Occipital Petalia and Albinism: A Study of Interhemispheric VEP Asymmetries in Albinism with No Nystagmus
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Participants
2.3. Data Analysis
2.3.1. Full Field Stimuli (Flash, Pattern Reversal, and Pattern Appearance)
2.3.2. Hemifield Pattern Reversal VEP Stimuli
3. Results
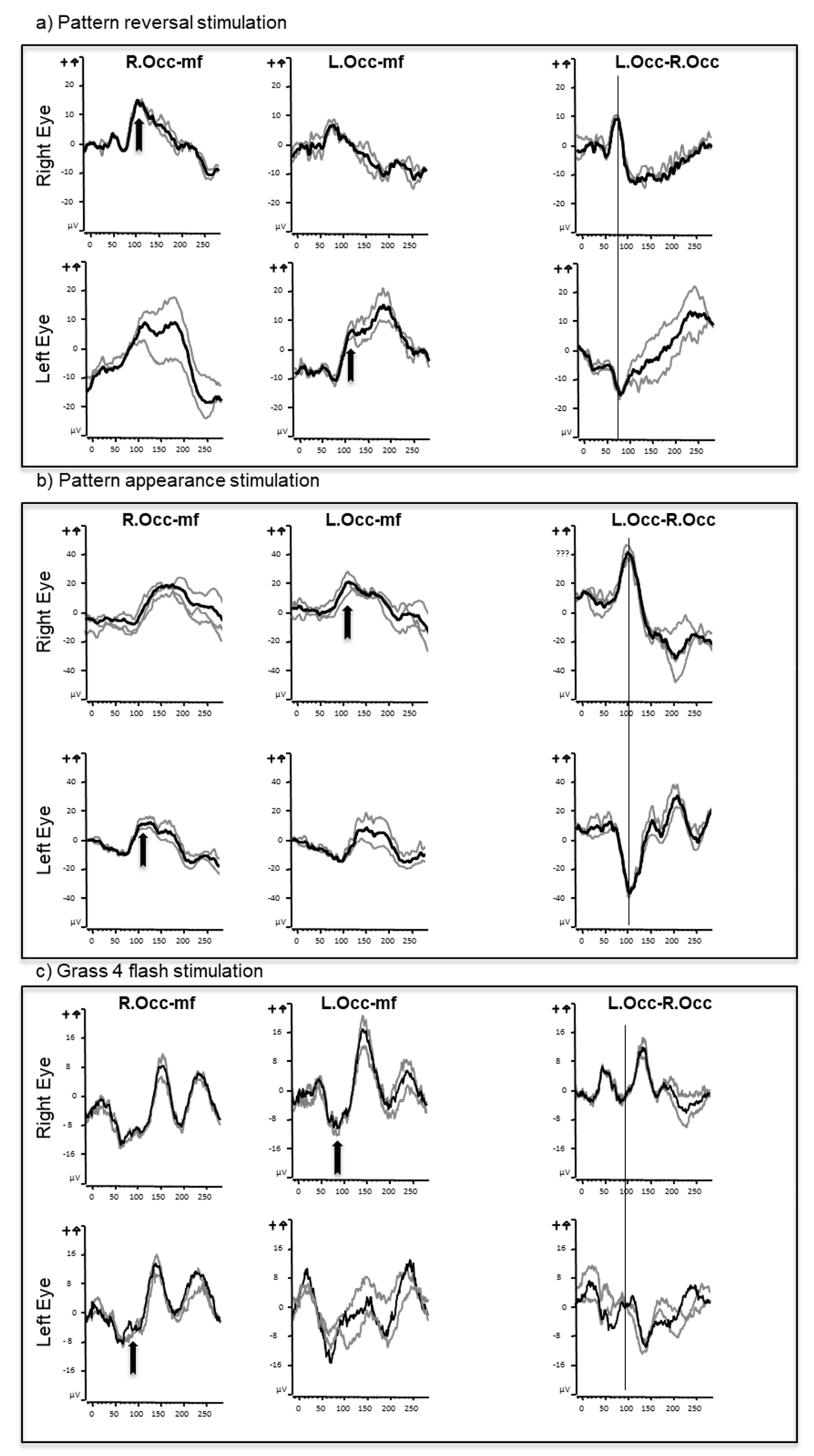
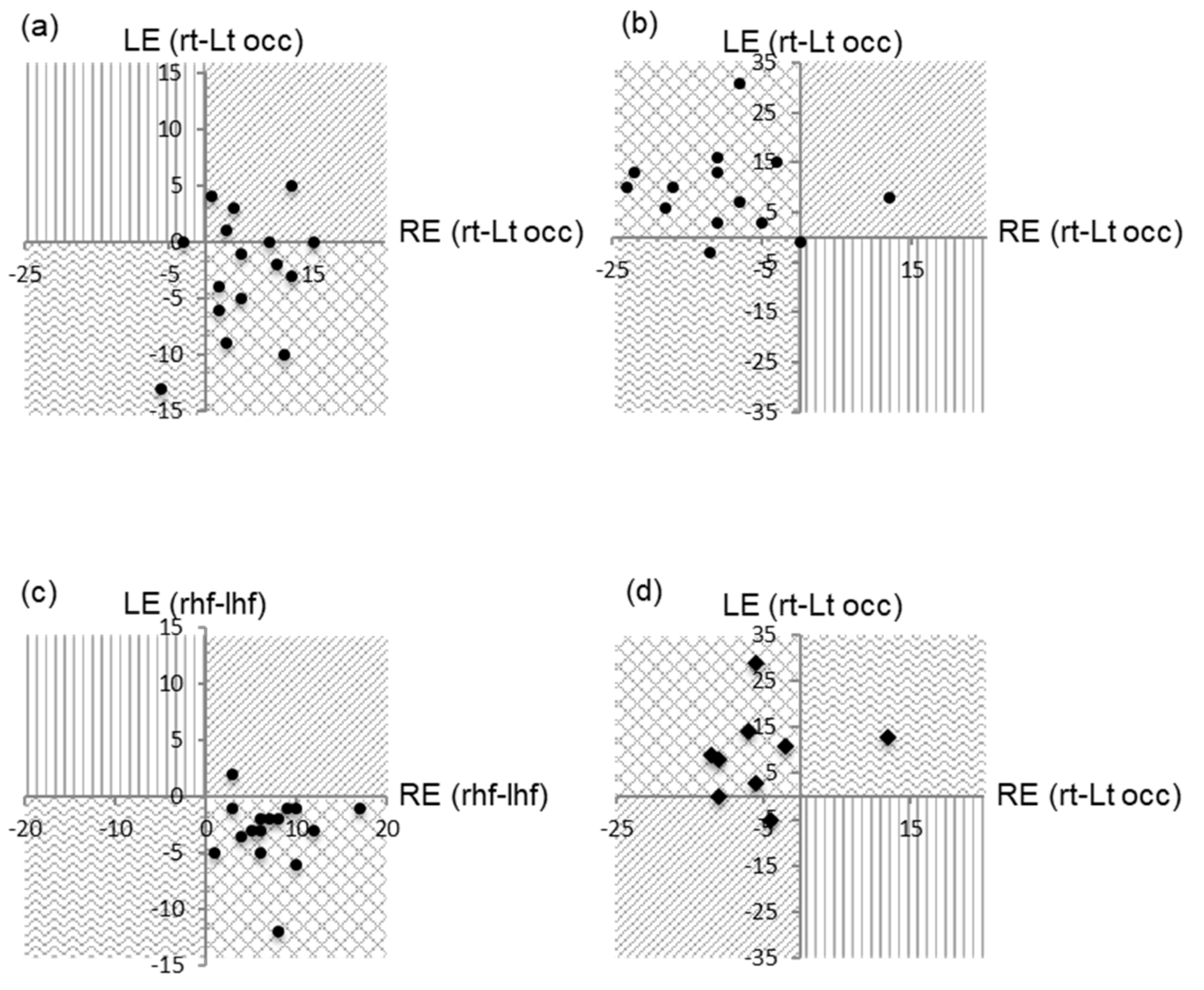
3.1. Full Field Pattern Reversal VEPs
3.2. Full Field Pattern Appearance VEPs
3.3. Flash VEPs
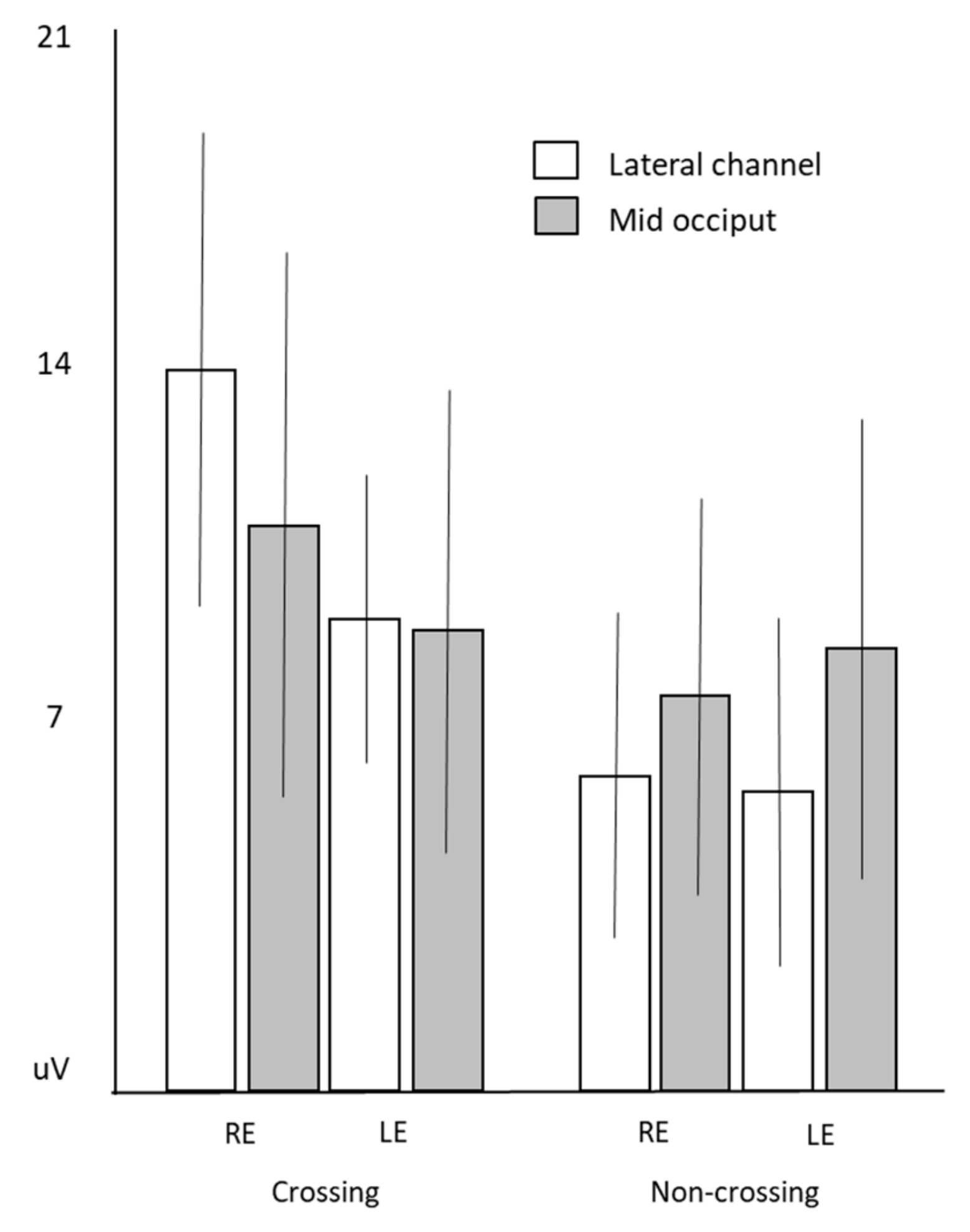
3.4. Hemifield prVEPs
4. Discussion
5. Conclusions
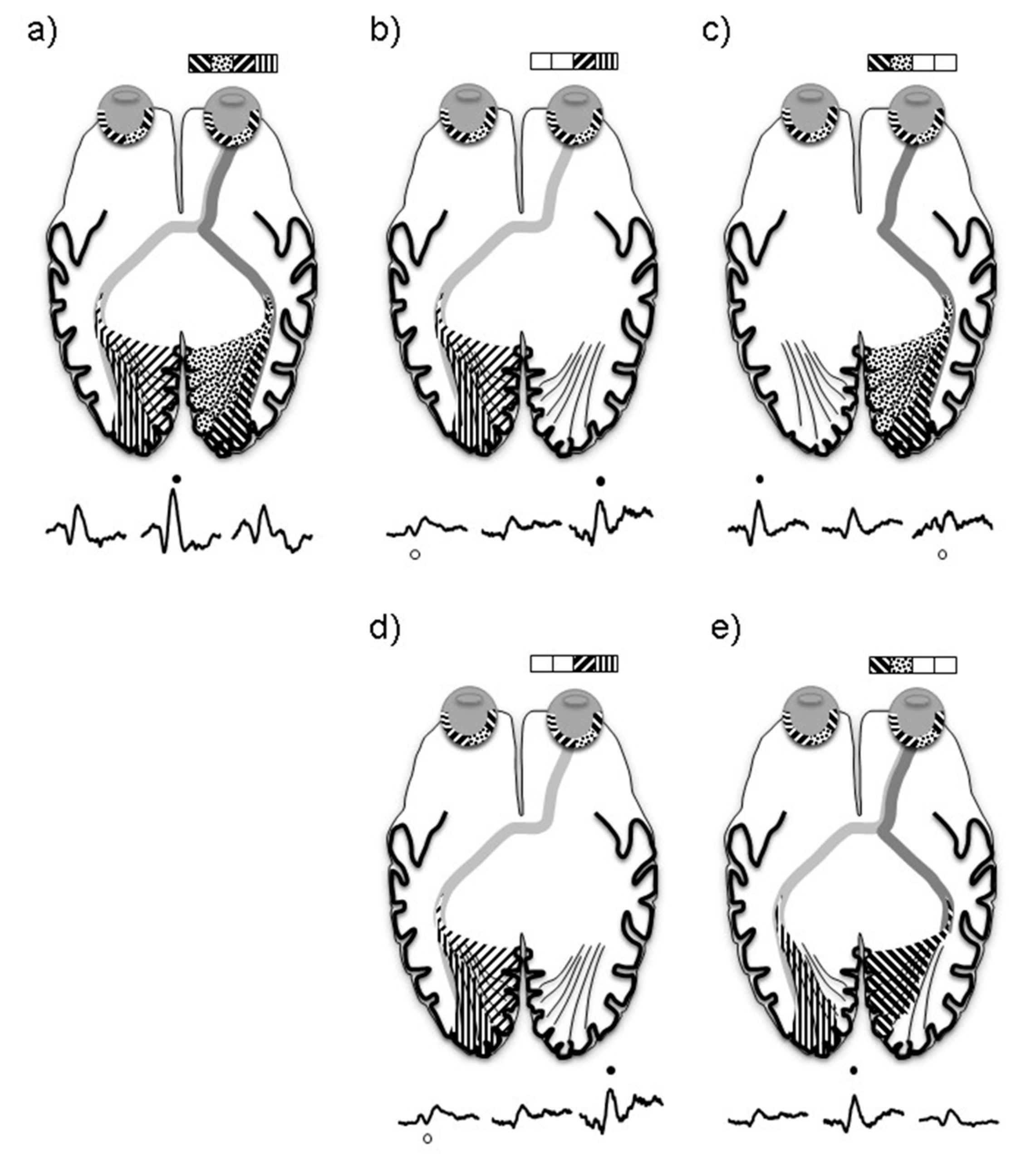
- The electrophysiological pattern of VEP trans-occipital asymmetries seen in normal subjects due to the influence of asymmetric occipital petalia is also evident in our cohort of albinos without nystagmus. As evidenced by the HF VEP studies, we were able to demonstrate that stimulation of the crossing fields in albinos produces larger responses from the left hemisphere compared to the right, which is like the controls. This functional VEP similarity is notable given recent MRI studies that have revealed decreased gyrification and gray matter volume mainly in the left hemisphere of albinos compared to controls.
- In cases where albinism is suspected, and no nystagmus is evident, hemifield stimulation should be attempted where possible as this study has shown it has superior detection sensitivity in detecting chiasmal misrouting.
- Studies combining functional MRI imaging and electrophysiological studies in subjects without nystagmus are needed to further investigate interhemispheric structural and functional correlations of visual pathway misrouting in albinism.
Author Contributions
Conflicts of Interest
References
- Kinnear, P.E.; Jay, B.; Witkop, C.J. Albinism. Surv. Ophthalmol. 1985, 30, 75–101. [Google Scholar] [CrossRef]
- Creel, D.J.; Summers, C.G.; King, R.A. Visual anomalies associated with albinism. Ophthalmic Paediatr. Genet. 1990, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.; Schaefer, T.; Krick, C.M.; Reith, W.; Backens, M.; Käsmann-Kellner, B. Configuration of the optic chiasm in humans with albinism as revealed by magnetic resonance imaging. Investig. Ophthalmol. Vis. Sci. 2003, 44, 16–21. [Google Scholar] [CrossRef]
- Bridge, H.; von dem Hagen, E.A.H.; Davies, G.; Chambers, C.; Gouws, A.; Hoffmann, M.; Morland, A.B. Changes in brain morphology in albinism reflect reduced visual acuity. Cortex 2014, 56, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.B.; Lorenz, B.; Morland, A.B.; Schmidtborn, L.C. Misrouting of the optic nerves in albinism: Estimation of the extent with visual evoked potentials. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3892–3898. [Google Scholar] [CrossRef] [PubMed]
- Poulter, J.A.; Al-Araimi, M.; Conte, I.; Van Genderen, M.M.; Sheridan, E.; Carr, I.M.; Parry, D.A.; Shires, M.; Carrella, S.; Bradbury, J.; et al. Recessive mutations in SLC38a8 cause foveal hypoplasia and optic nerve misrouting without albinism. Am. J. Hum. Genet. 2013, 93, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Van Genderen, M.M.; Riemslag, F.C.C.; Schuil, J.; Hoeben, F.P.; Stilma, J.S.; Meire, F.M. Chiasmal misrouting and foveal hypoplasia without albinism. Br. J. Ophthalmol. 2006, 90, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Brecelj, J.; Sustar, M.; Pečarič-Meglič, N.; Škrbec, M.; Stirn-Kranjc, B. VEP characteristics in children with achiasmia, in comparison to albino and healthy children. Doc. Ophthalmol. 2012, 124, 109–123. [Google Scholar] [CrossRef]
- Brecelj, J. Visual electrophysiology in the clinical evaluation of optic neuritis, chiasmal tumours, achiasmia, and ocular albinism: An overview. Doc. Ophthalmol. 2014, 129, 71–84. [Google Scholar] [CrossRef]
- Mellow, T.B.; Liasis, A.; Lyons, R.; Thompson, D. When do asymmetrical full-field pattern reversal visual evoked potentials indicate visual pathway dysfunction in children? Doc. Ophthalmol. 2011, 122, 9–18. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; Mcculloch, D.L.; Mizota, A.; Patrizia, A. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barrett, G.; Blumhardt, L.; Halliday, A.M.; Halliday, E.; Kriss, A. A paradox in the lateralisation of the visual evoked response. Nature 1976, 261, 253–255. [Google Scholar] [CrossRef]
- Sami, D.A.; Saunders, D.; Thompson, D.A.; Russell-Eggitt, I.M.; Nischal, K.K.; Jeffery, G.; Dattani, M.; Clement, R.A.; Liassis, A.; Taylor, D.S. The achiasmia spectrum: Congenitally reduced chiasmal decussation. Br. J. Ophthalmol. 2005, 89, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Korff, C.M.; Apkarian, P.; Bour, L.J.; Meuli, R.; Verrey, J.D.; Perez, E.R. Isolated absence of optic chiasm revealed by congenital nystagmus, MRI and VEPs. Neuropediatrics 2003, 34, 219–223. [Google Scholar] [PubMed]
- Hoffmann, M.; Kaule, F.; Levin, N.; Masuda, Y.; Kumar, A.; Gottlob, I.; Horiguchi, H.; Dougherty, R.; Stadler, J.; Wolynski, B.; et al. Plasticity and stability of the visual system in human achiasma. Neuron 2012, 75, 393–401. [Google Scholar] [CrossRef]
- Von Dem Hagen, E.A.H.; Hoffmann, M.B.; Morland, A.B. Identifying human albinism: A comparison of VEP and fMRI. Investig. Ophthalmol. Vis. Sci. 2008, 49, 238–249. [Google Scholar] [CrossRef][Green Version]
- Apkarian, P.; Reits, D.; Spekreijse, H.; Van Dorp, D. A decisive electrophysiological test for human albinism. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 513–531. [Google Scholar] [CrossRef]
- Soong, F.; Levin, A.V.; Westall, C.A. Comparison of techniques for detecting visually evoked potential asymmetry in albinism. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2000, 4, 302–310. [Google Scholar] [CrossRef]
- Dorey, S.; Neveu, M.; Burton, L.; Sloper, J.; Holder, G. The clinicla features of albinism and their correlation with visual evoked potentials. Br. J. Ophthalmol. 2003, 87, 767–772. [Google Scholar] [CrossRef]
- Lenassi, E.; Likar, K.; Stirn-Kranjc, B.; Brecelj, J. VEP maturation and visual acuity in infants and preschool children. Doc. Ophthalmol. 2008, 117, 111–120. [Google Scholar] [CrossRef]
- Saunders, K.J.; Brown, G.; McCulloch, D.L. Pattern-onset visual evoked potentials: More useful than reversal for patients with nystagmus. Doc. Ophthalmol. 1997, 94, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Timms, C.; Thompson, D.; Russell-Eggitt, I.; Clement, R. Saccadic instabilities in albinism without nystagmus. Exp. Brain Res. 2006, 175, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Grønskov, K.; Ek, J.; Sand, A.; Scheller, R.; Bygum, A.; Brixen, K.; Brondum-Nielsen, K.; Rosenberg, T. Birth prevalence and mutation spectrum in Danish patients with autosomal recessive albinism. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Blumhardt, L.D.; Barett, G.; Halliday, A.M. The assymetrical visual evoked potential to pattern reversl in one half field and its significance for the analysis of visual field defects. Br. J. Ophthalmol. 1977, 61, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Blumhardt, L.D.; Barrett, G.; Halliday, A.M.; Kriss, A. The effect of experimental “scotomata” on the ipsilatteral and contralateral responses to pattern-reversal in one half-field. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 376–392. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kuroiwa, Y. Amplitude asymmetry of hemifield pattern reversal VEPs in healthy subjects. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials 1990, 77, 81–85. [Google Scholar] [CrossRef]
- Kuroiwa, Y.; Celesia, G.G.; Tohgi, H. Amplitude difference between pattern-evoked potentials after left and right hemifield stimulation in normal subjects. Neurology 1987, 37, 795–799. [Google Scholar] [CrossRef]
- Neveu, M.M.; Von Dem Hagen, E.; Morland, A.B.; Jeffery, G. The fovea regulates symmetrical development of the visual cortex. J. Comp. Neurol. 2008, 506, 791–800. [Google Scholar] [CrossRef]
- Von Dem Hagen, E.A.; Houston, G.C.; Hoffmann, M.B.; Jeffery, G.; Morland, A.B. Retinal abnormalities in human albinism translate into a reduction of grey matter in the occipital cortex. Eur. J. Neurosci. 2005, 22, 2475–2480. [Google Scholar] [CrossRef]
- Mohammad, S.; Gottlob, I.; Kumar, A.; Thomas, M.; Degg, C.; Sheth, V.; Proudlock, F.A. The functional significance of foveal abnormalities in albinism measured using spectral-domain optical coherence tomography. Ophthalmology 2011, 118, 1645–1652. [Google Scholar] [CrossRef] [PubMed]

| Age | Sex | RVA | LVA | Albinism Type | Fovea Grade | Hemi Field Reversal | Flash | Full Field Reversal | Full Field Appearance | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.80 | F | 0.36 | 0.38 | OCA | 3 | ∞ | ∞ | ∞ | ∞ |
| 2 | 7.77 | M | 0.32 | 0.56 | OA | 3 | ∞ | ∞ | ∞ | ∞ |
| 3 | 6.12 | F | 0.48 | 0.56 | OCA | 4 | ∞ | ∞ | ∞ | → |
| 4 | 9.43 | F | 0.38 | 0.66 | OCA | 4 | ∞ | ∞ | ∞ | → |
| 5 | 8.27 | M | 0.26 | 0.2 | OA | 4 | ∞ | ∞ | ∞ | RE← LE = |
| 6 | 13.61 | M | 0.25 | 0.3 | OA | 2 | ∞ | ∞ | ∞ | |
| 7 | 7.80 | F | 0.36 | 0.2 | OCA | 4 | ∞ | ∞ | RE→ LE = | ∞ |
| 8 | 4.81 | M | 0.4 | 0.45 | OA | 2 | ∞ | ∞ | ← | |
| 9 | 9.43 | F | 0.34 | 0.2 | OCA | 4 | ∞ | ∞ | RE← LE = | ∞ |
| 10 | 5.85 | F | 0.44 | 0.56 | OCA | 3 | ∞ | ∞ | → | → |
| 11 | 6.81 | F | 0.3 | 0.3 | OCA | 2 | ∞ | ∞ | → | |
| 12 | 6.93 | M | 0.45 | 0.525 | OA | 4 | ∞ | ∞ | → | |
| 13 | 9.45 | F | 0.3 | 0.45 | OCA | 1 | → | ∞ | → | |
| 14 | 13.08 | F | 0.38 | 0.3 | HPS | 2 | ∞ | → | ∞ | |
| 15 | 7.75 | M | 0.575 | 0.55 | OA | 3 | ∞ | ← | ∞ | ∞ |
| 16 | 5.1 | F | 0.3 | 0.6 | OCA | 1 | ∞ | ∞ | ∞ | |
| Percentage detection rate of crossed asymmetry in total group (n = 16) | 93.75% | 87.5% | 56.25% | |||||||
| Percentage detection rate of crossed asymmetry in group that had all stimuli (n = 9) | 100% | 88.88% | 66.66% | 55.55% | ||||||
| Right Eye | Left Eye | |||||||
|---|---|---|---|---|---|---|---|---|
| Amplitude ± SD (uV) | Latency ± SD (ms) | Amplitude ± SD (uV) | Latency ± SD (ms) | |||||
| iP1 | mP1 | iP1 | mP1 | iP1 | mP1 | LE iP1 | LE mP1 | |
| Reversal | 11.6 ± 5.3 | 15.4 ± 5.3 | 103.9 ± 5.1 | 103.0 ± 5.1 | 10.0 ± 4.7 | 14.8 ± 5.0 | 103.0 ± 5.0 | 102.1 ± 6.1 |
| Onset | 18.0 ± 5.8 | 24.6 ± 6.1 | 112.4 ± 18.4 | 109.2 ± 8.2 | 18.9 ± 10.7 | 25.1 ± 8.0 | 114.0 ± 11.2 | 125.8 ± 11.4 |
| Flash | 15.5 ± 11.7 | 23.9 ± 11.8 | 115.1 ± 19.0 | 117.1 ± 20.6 | 14.5 ± 10.4 | 22.5 ± 10.9 | 118.1 ± 20.3 | 117.3 ± 20.0 |
| R Half Field | 12.3 uV ± 3.8 | 9.6 ± 4.6 | 100.7 ± 3.4 | 100.8 ± 4.8 | 6.1 uV ± 3.0 | 7.6 uV ± 3.7 | 104.1 ± 3.9 | 104.1 ± 3.9 |
| L Half Field | 5.1 uV ± 2.9 | 7.6 ± 3.4 | 106.44 ± 9.9 | 103.6 ± 10.6 | 9.3 uV ± 2.7 | 9.1 uV ± 2.59 | 99.6.1 ± 6.1 | 99.6.1 ± 6.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liasis, A.; Handley, S.E.; Nischal, K.K. Occipital Petalia and Albinism: A Study of Interhemispheric VEP Asymmetries in Albinism with No Nystagmus. J. Clin. Med. 2019, 8, 802. https://doi.org/10.3390/jcm8060802
Liasis A, Handley SE, Nischal KK. Occipital Petalia and Albinism: A Study of Interhemispheric VEP Asymmetries in Albinism with No Nystagmus. Journal of Clinical Medicine. 2019; 8(6):802. https://doi.org/10.3390/jcm8060802
Chicago/Turabian StyleLiasis, Alkiviades, Sian E. Handley, and Ken K. Nischal. 2019. "Occipital Petalia and Albinism: A Study of Interhemispheric VEP Asymmetries in Albinism with No Nystagmus" Journal of Clinical Medicine 8, no. 6: 802. https://doi.org/10.3390/jcm8060802
APA StyleLiasis, A., Handley, S. E., & Nischal, K. K. (2019). Occipital Petalia and Albinism: A Study of Interhemispheric VEP Asymmetries in Albinism with No Nystagmus. Journal of Clinical Medicine, 8(6), 802. https://doi.org/10.3390/jcm8060802





