Drop of Butyrylcholinesterase Activity after Cyclophosphamide Conditioning as a Predictive Marker of Liver Transplant-Related Complications and Its Correlation with Transplant-Related Mortality in Pediatric Hematopoietic Stem Cell Recipients
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. HSCT Procedure
2.3. Liver Disease at Transplant Assessment
2.4. Prophylaxis of Transplant-Related Liver Complications
2.5. Criteria for the Diagnosis of Transplant-Related Liver Complications
2.6. Statistical Analysis
2.7. Analysis of Biochemical Parameters
3. Results
3.1. Demographic Features
3.2. Analysis of the Suitability of the Prognostic Markers Chosen for the Study
3.3. Analysis of the Relationship between Patients’ Variables and the Reduction in the Serum BChE Activity Level
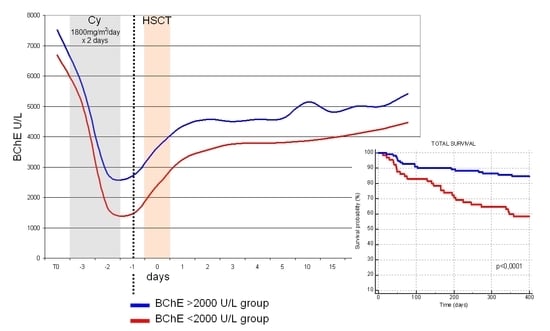
3.4. Analysis of the Relationship between the Reduction in Serum BChE Activity Level and the Transplant Outcomes
3.5. Analysis of Patient and Disease Variables Associated with the Risk of Severe Liver Damage Development
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
Consent for Publication
References
- Niederwieser, D.; Baldomero, H.; Szer, J.; Gratwohl, M.; Aljurf, M.; Atsuta, Y.; Bouzas, L.F.; Confer, D.; Greinix, H.; Horowitz, M.; et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016, 51, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, S.; Smith, F.O. Hematopoietic stem cell transplantation for childhood malignancies of myeloid origin. Bone Marrow Transplant. 2008, 41, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessop, H.; Farge, D.; Saccardi, R.; Alexander, T.; Rovira, M.; Sharrack, B.; Greco, R.; Wulffraat, N.; Moore, J.; Kazmi, M.; et al. General information for patients and carers considering haematopoietic stem cell transplantation (HSCT) for severe autoimmune diseases (ADs): A position statement from the EBMT Autoimmune Diseases Working Party (ADWP), the EBMT Nurses Group, the EBMT Patient, Family and Donor Committee and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. 2019. [Google Scholar] [CrossRef]
- Thorvaldson, L.; Remberger, M.; Winiarski, J.; Omazic, B.; Fischler, B.; Sundin, M. HLA, GVHD, and parenteral nutrition are risk factors for hepatic complications in pediatric HSCT. Pediatr. Transplant. 2016, 20, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S.I.; Myerson, D.; Spurgeon, C.L.; Sullivan, K.M.; Storer, B.; Schoch, H.G.; Kim, S.; Flowers, M.E.; McDonald, G.B. Hepatitis C virus infection and bone marrow transplantation: A cohort study with 10-year follow-up. Hepatology 1999, 29, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, H.; Kurosawa, S.; Yakushijin, K.; Taniguchi, S.; Murata, M.; Ikegame, K.; Kobayashi, T.; Eto, T.; Miyamura, K.; Sakamaki, H. Impact of hepatitis C virus infection on clinical outcome in recipients after allogeneic hematopoietic cell transplantation. Am. J. Hematol. 2013, 88, 477–484. [Google Scholar] [CrossRef]
- McDonald, G.B.; Frieze, D. A problem-oriented approach to liver disease in oncology patients. Gut 2008, 57, 987–1003. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kawabata, H.; Kanda, J.; Uchiyama, T.; Mizumoto, C.; Kondo, T.; Yamashita, K.; Ichinohe, T.; Ishikawa, T.; Kadowaki, N.; et al. Differing impacts of pretransplant serum ferritin and C-reactive protein levels on the incidence of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2013, 97, 109–116. [Google Scholar] [CrossRef]
- Maximova, N.; Sonzogni, A.; Matarazzo, L.; Ghirardi, A.; D’Antiga, L. Vanishing Bile Ducts in the Long Term after Pediatric Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018, 24, 2250–2258. [Google Scholar] [CrossRef] [Green Version]
- Norvell, J.P. Liver disease after hematopoietic cell transplantation in adults. Transplant. Rev. 2015, 29, 8–15. [Google Scholar] [CrossRef]
- Coppell, J.A.; Richardson, P.G.; Soiffer, R.; Martin, P.L.; Kernan, N.A.; Chen, A.; Guinan, E.; Vogelsang, G.; Krishnan, A.; Giralt, S.; et al. Hepatic veno-occlusive disease following stem cell transplantation: Incidence, clinical course, and outcome. Biol. Blood Marrow Transplant. 2010, 16, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.L.; Huang, L.W.; Yin, J.; Zhang, K.N.; Xiao, K.; Qing, G.Z. Association between early serum cholinesterase activity and 30-day mortality in sepsis-3 patients: A retrospective cohort study. PLoS ONE 2018, 13, e0203128. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, X.; Tan, Q.; Yang, H.; Qi, X. Low Serum-Butyrylcholinesterase Activity as a Prognostic Marker of Mortality Associates with Poor Cardiac Function in Acute Myocardial Infarction. Clin. Lab. 2016, 62, 1093–1099. [Google Scholar] [CrossRef]
- Ba, L.; Wu, D.Q.; Qian, A.Y.; Zhang, M.; Xiong, B. Dynamic changes of serum cholinesterase activity after severe trauma. J. Zhejiang Univ. Sci. B 2014, 15, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Chiarla, C.; Giovannini, I.; Giuliante, F.; Vellone, M.; Ardito, F.; Nuzzo, G. Plasma cholinesterase correlations in acute surgical and critical illness. Minerva Chir. 2011, 66, 323–327. [Google Scholar] [PubMed]
- Lampón, N.; Hermida-Cadahia, E.F.; Riveiro, A.; Tutor, J.C. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann. Hepatol. 2012, 11, 356–363. [Google Scholar] [CrossRef]
- Feng, W.; Tang, C.; Guo, H.; Bao, Y.; Wen, X.; Xue, T.; Gong, H. Prognostic value of serum cholinesterase activities in sepsis patients. Hepato-Gastroenterology 2013, 60, 1001–1005. [Google Scholar]
- Arndt, C.; Beck, J.F.; Gruhn, B. A pediatric prognostic score for patients undergoing allogeneic hematopoietic stem cell transplantation. Eur. J. Haematol. 2014, 93, 509–515. [Google Scholar] [CrossRef]
- Maximova, N.; Schillani, G.; Simeone, R.; Maestro, A.; Zanon, D. Comparison of Efficacy and Safety of Caspofungin Versus Micafungin in Pediatric Allogeneic Stem Cell Transplant Recipients: A Retrospective Analysis. Adv. Ther. 2017, 34, 1184–1199. [Google Scholar] [CrossRef]
- Jensen, P.D.; Jensen, F.T.; Christensen, T.; Ellegaard, J. Evaluation of transfusional iron overload before and during iron chelation by magnetic resonance imaging of the liver and determination of serum ferritin in adult non-thalassaemic patients. Br. J. Haematol. 1995, 89, 880–889. [Google Scholar] [CrossRef]
- Maximova, N.; Gregori, M.; Zennaro, F.; Sonzogni, A.; Simeone, R.; Zanon, D. Hepatic Gadolinium Deposition and Reversibility after Contrast Agent-enhanced MR Imaging of Pediatric Hematopoietic Stem Cell Transplant Recipients. Radiology 2016, 281, 418–426. [Google Scholar] [CrossRef]
- Yoon, J.H.; Yoo, K.H.; Sung, K.W.; Jung, C.W.; Kim, J.S.; Hahn, S.M.; Kang, H.J.; Lee, J.H.; Im, H.J.; Ahn, J.S.; et al. Validation of treatment outcomes according to revised severity criteria from European Society for Blood and Marrow Transplantation (EBMT) for sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD). Bone Marrow Transplant. 2019. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Bahloul, M.; Baccouch, N.; Chtara, K.; Turki, M.; Turki, O.; Hamida, C.B.; Chelly, H.; Ayedi, F.; Chaari, A.; Bouaziz, M. Value of Serum Cholinesterase Activity in the Diagnosis of Septic Shock Due to Bacterial Infections. J. Intensive Care Med. 2017, 32, 346–352. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Yule, S.M.; Boddy, A.V.; Cole, M.; Price, L.; Wyllie, R.; Tasso, M.J.; Pearson, A.D.; Idle, J.R. Cyclophosphamide metabolism in children. Cancer Res. 1995, 55, 803–809. [Google Scholar]
- Shokrzadeh, M.; Ahmadi, A.; Naghshvar, F.; Chabra, A.; Jafarinejhad, M. Prophylactic efficacy of melatonin on cyclophosphamide-induced liver toxicity in mice. BioMed Res. Int. 2014, 2014, 470425. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, Z.; Liu, W.; Liu, B.; Zhang, R.; Wang, W.; Zheng, W.; Xu, F.; Wang, J.; Chen, Y. Protective effect of ALDH2 against cyclophosphamide-induced acute hepatotoxicity via attenuating oxidative stress and reactive aldehydes. Biochem. Biophys. Res. Commun. 2018, 499, 93–98. [Google Scholar] [CrossRef]
- Bodur, E. Human serum butyrylcholinesterase interactions with cisplatin and cyclophosphamide. Biochimie 2010, 92, 979–984. [Google Scholar] [CrossRef]
- Zsigmond, E.K.; Robins, G. The effect of a series of anti-cancer drugs on plasma cholinesterase activity. Can. Anaesth. Soc. J. 1972, 19, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Bellardo, A.; Pecchio, F.; Manca, A.; Sartoris, S.; Airoldi, M.; Fazio, M. Administration of cyclophosphamide determines the lowering of serum cholinesterase levels. Boll. Soc. Ital. Biol. Sper. 1980, 56, 1329–1333. [Google Scholar]
- Wang, R.I.; Ross, C.A. Prolonged apnea following succinylcholine in cancer patients receiving AB-132. Anesthesiology 1963, 24, 363–367. [Google Scholar] [CrossRef]
- Al-Jafari, A.A.; Duhaiman, A.S.; Kamal, M.A. Inhibition of human acetylcholinesterase by cyclophosphamide. Toxicology 1995, 96, 1–6. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Jabbour, E.J.; Mohty, M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2019. [Google Scholar] [CrossRef]
- Maximova, N.; Gregori, M.; Boz, G.; Simeone, R.; Zanon, D.; Schillani, G.; Zennaro, F. MRI-based evaluation of multiorgan iron overload is a predictor of adverse outcomes in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Oncotarget 2017, 8, 79650–79661. [Google Scholar] [CrossRef] [Green Version]
- Barker, C.C.; Butzner, J.D.; Anderson, R.A.; Brant, R.; Sauve, R.S. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2003, 32, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Maximova, N.; Ferrara, G.; Minute, M.; Pizzol, A.; Kiren, V.; Montico, M.; Gregori, M.; Tamaro, P. Experience from a single paediatric transplant centre with identification of some protective and risk factors concerning the development of hepatic veno-occlusive disease in children after allogeneic hematopoietic stem cell transplant. Int. J. Hematol. 2014, 99, 766–772. [Google Scholar] [CrossRef]
- Dalle, J.H.; Giralt, S.A. Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation: Risk Factors and Stratification, Prophylaxis, and Treatment. Biol. Blood Marrow Transplant. 2016, 22, 400–409. [Google Scholar] [CrossRef]
- Anasetti, C. What are the most important donor and recipient factors affecting the outcome of related and unrelated allogeneic transplantation? Best Pract. Res. Clin. Haematol. 2008, 21, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Altès, A.; Remacha, A.F.; Sureda, A.; Martino, R.; Briones, J.; Canals, C.; Brunet, S.; Sierra, J.; Gimferrer, E. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002, 29, 987–989. [Google Scholar] [CrossRef] [Green Version]
- Morabito, F.; Gentile, M.; Gay, F.; Bringhen, S.; Mazzone, C.; Vigna, E.; Musto, P.; Di Raimondo, F.; Palumbo, A. Insights into defibrotide: An updated review. Expert Opin. Biol. Ther. 2009, 9, 763–772. [Google Scholar] [CrossRef]
- Palomo, M.; Diaz-Ricart, M.; Rovira, M.; Escolar, G.; Carreras, E. Defibrotide prevents the activation of macrovascular and microvascular endothelia caused by soluble factors released to blood by autologous hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 497–506. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives—A position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015, 50, 781–789. [Google Scholar] [CrossRef]
- Richardson, P.G.; Carreras, E.; Iacobelli, M.; Nejadnik, B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018, 2, 1495–1509. [Google Scholar] [CrossRef]
- Defitelio (Defibrotide Sodium). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/defitelio-defibrotide-sodium (accessed on 28 October 2018).
- Park, Y.D.; Yasui, M.; Yoshimoto, T.; Chayama, K.; Shimono, T.; Okamura, T.; Inoue, M.; Yumura-Yagi, K.; Kawa-Ha, K. Changes in hemostatic parameters in hepatic veno-occlusive disease following bone marrow transplantation. Bone Marrow Transplant. 1997, 19, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Pihusch, M.; Wegner, H.; Goehring, P.; Salat, C.; Pihusch, V.; Hiller, E.; Andreesen, R.; Kolb, H.J.; Holler, E.; Pihusch, R. Diagnosis of hepatic veno-occlusive disease by plasminogen activator inhibitor-1 plasma antigen levels: A prospective analysis in 350 allogeneic hematopoietic stem cell recipients. Transplantation 2005, 80, 1376–1382. [Google Scholar] [CrossRef]
- Ravaioli, F.; Colecchia, A.; Alemanni, L.V.; Vestito, A.; Dajti, E.; Marasco, G.; Sessa, M.; Pession, A.; Bonifazi, F.; Festi, D. Role of imaging techniques in liver veno-occlusive disease diagnosis: Recent advances and literature review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 463–484. [Google Scholar] [CrossRef]
- Colecchia, A.; Ravaioli, F.; Sessa, M.; Alemanni, V.L.; Dajti, E.; Marasco, G.; Vestito, A.; Zagari, R.M.; Barbato, F.; Arpinati, M.; et al. Liver Stiffness Measurement Allows Early Diagnosis of Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome in Adult Patients Who Undergo Hematopoietic Stem Cell Transplantation: Results from a Monocentric Prospective Study. Biol. Blood Marrow Transplant. 2019, 25, 995–1003. [Google Scholar] [CrossRef] [Green Version]




| Pre-Transplant Baseline Characteristics | Whole Cohort |
|---|---|
| Number of patients (%) | 176 (100) |
| Sex: | |
| Male (%) | 110 (62.5) |
| Female (%) | 66 (37.5) |
| Age at transplant, years (mean [± SD]) | 9.2 (5.1) |
| Underlying disease, number (%): | |
| Acute lymphoblastic leukemia | 65 (36.9) |
| Acute myeloid leukemia | 33 (18.8) |
| Myelodysplastic syndrome | 22 (11.9) |
| Solid tumour | 22 (11.9) |
| Nonmalignant disorders | 34 (19.3) |
| Disease risk, number (%): * | |
| Low | 109 (61.9) |
| High | 67 (38.1) |
| Myeloablative conditioning, number (%): | |
| MCHT-based | 113 (64.2) |
| TBI-based | 63 (35.8) |
| Cyclophosphamide total dose, mg/m2, (mean [± SD]) | 3499.4 (217.7) |
| Type of transplantation, number (%): | |
| Allogeneic | 162 (92.0) |
| Autologous | 14 (8.0) |
| Graft source, number (%): | |
| Bone marrow | 127 (72.2) |
| Peripheral blood stem cells | 39 (22.2) |
| Umbilical cord blood | 10 (5.7) |
| Allogeneic donor type, number (%): | |
| Matched related donor | 51 (31.5) |
| Matched unrelated donor | 77 (47.5) |
| Haploidentical donor | 34 (21.0) |
| Liver disorders at transplant, number (%): | |
| Siderosis | 126 (71.6) |
| Viral hepatitis | 11 (6.3) |
| Metabolic liver disease | 11 (6.3) |
| Autoimmune hepatitis | 5 (2.8) |
| Biochemical Markers * | AUC—ROC (95% CI) | ROC—Significant Value (p) | Maximum Youden Index (Cut-Off) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| C-reactive protein (mg/dL) | 0.591 (0.514–0.664) | NS | 0.5 | 86.2 | 34.7 |
| γGT (U/mL) | 0.607 (0.530–0.681) | NS | 77 | 27.6 | 93.7 |
| ALT (U/mL) | 0.598 (0.521–0.672) | NS | 28 | 55.2 | 65.5 |
| AST (U/mL) | 0.554 (0.477–0.630) | NS | 20 | 62.1 | 57.2 |
| Total bilirubin (mg/dL) | 0.583 (0.506–0.657) | NS | 0.29 | 100 | 22.1 |
| BChE (U/L) | 0.800 (0.733–0.857) | <0.0001 | 1799 | 72.4 | 80.8 |
| Variables | BChE > 2000 U/L | BChE < 2000 U/L | p-Value * |
|---|---|---|---|
| Number of patients (%) | 111 (63.1) | 65 (36.9) | - |
| Gender: | |||
| Male (%) | 73 (65.8) | 41 (63.1) | NS |
| Female (%) | 38 (34.2) | 24 (36.9) | - |
| Age at transplant, (years, median [IQR]) | 9 (4–13) | 9 (5–14) | NS ** |
| Underlying disease, number (%): | |||
| Acute lymphoblastic leukemia | 36 (32.4) | 29 (44.6) | NS |
| Acute myeloid leukemia | 25 (22.5) | 8 (12.3) | NS |
| Myelodysplastic syndrome | 16 (14.4) | 6 (9.2) | NS |
| Solid tumor | 11 (9.9) | 11 (16.9) | NS |
| Nonmalignant disorders | 23 (20.7) | 11 (16.9) | NS |
| Disease risk, number (%): | |||
| Low | 86 (77.5) | 24 (36.9) | <0.0001 |
| High | 25 (22.5) | 41 (63.1) | - |
| Myeloablative conditioning, number (%): | |||
| MCHT-based | 78 (70.3) | 35 (53.8) | <0.05 |
| TBI-based | 33 (29.7) | 30 (46.2) | - |
| Liver iron overload, number (%): | |||
| Absent | 34 (30.6) | 16 (24.6) | NS |
| Mild | 22 (19.8) | 9 (13.8) | NS |
| Moderate | 22 (19.8) | 8 (12.3) | NS |
| Severe | 33 (29.7) | 32 (49.2) | <0.05 |
| Baseline BChE value, (U/L, median [IQR]) | 7789 (6028–9218) | 6780 (4889–8031) | <0.05 ** |
| Serum ferritin, (μg/L, median [IQR]) | 989 (343–2012) | 1680 (402–2877) | NS ** |
| Liver disease, number (%) | 16 (14.4) | 17 (26.1) | NS |
| Defibrotide prophylaxis, number (%) | 41 (36.9) | 27 (41.5) | NS |
| UDCA prophylaxis, number (%) | 109 (98.2) | 60 (92.3) | NS |
| HDNAC prophylaxis, number (%) | 72 (64.8) | 39 (60.0) | NS |
| Variables | BChE Drop < 70% | BChE Drop > 70% | p-Value * |
|---|---|---|---|
| Number of patients (%) | 94 (53.4) | 82 (46.6) | - |
| Gender: | |||
| Male (%) | 66 (70.2) | 48 (58.5) | NS |
| Female (%) | 28 (29.8) | 34 (41.5) | - |
| Age at transplant, (years, median [IQR]) | 7 (4–13) | 10 (6–13) | NS ** |
| Underlying disease, number (%): | |||
| Acute lymphoblastic leukemia | 26 (27.7) | 39 (47.6) | <0.001 |
| Acute myeloid leukemia | 20 (19.7) | 13 (9.7) | NS |
| Myelodysplastic syndrome | 16 (17.0) | 6 (7.3) | NS |
| Solid tumor | 12 (12.8) | 10 (12.2) | NS |
| Nonmalignant disorders | 20 (19.7) | 14 (17.1) | NS |
| Disease risk, number (%): | |||
| Low | 67 (71.3) | 34 (41.5) | <0.0001 |
| High | 27 (28.7) | 48 (58.5) | - |
| Myeloablative conditioning, number (%): | |||
| MCHT-based | 71 (75.5) | 42 (51.2) | <0.001 |
| TBI-based | 23 (24.4) | 40 (48.8) | - |
| Liver iron overload, number (%): | |||
| Absent | 29 (30.9) | 21 (25.6) | NS |
| Mild | 19 (20.2) | 12 (14.6) | NS |
| Moderate | 20 (21.3) | 10 (12.2) | NS |
| Severe | 26 (27.7) | 39 (47.6) | <0.05 |
| Baseline BChE value, (U/L, median [IQR]) | 6815 (5115–8361) | 7897 (6706–9408) | <0.001 ** |
| Serum ferritin, (μg/L, median [IQR]) | 950.1 (322–2135) | 1474.4 (406–2440) | NS ** |
| Liver disease, number (%): | 19 (20.2) | 16 (19.5) | NS |
| Defibrotide prophylaxis, number (%) | 31 (33.0) | 37 (45.1) | NS |
| UDCA prophylaxis, number (%) | 92 (97.9) | 77 (93.9) | NS |
| HDNAC prophylaxis, number (%) | 58 (61.7) | 53 (64.6) | NS |
| Variables | BChE > 2000 U/L | BChE < 2000 U/L | p-Value * |
|---|---|---|---|
| Patients, number (%) | 111 (63.1) | 65 (36.9) | - |
| Liver dysfunction, number (%) | 47 (42.3) | 42 (64.6) | <0.05 |
| SOS, number (%) | 5 (4.5) | 11 (16.9) | <0.05 |
| Allogeneic recipients, number (%) | 103 (58.5) | 59 (33.5) | - |
| Liver GVHD, number (%): # | |||
| Absent | 68 (66.0) | 33 (55.9) | NS |
| I grade | 12 (11.6) | 4 (6.8) | NS |
| II grade | 8 (7.8) | 8 (13.6) | NS |
| III grade | 10 (9.7) | 7 (11.9) | NS |
| IV grade | 5 (4.9) | 7 (11.9) | NS |
| Overall survival, number (%) | 94 (84.7) | 38 (58.5) | <0.001 |
| Death, number (%): | 17 (15.3) | 27 (41.5) | <0.001 |
| Transplant-related | 9 (8.1) | 15 (23.1) | <0.05 |
| Relapse | 8 (7.2) | 12 (18.5) | <0.05 |
| Variables | BChE Drop < 70% | BChE Drop > 70% | p-Value * |
|---|---|---|---|
| Patients, number (%) | 94 (53.4) | 82 (46.6) | - |
| Liver dysfunction, number (%) | 53 (56.4) | 36 (43.9) | NS |
| SOS, number (%) | 2 (2.1) | 14 (17.1) | <0.005 |
| Allogeneic recipients, number (%) | 86 (48.9) | 76 (43.2) | - |
| Liver GVHD, number (%): # | |||
| Absent | 57 (66.3) | 44 (57.9) | NS |
| I grade | 8 (9.3) | 8 (10.5) | NS |
| II grade | 8 (9.3) | 8 (10.5) | NS |
| III grade | 7 (8.1) | 10 (13.2) | NS |
| IV grade | 6 (7.0) | 6 (7.9) | NS |
| Overall survival, number (%) | 75 (79.8) | 57 (75.0) | NS |
| Death, number (%): | 19 (20.2) | 25 (30.5) | NS |
| Transplant-related | 9 (9.6) | 15 (18.3) | NS |
| Relapse | 10 (10.6) | 10 (12.2) | NS |
| Variables | Severe Liver Damage | Liver Damage Absent or Mild | p-Value * |
|---|---|---|---|
| Number of patients (%) | 44 (25) | 132 (75) | - |
| Underlying disease, number (%): | |||
| Acute lymphoblastic leukemia | 18 (40.9) | 47 (35.6) | NS |
| Acute myeloid leukemia | 11 (25) | 22 (16.7) | NS |
| Other | 15 (34.1) | 63 (47.7) | NS |
| Disease risk, number (%): | |||
| Low | 12 (27.3) | 97 (73.5) | <0.0001 |
| High | 32 (72.7) | 35 (26.5) | - |
| Myeloablative conditioning, number (%) | |||
| MCHT-based | 31 (70.5) | 82 (62.1) | NS |
| TBI-based | 13 (29.5) | 50 (37.9) | NS |
| Iron overload, number (%) | |||
| From absent to moderate | 13 (29.5) | 98 (74.2) | <0.0001 |
| Severe | 31 (70.5) | 34 (25.8) | <0.0001 |
| Baseline BChE value, (U/L, median [IQR]) | 6815 (4934–8640) | 7655(5773–8827) | NS ** |
| Serum ferritin, (μg/L, median [IQR]) | 2052 (1329–3112) | 974 (329–2048) | <0.05 ** |
| Pre-transplant liver disease, number (%): | 9 (20.4) | 28 (21.2) | NS |
| Defibrotide prophylaxis, number (%) | - | 68 (51.5) | <0.0001 |
| HDNAC prophylaxis, number (%) | 25 (56.8) | 86 (65.2) | NS |
| Overall survival, number (%) | 19 (43.2) | 113 (85.6) | <0.0001 |
| Death, number (%): | 25 (56.8) | 19 (14.4) | <0.0001 |
| Transplant-related | 23 (52.3) | 1 (0.8) | <0.0001 |
| Relapse | 2 (4.5) | 18 (13.6) | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maximova, N.; Caddeo, G.; Zanon, D.; Maestro, A.; Simeone, R. Drop of Butyrylcholinesterase Activity after Cyclophosphamide Conditioning as a Predictive Marker of Liver Transplant-Related Complications and Its Correlation with Transplant-Related Mortality in Pediatric Hematopoietic Stem Cell Recipients. J. Clin. Med. 2019, 8, 825. https://doi.org/10.3390/jcm8060825
Maximova N, Caddeo G, Zanon D, Maestro A, Simeone R. Drop of Butyrylcholinesterase Activity after Cyclophosphamide Conditioning as a Predictive Marker of Liver Transplant-Related Complications and Its Correlation with Transplant-Related Mortality in Pediatric Hematopoietic Stem Cell Recipients. Journal of Clinical Medicine. 2019; 8(6):825. https://doi.org/10.3390/jcm8060825
Chicago/Turabian StyleMaximova, Natalia, Giulia Caddeo, Davide Zanon, Alessandra Maestro, and Roberto Simeone. 2019. "Drop of Butyrylcholinesterase Activity after Cyclophosphamide Conditioning as a Predictive Marker of Liver Transplant-Related Complications and Its Correlation with Transplant-Related Mortality in Pediatric Hematopoietic Stem Cell Recipients" Journal of Clinical Medicine 8, no. 6: 825. https://doi.org/10.3390/jcm8060825
APA StyleMaximova, N., Caddeo, G., Zanon, D., Maestro, A., & Simeone, R. (2019). Drop of Butyrylcholinesterase Activity after Cyclophosphamide Conditioning as a Predictive Marker of Liver Transplant-Related Complications and Its Correlation with Transplant-Related Mortality in Pediatric Hematopoietic Stem Cell Recipients. Journal of Clinical Medicine, 8(6), 825. https://doi.org/10.3390/jcm8060825