instr. Course Lect. 2008, 57, 563–571. [Google Scholar]
- Verhaegen, J.; Clockaerts, S.; Van Osch, G.J.; Somville, J.; Verdonk, P.; Mertens, P. TruFit Plug for Repair of Osteochondral Defects-Where Is the Evidence? Systematic Review of Literature. Cartilage 2015, 6, 12–19. [Google Scholar] [CrossRef]
- De Luca, P.; Kouroupis, D.; Viganò, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Contains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef]
- Kon, E.; Robinson, D.; Verdonk, P.; Drobnic, M.; Patrascu, J.M.; Dulic, O.; Gavrilovic, G.; Filardo, G. A novel aragonite-based scaffold for osteochondral regeneration: Early experience on human implants and technical developments. Injury 2016, 47, S27–S32. [Google Scholar] [CrossRef]
- Andrade, R.; Vasta, S.; Pereira, R.; Pereira, H.; Papalia, R.; Karahan, M.; Oliveira, J.M.; Reis, R.L.; Espregueira-Mendes, J. Knee donor-site morbidity after mosaicplasty—A systematic review. J. Exp. Orthop. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Pareek, A.; King, A.H.; Johnson, N.R.; Stuart, M.J.; Williams, R.J., 3rd. Return to sport after the surgical management of articular cartilage lesions in the knee: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
| Article | Type of Study | Number of Patients/Mean Age | Location | Lesion Size | Follow-Up | Clinical Results | Radiologic Results/Histological Evaluation | Complications/Reoperation | |
|---|---|---|---|---|---|---|---|---|---|
| Preop | Postop | Postop | |||||||
| Delcogliano et al. 2014, KSSTA [17] | Case Series, Level IV | 19 patients; 33 ± 15 years | 20 lesions: 10 MFC, 7 LFC, 3 tibial plateau | 5.2 ±1.6 cm2. (4–8 cm2); mean depth of 8 mm (6–9 mm) | 12 and 24 months | IKDC: 35.7 ± 6.3 Tegner: 2 (0–4) EQ-VAS: 3.1 ± 1.1 | IKDC: 12 months: 67.7 ± 13.4 (p < 0.0005) * 24 months: 72.8 ± 12.4 (p < 0.0005) *† Tegner: 24 months: 5 (1–7) (p < 0.0005) * EQ-VAS 24 months: 7.3 ± 1.1(p < 0.0005) * | MOCART 24 months: 63.2 ± 11.7 | 2 failures: 12 and 24 months after surgery No complications |
| Brix et al. 2016, Int Orthop [18] | Case Series, Level IV | 8 patients; 37 years | 8 lesions: 5 MFC, 3 LFC | 2.07 cm2 (1.5 to 3.75 cm2) | 12,18 and 24 months | IKDC Lysholm Cincinnati | IKDC: 6,12,18 and 24 months: p = 0.30 Tegner–Lysholm: 6,12,18 and 24 months: p = 0.176 Cincinnati 6,12,18 and 24 months: p = 0.53 | MOCART 18 months: 69 (60–100) T2 mapping at 18 months: 0.9874 | 2 complications: resurgery at 7 and 24 months not for scaffold failure. In the first case the re-arthroscopy showed a novel chondral lesion on the lateral condyle. In the second case an infrapatellar ossicle was removed No failures |
| Berruto et al. 2016, The Knee [19] | Case Series, Level IV | 11 patients; 52.1± 9.6 years | 11 lesions: 9 MFC, 2 LFC | 3.47 ± 1.75 cm2 (range 1.5 to 7.5 cm2) | 12 and 24 months | IKDC: 40.54 ± 15.0 (17–57) Lysholm: 49.7 ± 17.9 (22–88) Tegner: 4 ± 1.1 (2–6)(pre-injury) VAS: 6.3 ± 2.5 (3–8) | IKDC: 12 months: 65.72 ± 14.8 * (p = 0.014) 24 months: 63.90 ± 19.9 * (p = 0.03) Lysholm: 12 months: 85.4 ± 12.1 * (p = 0.01) 24 months: 86.6 ± 12.7 * (p = 0.04) Tegner: 12 months: 3.7 ± 1.3 ( p > 0.05) 24 months: 3.8 ± 1.7 ( p > 0.05) VAS: 12 months: 2.2 ± 2.1 * (p = 0.02) 24 months: 1.6 ± 2.7 * (p < 0.05) | N.A. | 2 failures: 2 condylar collapse and subsequent knee arthroplasty at 18 months after implantation |
| Kon et al. 2014, Injury [20] | Case Series, Level IV | 11 patients; 37.3 ± 11.0 years | 13 lesions: 11 tibial plateau, 1 MFC, 1 LFC | 5.1 ±2.7 cm2 (3.0–12.5 cm2) | 6,12 and 24 months | IKDC: 42.5 ± 10.2 Tegner: 2.3 ± 2.1 | IKDC: 6 months: 58.3 ± 14.1 * (p < 0.05) 12 months: 69.8 ± 19.0 *† (p = 0.03) 24 months: 68.4 ± 17.0 * Tegner: 12 months 4.8 ± 2.4 * (p < 0.05) 24 months: 5.3 ± 2.5 * | N.A. | 3 minor complications: fever during the first week spontaneously resolved. |
| Di Martino et al. 2015, Injury [21] | Case Series, Level IV | 23 patients; 38.0 ±8.2 years | 23 lesions: 12 MFC, 9 LFC, 1 tibial plateau, 1 patella | 3.2 ± 1.9 cm2 | 12 and 24 months | IKDC: 42.8 ± 13.8 Tegner score: 3.3 ± 2.7 (before injury 6.1 ± 2.6) | IKDC: 12 months: 74.3 ± 17.4 *(p < 0.0005) 24 months 74.9 ± 2 0.4 * Tegner: 12 months: 4.6 ± 2.2 * (p < 0.0005) 24 months: 4.7 ± 2.1 * | Mocart score: 12 monhts: 72.9 ± 13.6 24 months: 70.8 ± 13.2 | 2 complications: 2 patients underwent knee mobilization under narcosis at 2 and 4 months 2 failures |
| Filardo et al. 2013, The Knee [22] | Comparative study, Level III | 33 patients, 39.5 ± 10.6 years | 47 lesions: 11 MFC, 9 LFC, 13 trochlea, 9 patella, 5 tibial plateau | 4.5 ± 2.7 cm2 | 12 and 24 months | IKDC: 40.4 ± 14.1 VAS: 4.0 ± 2.2 Tegner: 1.9 ± 1.8 | IKDC: 12 months: 69.6 ± 17.0 * (p < 0.0005) 24 months: 75.5 ± 15.0 *† (p = 0.038) VAS: 12 months: 4.7 ± 3.0 24 months: 7.3 ± 1.5 *† (p < 0.0005) Tegner: 12 months: 4.0 ± 1.8 * (p < 0.0005) 24 months: 4.5 ± 1.7 * (p = 0.09) | N.A. | No failure 2 complications: 2 arthroscopic regularization/shaving due to partial detachment of the scaffold |
| Berruto et al. 2014, Am J Sports Med [23] | Case Series, Level IV | 49 patients; 37.6 ± 14 years | 49 lesions: 33 MFC, 11 LFC, 4 tibial plateau, 1 trochlea | 4.35 cm2 (3–8.25 cm2) | 6,12 and 36 months | IKDC: 45.45 ± 19.29 VAS: 6.69 ± 1.88 Tegner: 2.20 ± 0.67 | IKDC: 12 months: 70.86 ± 18.08 * (p < 0.001) 24 months: 75.42 ± 19.31 *†(p < 0.05) 36 months: 76.14 ± 18.53 * VAS: 12 months: 2.55 ± 2.38 * (p < 0.05) 24 months: 1.96 ± 2.47 * 36 months: 2.1 ± 2.23 * Tegner score: 24 months: 4.9 ± 1.73 * 36 months: 5.06 ± 1.65 * | MOCART 24 months: 70% showed complete filling of the lesion, 63.3% had an intact articular surface, and 86% had mild or no effusion Histological evaluation: In all specimens, histological examination revealed no residual scaffold. Both subchondral bone and the mineralization process appeared normal in all specimens evaluated with Mallory trichrome | 6 minor complications (swelling and bleeding) 5 failures |
| Perdisa et al. 2017, Am J Sports Med [24] | Case Series, Level IV | 34 patients; 30.0 ± 10 years | 34 patellar lesion | 2.1 ± 1 cm2 | 12 and 24 months | IKDC: 39.5 ± 14.5 Tegner: 1.8 ± 1.0 | IKDC: 12 months: 61.9 ±14.5 * (p < 0.0005) 24 months: 67.6 ± 17.4 *† (p = 0.02) Tegner: 12 months: 3.3 ± 1.5 * (p < 0.0005) 24 months:3.3 ± 1.1 * | MOCART: 12 months: 79.8 ± 12.6 24 months: 83.5 ± 11.4 | 2 failures, underwent realignment procedures |
| Filardo et al., 2013, Am J Sports Med [25] | Case Series, Level IV | 27 patients; 25.5 ± 7.7 years | 27 lesions: 17 MFC, 10 LFC | 3.4 ± 2.2cm2 | 12 and 24 months | IKDC: 48.4 ± 17.8 Tegner: 2.4 ± 1.7 (5.7 ± 2.3 before onset of symptoms) | IKDC: 12 months: 76.0 ±12.8 * (p < 0.0005) 24 months: 82.3 ± 12.8 *† (p < 0.0005) Tegner: 12 months:3.6 ± 1.2 * (p = 0.01) 24 months:4.5 ± 1.6 *† (p = 0.01) | MOCART: 12 months: 66.9 ± 12.8 24 months: 67.0 ± 25.7 | 5 adverse event: -3 patients had joint stiffness treated with knee mobilization under anesthesia -2 fever |
| Kon et al. 2014, Am J Sports Med [26] | Case Series, Level IV | 27 patients; 34.9 ± 10.2 years | 30 lesions: 7 MFC, 5 LFC, 11 patella, 5 trochlea 2 tibial plateau | 2.9 ± 1.3 cm2 | 24 and 60 months | IKDC: 40.0 ± 15.0 Tegner:1.6 ± 1.1 (5.2 ± 2.6 before onset of symptoms) | IKDC: 24 months: 76.5 ±14. 5 * (p < 0.0005) 60 months: 77.1 ±18.0 * (p < 0.000) Tegner: 24 months: 4.0 ± 1.8 * (p < 0.0005) 60 months:4.1 ± 1.9 * (p < 0.0005) | MOCART: 24 months:68.0 ± 13.8 60 months: 74.8 ± 12.3 † | None |
| Kon et al. 2011, Am J Sports Med [27] | Case Series, Level IV | 28 patients; 35.3 ± 10.2 years | 34 lesions: 8 MFC, 5 LFC, 12 patella, 7 trochlea, 2 tibial plateau | 2.9 ± 1.3 cm2 | 6,12 and 24 months | IKDC Tegner:1.6 ± 1.1 (5.2 ± 2.5 before onset of symptoms) | IKDC: 6 months: p < 0.0005 * 12 months: p < 0.0005 * 24 months: p < 0.005 *† Tegner: 12 months:4.0 ± 1.6 * (p < 0.0005) 24 months: 4.0 ± 1.6 * (p < 0.0005) | MOCART: 24 months: 79.2 (40–95) Histological evaluation: at the 2-year follow-up showed complete biomaterial reabsorption and a hyaline-like tissue with a strong proteoglycan content and presence of collagen type II | 10 complications: Swelling during the first month was observed in 6 patients. One patient experienced bleeding during the first 3 days after surgery. Two patients developed a fever during the first 3 weeks. All adverse events resolved within 1 month after surgery, with the exception of 2 patients with joint stiffness who were reoperated on arthroscopically, one at 2 months and the other at 5 months. One patient affected by multiple lesions had loosening of one of the grafts, which was removed, and another patient was reoperated for graft hypertrophy. |
| Kon et al. 2014, J Mater Sci: Mater Med [28] | Case Series, level IV | 79 patients; 31.0 ± 11.3 years | 82 lesions: 41 MFC, 26 LFC, 15 trochlea | 3.2 ± 2.0 cm2 | 12 and 24 months | IKDC: 47.4 ± 17.1 Tegner: 2.9 ± 2.0 (6.3 ± 2.2 before the onset of symptoms) | IKDC: 12 months: 72.1 ±18. 9 * (p < 0.0005) 24 months: 76.2 ±19.6 *†(p = 0.004) Tegner: 12 months: 3.8 ± 1.6 * (p < 0.0005) 24 months: 4.4 ± 1.9 *† (p < 0.0005) | MOCART: 12 months; median 70 24 months: median 80† | 17 patients reported swelling 9 resurgery due to stiffness |
| Marcacci et al. 2013, KSSTA [29] | Case Series, Level IV | 43 patients; 40.1 ± 11 | 43 Femoral condyles | 4.6 ± 2.1 cm2 | 36 months | IKDC: 47.3 ± 17.1 VAS: 6.1 ± 2.0 Tegner: 2 (1–5) 6 (3–10 before onset of symptoms) | IKDC: 79.6 ± 16.1 * (p < 0.0005) VAS: 2.3 ± 2.2 * (p < 0.0005) Tegner: 4 (3–10) * (p < 0.0005) | N.A. | None |
| Mathis et al. 2018, KSSTA [30] | Case Series, Level IV | 14 patients; 33 ± 10 years | 14 lesions: 8 MFC, 2 CFL. 2 trochlea, 2 patella. | 1.0–3.5 cm2 | 12 months | Lysholm: 65.6 ± 12.6 Tegner: 6.0 (3-9) | Lysholm: 90.1 ± 10.0 * (p < 0.001) Tegner: 4.5 (p < 0.01) * | SPECT/CT: A complete filling of the defect was shown in 14%, a partial filling in 14% and only minor filling was seen in 72%. | None |
| Perdisa et al.2018, Am J Sport Med [31] | Case Series, Level IV | 27 patients; 25.5± 7.7 years | 27 lesions: 17 MFC, 10 LFC | 3.4 ± 2.2cm2 | 12,24,36,48 and 60 months after surgery | IKDC: 48.4 ± 17.8 Tegner: 2.4 ± 1.7 5.7 ± 2.2 (before onset of symptoms) | IKDC: 12 months: p < 0.0005 vs preop * 24 months: p < 0.0005 vs preop * 36 months: p < 0.0005 vs preop * 48 months: p < 0.0005 vs preop * 60 months: p < 0.0005 vs preop and vs 24 months * Tegner: 12 months: non-significant improvement vs preop 24 months: 4.4 ± 1.6 (p = 0.001) * 60 months: 5.0 ± 1.7 (p < 0.0005)* | MOCART: 24 months: 74.2 ±16.2 60 months: 81.4 ±11.8 | None |
| Verdonk et al., 2015, Bone Joint J [32] | Case Series, Level IV | 38 patients; 30.5 ±11.9 years | 38 lesions: 23 MFC, 7 LFC, 5 patella, 3 trochlea | 3.7 ±2.4 cm2 | 3,6,12,18,24 months | KOOS: 213.9 ± 88.3 Tegner: 3.1 ± 2.5 | KOOS: 3 months: 261.2 ± 98.8 * (p = 0.01) 6 months: 295.9 ± 106.1 * (p = 0.01) 12 months: 328.2 ± 105.7 * (p = 0.01) 18 months: 335.8 ± 100.9 * (p = 0.01) 24 months: 356.1 ± 96.9 *(p = 0.01) Tegner: 3 months: 1.9 ± 1.9 * (p = 0.01) 6 months: 2.5 ± 1.9 (p = 0.09) 12 months: 3.4 ± 1.9 (p = 0.46) 18 months: 3.4 ± 1.8 (p = 0.27) 24 months: 3.8 ± 1.9 * (p = 0.03) | MOCART: Significant improvement at 3, 12 and 24 months after surgery. | 2 failures: total knee arthroplasty at 14 and 20 months 3 complications: -1 further arthroscopy due to hypertrophy -2 joint stiffness treated with knee mobilization under anesthesia |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosi, R.; Valli, F.; De Luca, P.; Ursino, N.; Usuelli, F.G. MaioRegen Osteochondral Substitute for the Treatment of Knee Defects: A Systematic Review of the Literature. J. Clin. Med. 2019, 8, 783. https://doi.org/10.3390/jcm8060783
D’Ambrosi R, Valli F, De Luca P, Ursino N, Usuelli FG. MaioRegen Osteochondral Substitute for the Treatment of Knee Defects: A Systematic Review of the Literature. Journal of Clinical Medicine. 2019; 8(6):783. https://doi.org/10.3390/jcm8060783
Chicago/Turabian StyleD’Ambrosi, Riccardo, Federico Valli, Paola De Luca, Nicola Ursino, and Federico Giuseppe Usuelli. 2019. "MaioRegen Osteochondral Substitute for the Treatment of Knee Defects: A Systematic Review of the Literature" Journal of Clinical Medicine 8, no. 6: 783. https://doi.org/10.3390/jcm8060783
APA StyleD’Ambrosi, R., Valli, F., De Luca, P., Ursino, N., & Usuelli, F. G. (2019). MaioRegen Osteochondral Substitute for the Treatment of Knee Defects: A Systematic Review of the Literature. Journal of Clinical Medicine, 8(6), 783. https://doi.org/10.3390/jcm8060783






