Post-Infectious Myocardial Infarction: New Insights for Improved Screening
Abstract
:1. Background
2. Methods
2.1. Patients
2.2. Definitions
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Angiographic Data
3.3. Hospital Outcomes
4. Discussion
4.1. Type of Infection and MI
4.2. Prognosis of Respiratory Tract Infection in MI
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, P.R.; Khaw, K.T.; Plummer, M.; Foley, A.; Meade, T.W. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: Winter infections and death from cardiovascular disease. Lancet Lond. Engl. 1994, 343, 435–439. [Google Scholar] [CrossRef]
- Barnes, M.; Heywood, A.E.; Mahimbo, A.; Rahman, B.; Newall, A.T.; Macintyre, C.R. Acute myocardial infarction and influenza: A meta-analysis of case-control studies. Heart Br. Card. Soc. 2015, 101, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Collier, J.; Bhagat, K. Infection, inflammation, and infarction: Does acute endothelial dysfunction provide a link? Lancet Lond. Engl. 1997, 349, 1391–1392. [Google Scholar] [CrossRef]
- Cangemi, R.; Casciaro, M.; Rossi, E.; Calvieri, C.; Bucci, T.; Calabrese, C.M.; Taliani, G.; Falcone, M.; Palange, P.; Bertazzoni, G.; et al. Platelet Activation Is Associated with Myocardial Infarction in Patients with Pneumonia. J. Am. Coll. Cardiol. 2014, 64, 1917–1925. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar]
- Sandoval, Y.; Jaffe, A.S. Type 2 Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 1846–1860. [Google Scholar] [CrossRef]
- Baron, T.; Hambraeus, K.; Sundström, J.; Erlinge, D.; Jernberg, T.; Lindahl, B.; TOTAL-AMI study group. Type 2 myocardial infarction in clinical practice. Heart 2015, 101, 101–106. [Google Scholar] [CrossRef]
- Landes, U.; Bental, T.; Orvin, K.; Vaknin-Assa, H.; Rechavia, E.; Iakobishvili, Z.; Lev, E.; Assali, A.; Kornowski, R. Type 2 myocardial infarction: A descriptive analysis and comparison with type 1 myocardial infarction. J. Cardiol. 2016, 67, 51–56. [Google Scholar] [CrossRef]
- Stein, G.Y.; Herscovici, G.; Korenfeld, R.; Matetzky, S.; Gottlieb, S.; Alon, D.; Gevrielov-Yusim, N.; Iakobishvili, Z.; Fuchs, S. Type-II Myocardial Infarction—Patient Characteristics, Management and Outcomes. PLoS ONE 2014, 9, e84285. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cangemi, R.; Falcone, M.; Taliani, G.; Pieralli, F.; Vannucchi, V.; Nozzoli, C.; Venditti, M.; Chirinos, J.A.; Corrales-Medina, V.F.; et al. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association Between Hospitalization for Pneumonia and Subsequent Risk of Cardiovascular Disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Aliberti, S.; Mirsaeidi, M.; Peyrani, P.; Filardo, G.; Amir, A.; Moffett, B.; Gordon, J.; Blasi, F.; Bordon, J. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Steg, P.G.; Ravisy, J.; Lorgis, L.; Laurent, Y.; Sicard, P.; Janin-Manificat, L.; Beer, J.-C.; Makki, H.; Lagrost, A.-C.; et al. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation 2008, 118, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Thygesen, K.; Alpert, J.S.; et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Saaby, L.; Poulsen, T.S.; Hosbond, S.; Larsen, T.B.; Pyndt Diederichsen, A.C.; Hallas, J.; Thygesen, K.; Mickley, H. Classification of Myocardial Infarction: Frequency and Features of Type 2 Myocardial Infarction. Am. J. Med. 2013, 126, 789–797. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, M.M.; Lillo, J.A.; Diez De Los Ríos, M.J.; Rodríguez, M.; Dayaldasani, A.; Yahyaoui, R.; Pérez, V. Analytical performance of a sensitive assay for cardiac troponin I with loci technology. Clin. Biochem. 2010, 43, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Morel, M.-A.; Kappetein, A.P.; Morice, M.-C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroInterv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2005, 1, 219–227. [Google Scholar]
- Madjid, M.; Casscells, S.W. Of birds and men: Cardiologists’ role in influenza pandemics. Lancet Lond. Engl. 2004, 364, 1309. [Google Scholar] [CrossRef]
- Madjid, M.; Miller, C.C.; Zarubaev, V.V.; Marinich, I.G.; Kiselev, O.I.; Lobzin, Y.V.; Filippov, A.E.; Casscells, S.W. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: Results from 8 years of autopsies in 34,892 subjects. Eur. Heart J. 2007, 28, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Serpa, J.; Rueda, A.M.; Giordano, T.P.; Bozkurt, B.; Madjid, M.; Tweardy, D.; Musher, D.M. Acute Bacterial Pneumonia is Associated With the Occurrence of Acute Coronary Syndromes. Medicine (Baltimore) 2009, 88, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Suh, K.N.; Rose, G.; Chirinos, J.A.; Doucette, S.; Cameron, D.W.; Fergusson, D.A. Cardiac Complications in Patients with Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis of Observational Studies. PLoS Med. 2011, 8, e1001048. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.S.; Jaber, R.; Lindenauer, P.K.; Garb, J.L.; Fitzgerald, J.; Rothberg, M.B. Death among patients hospitalized with pneumonia: Implications for hospital outcome measures. JAMA Intern. Med. 2015, 175, 851–853. [Google Scholar] [CrossRef]
- Jafarzadeh, S.R.; Thomas, B.S.; Warren, D.K.; Gill, J.; Fraser, V.J. Longitudinal Study of the Effects of Bacteremia and Sepsis on 5-year Risk of Cardiovascular Events. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Rae, N.; Finch, S.; Chalmers, J.D. Cardiovascular disease as a complication of community-acquired pneumonia. Curr. Opin. Pulm. Med. 2016, 22, 212–218. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. Prevalence, pathogenesis, therapy, and prevention of cardiovascular events in patients with community-acquired pneumonia. Pneumonia 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Della Valle, P.; Calvieri, C.; Taliani, G.; Ferroni, P.; Falcone, M.; Carnevale, R.; Bartimoccia, S.; D’Angelo, A.; Violi, F.; et al. Low-grade endotoxemia and clotting activation in the early phase of pneumonia. Respirol. Carlton Vic. 2016, 21, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Cangemi, R.; Farcomeni, A.; Calvieri, C.; Barillà, F.; Scarpellini, M.G.; Bertazzoni, G.; Palange, P.; Taliani, G.; et al. Lower Mortality Rate in Elderly Patients With Community-Onset Pneumonia on Treatment With Aspirin. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2015, 4. [Google Scholar] [CrossRef]
- Oz, F.; Gul, S.; Kaya, M.G.; Yazici, M.; Bulut, I.; Elitok, A.; Ersin, G.; Abakay, O.; Akkoyun, C.D.; Oncul, A.; et al. Does aspirin use prevent acute coronary syndrome in patients with pneumonia: Multicenter prospective randomized trial. Coron. Artery Dis. 2013, 24, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Putot, A.; Derrida, S.B.; Zeller, M.; Avondo, A.; Ray, P.; Manckoundia, P.; Cottin, Y. Short-Term Prognosis of Myocardial Injury, Type 1 and Type 2 Myocardial Infarction in the Emergency Unit. Am. J. Med. 2018, 131, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Putot, A.; Jeanmichel, M.; Chagué, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Type 2 Myocardial Infarction: A Geriatric Population-based Model of Pathogenesis. Aging Dis. 2019, in press. [Google Scholar]
- Ewig, S.; Birkner, N.; Strauss, R.; Schaefer, E.; Pauletzki, J.; Bischoff, H.; Schraeder, P.; Welte, T.; Hoeffken, G. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009, 64, 1062–1069. [Google Scholar] [CrossRef] [Green Version]
- Ruane, L.; Buckley, T.; Hoo, S.Y.S.; Hansen, P.S.; McCormack, C.; Shaw, E.; Fethney, J.; Tofler, G.H. Triggering of acute myocardial infarction by respiratory infection: Infarction and respiratory infection. Intern. Med. J. 2017, 47, 522–529. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Gupta, N.; Guo, Y.; Bangalore, S. Comparison of Outcomes of Patients with Sepsis with Versus Without Acute Myocardial Infarction and Comparison of Invasive Versus Noninvasive Management of the Patients with Infarction. Am. J. Cardiol. 2016, 117, 1065–1071. [Google Scholar] [CrossRef]
- Nowak, R.M.; Jacobsen, G.; Christenson, R.H.; Moyer, M.; Hudson, M.; McCord, J. Differentiating type 1 and 2 acute myocardial infarctions using the N-terminal pro B-type natriuretic peptide/cardiac troponin T ratio. Am. J. Emerg. Med. 2018, 36, 1849–1854. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Fan, S.; Xing, J.; Liu, H. BNP and NT-proBNP levels in patients with sepsis. Front. Biosci. Landmark Ed. 2013, 18, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Saaby, L.; Poulsen, T.S.; Diederichsen, A.C.P.; Hosbond, S.; Diederichsen, S.Z.; Larsen, T.B.; Schmidt, H.; Gerke, O.; Hallas, J.; et al. Comparison of mortality in patients with acute myocardial infarction accidentally admitted to non-cardiology departments versus that in patients admitted to coronary care units. Am. J. Cardiol. 2014, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, A.; Thomsen, R.W.; Ulrichsen, S.P.; Sørensen, H.T. Chronic heart failure and risk of hospitalization with pneumonia: A population-based study. Eur. J. Intern. Med. 2013, 24, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Blasi, F.; Dartois, N.; Akova, M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015, 70, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Kasatpibal, N.; Riis, A.; Nørgaard, M.; Sørensen, H.T. The Impact of Pre-existing Heart Failure on Pneumonia Prognosis: Population-based Cohort Study. J. Gen. Intern. Med. 2008, 23, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]

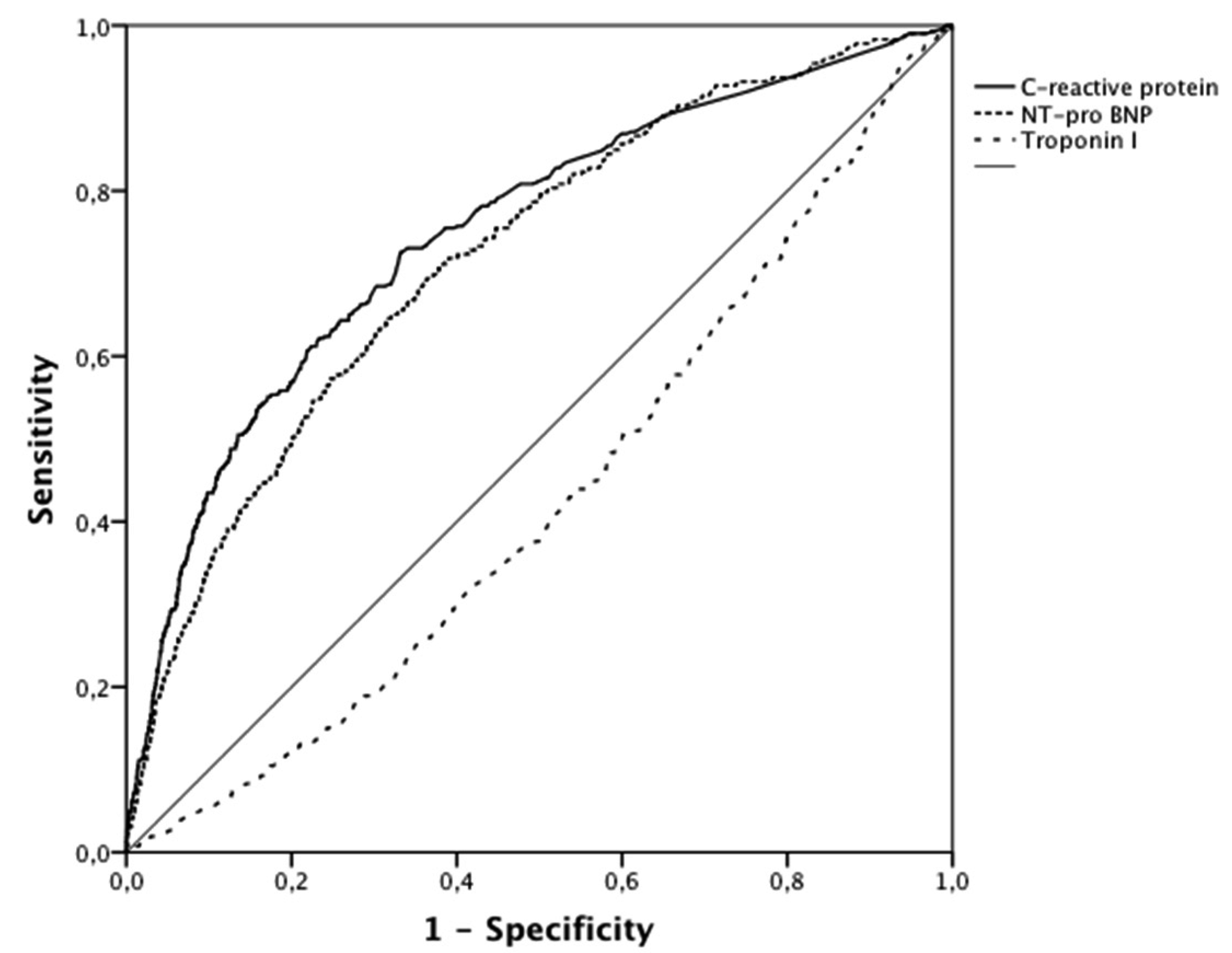
| AUC (95% CI) | Cutoff | Sensitivity | Specificity | p-Value | |
|---|---|---|---|---|---|
| C-reactive protein | 0.75 (0.72–0.78) | 8.6 mg/L | 73% | 67% | <0.001 |
| NT- pro BNP | 0.72 (0.69–0.75) | 1944 pg/mL | 65% | 68% | <0.001 |
| Troponin I | 0.43 (0.40–0.46) | 12 µg/L* | 50%* | 62%* | <0.001 |


| Post-Infectious MI n = 466 | Other MI n = 4107 | p-Value | |
|---|---|---|---|
| Risk factors and comorbidities | |||
| Age, years | 78 (66–85) | 68 (57–80) | <0.001 |
| Female | 188 (40) | 1166 (28) | <0.001 |
| BMI, kg/m² | 26 (23–30) | 26 (24–30) | 0.07 |
| Hypertension | 343 (74) | 2454 (60) | <0.001 |
| Hypercholesterolemia | 249 (53) | 2144 (52) | 0.4 |
| Family history of CAD | 134 (28) | 1330 (32) | 0.03 |
| Smoking | 78 (17) | 1232 (30) | <0.001 |
| Diabetes | 186 (40) | 1026 (25) | <0.001 |
| Chronic renal failure | 66 (14) | 217 (5) | <0.001 |
| COPD | 81 (17) | 291 (7) | <0.001 |
| Neoplasia | 90 (19) | 565 (14) | 0.003 |
| Cardiovascular history | |||
| CAD | 168 (36) | 985 (24) | <0.001 |
| Stroke | 56 (12) | 315 (8) | 0.003 |
| PAD | 84 (18) | 316 (8) | <0.001 |
| HF | 61 (13) | 187 (5) | <0.001 |
| Atrial fibrillation | 100 (21) | 400 (10) | <0.001 |
| Aortic stenosis | 50 (11) | 189 (4) | <0.001 |
| Type of MI | |||
| Type 1 | 130 (28) | 3580 (87) | <0.001 |
| Type 2 | 336 (72) | 527 (13) | <0.001 |
| Clinical data at admission | |||
| HR, beats/min | 83 (70–100) | 78 (67–90) | <0.001 |
| SBP, mmHg | 131 (114–154) | 140 (120–160) | <0.001 |
| DBP, mmHg | 72 (62–87) | 80 (70–93) | <0.001 |
| Anterior wall location | 179 (38) | 1457 (35) | 0.2 |
| GRACE risk score | 177 (151–199) | 144 (121–173) | <0.001 |
| Acute HF | 252 (54) | 957 (23) | <0.001 |
| LVEF, % | 45 (35–55) | 55 (45–60) | <0.001 |
| ECG at admission | |||
| STEMI | 181 (39) | 1969 (48) | <0.001 |
| AF/Flutter | 64 (14) | 362 (9) | <0.001 |
| LBBB | 48 (10) | 233 (6) | <0.001 |
| Biological data | |||
| Hemoglobin, g/100mL | 12.8 (11.6-14.3) | 14.2(12.9–15.3) | <0.001 |
| Leucocytes, G/L | 12.6 (9.9-14.7) | 12.0 (9.7–14.1) | <0.001 |
| CRP, mg/L | 33 (7–103) | 5 (3–13) | <0.001 |
| Creatinine, µmol/L | 93 (70–130) | 83 (70–104) | 0.003 |
| eGFR, mL/min | 60 (39–84) | 77 (57–93) | <0.001 |
| Troponin I peak, µg/L | 7 (2–29) | 13 (3–58) | <0.001 |
| NT-proBNP, pg/mL | 3800 (920–12772) | 664 (164–2685) | <0.001 |
| CK peak, IU/L | 340 (131–991) | 496 (189–1457) | <0.001 |
| Angiographic data | |||
| Coronary angiography | 365 (78) | 4006 (97) | <0.001 |
| Non-obstructive/normal | 53 (14) | 246 (6) | 0.005 |
| 3-vessel disease | 132 (36) | 1216 (30) | 0.02 |
| SYNTAX score | 11 (3–20) | 10 [5,6,7,8,9,10,11,12,13,14,15,16,17,18] | 0.8 |
| Acute management | |||
| PCI | 190 (41) | 2994 (73) | <0.001 |
| CABG | 193 (5) | 18 (4) | 0.4 |
| Hospital outcomes | |||
| ICU stay (days) | 4 (3–6) | 4 (3–5) | <0.001 |
| Hospital stay (days) | 13 (7–21) | 9 (7–12) | <0.001 |
| All cause death | 54 (11) | 234 (6) | <0.001 |
| Cardiovascular death | 48 (10) | 210 (5) | <0.001 |
| Re-infarction | 11 (2) | 136 (3) | 0.3 |
| Severe HF | 162 (35) | 511 (12) | <0.001 |
| MI Characteristics | |
|---|---|
| Ischemic chest pain | 256 (55) |
| New ECG abnormalities | 384 (82) |
| ST segment elevation | 181 (39) |
| ST segment depression | 121 (26) |
| T wave inversion | 112 (24) |
| New LBBB | 21 (5) |
| New pathological Q waves | 111 (24) |
| Imaging evidence of ischemia | 59 (13) |
| Infection characteristics | |
| Temperature >39°C | 134 (29) |
| Respiratory rate > 24/min | 153 (33) |
| Leucocytes > 12 × 109/L | 151 (32) |
| Heart rate > 100/min | 114 (24) |
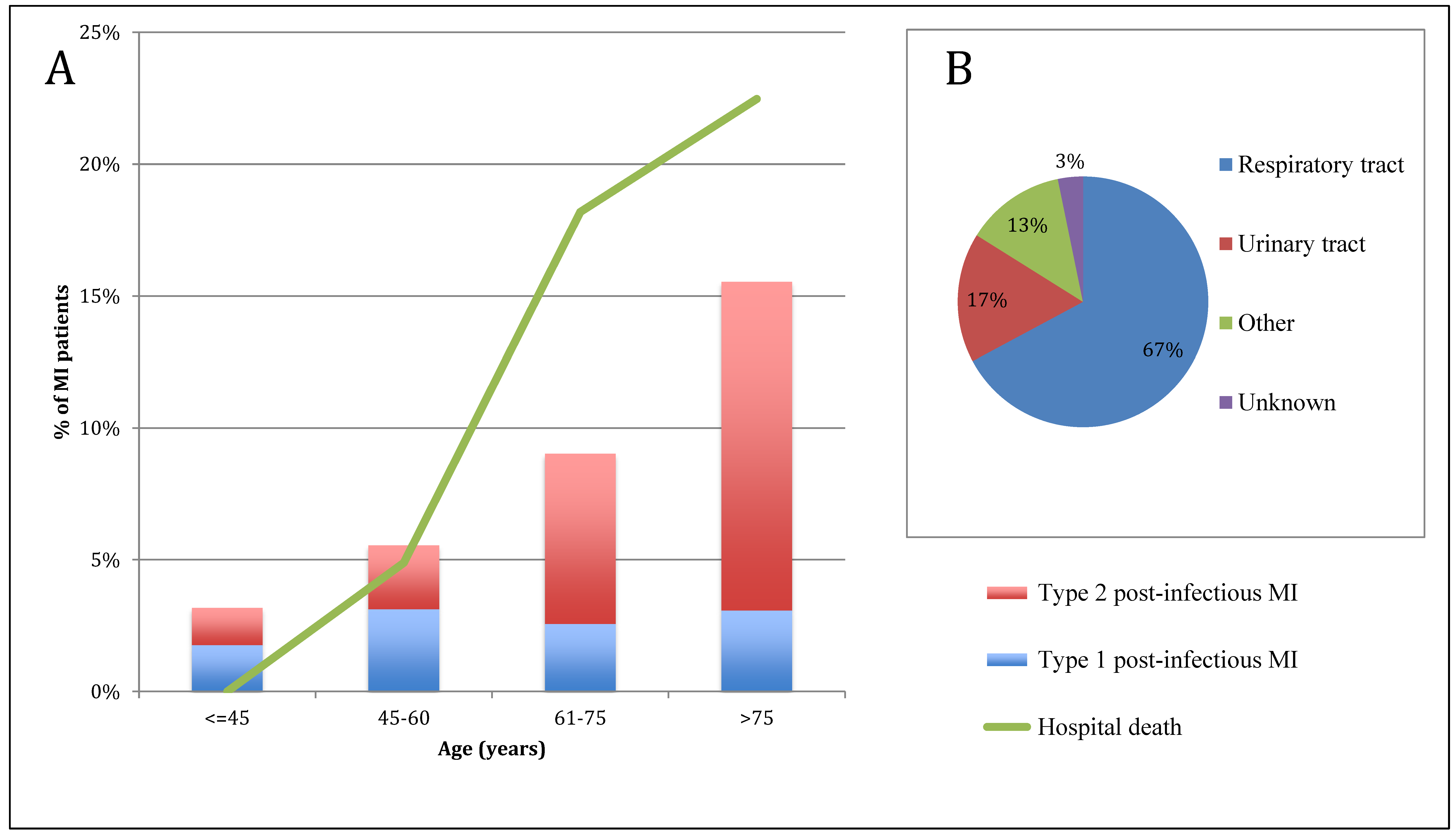
| Respiratory tract infection | 313 (67) |
| Acute bronchitis | 163 (35) |
| With microbial identification: | 12 |
| Influenzae virus | 5 |
| Parainfluenzae virus | 2 |
| Metapneumovirus | 1 |
| Rhinovirus | 2 |
| RSV | 2 |
| Acute pneumonia | 150 (32) |
| With microbial identification: | 25 |
| Streptococcus pneumoniae | 11 |
| Other Streptococcus spp. | 3 |
| Enterococcus spp. | 2 |
| Haemophilus influenzae | 2 |
| Escherichia coli | 2 |
| Pseudomonas aeruginosa | 1 |
| Citrobacter koseri | 1 |
| Hafnia alvei | 1 |
| Moraxella catarrhalis | 1 |
| Aspergillus fulmigatus | 1 |
| Urinary tract infection | 78 (17) |
| Other site infection | 60 (13) |
| Undetermined infection | 15 (3) |
| Severe HF (n = 673) | CV Mortality (n = 258) | All-Cause Mortality (n = 288) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Post-infectious MI (vs. other MI) | 1.22 | 0.85–1.76 | 0.3 | 0.87 | 0.50–1.50 | 0.6 | 0.72 | 0.43–1.20 | 0.2 |
| Type 1 MI (vs. Type 2) | 0.41 | 0.30–0.55 | <0.001 | 0.69 | 0.44–1.08 | 0.1 | 0.53 | 0.35–0.80 | 0.003 |
| GRACE Score (per point) | 1.04 | 1.03–1.04 | <0.001 | 1.03 | 1.03–1.04 | <0.001 | 1.03 | 1.03–1.04 | <0.001 |
| LVEF (per 10 %) | 0.61 | 0.56–0.67 | <0.001 | 0.66 | 0.58–0.75 | <0.001 | 0.68 | 0.60–0.76 | <0.001 |
| Troponin (per 10 µg/L) | 1.01 | 1.00–1.02 | <0.001 | 1.02 | 1.01–1.03 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| GRACE Score (per point) | 1.02 | 1.01–1.03 | <0.001 | 1.02 | 1.01–1.03 | 0.002 |
| Type 1 MI (vs. Type 2) | 0.98 | 0.53–1.87 | 1 | 2.44 | 1.12–5.29 | 0.02 |
| RTI (vs. other infection) | 1.46 | 0.77–2.76 | 0.3 | 2.89 | 1.19–6.99 | 0.02 |
| LVEF (per 10 %) | 0.66 | 0.52–0.83 | <0.001 | 0.74 | 0.55–0.99 | 0.04 |
| CRP (per 10 mg/L) | 1.06 | 1.03–1.09 | <0.001 | 1.05 | 1.02–1.09 | 0.005 |
| NT-proBNP (per 1000 pg/mL) | 1.03 | 1.01–1.04 | 0.006 | 1.02 | 1.01–1.03 | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putot, A.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Post-Infectious Myocardial Infarction: New Insights for Improved Screening. J. Clin. Med. 2019, 8, 827. https://doi.org/10.3390/jcm8060827
Putot A, Chague F, Manckoundia P, Cottin Y, Zeller M. Post-Infectious Myocardial Infarction: New Insights for Improved Screening. Journal of Clinical Medicine. 2019; 8(6):827. https://doi.org/10.3390/jcm8060827
Chicago/Turabian StylePutot, Alain, Frédéric Chague, Patrick Manckoundia, Yves Cottin, and Marianne Zeller. 2019. "Post-Infectious Myocardial Infarction: New Insights for Improved Screening" Journal of Clinical Medicine 8, no. 6: 827. https://doi.org/10.3390/jcm8060827
APA StylePutot, A., Chague, F., Manckoundia, P., Cottin, Y., & Zeller, M. (2019). Post-Infectious Myocardial Infarction: New Insights for Improved Screening. Journal of Clinical Medicine, 8(6), 827. https://doi.org/10.3390/jcm8060827






