Clinical Value of Whole Blood Procalcitonin Using Point of Care Testing, Quick Sequential Organ Failure Assessment Score, C-Reactive Protein and Lactate in Emergency Department Patients with Suspected Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
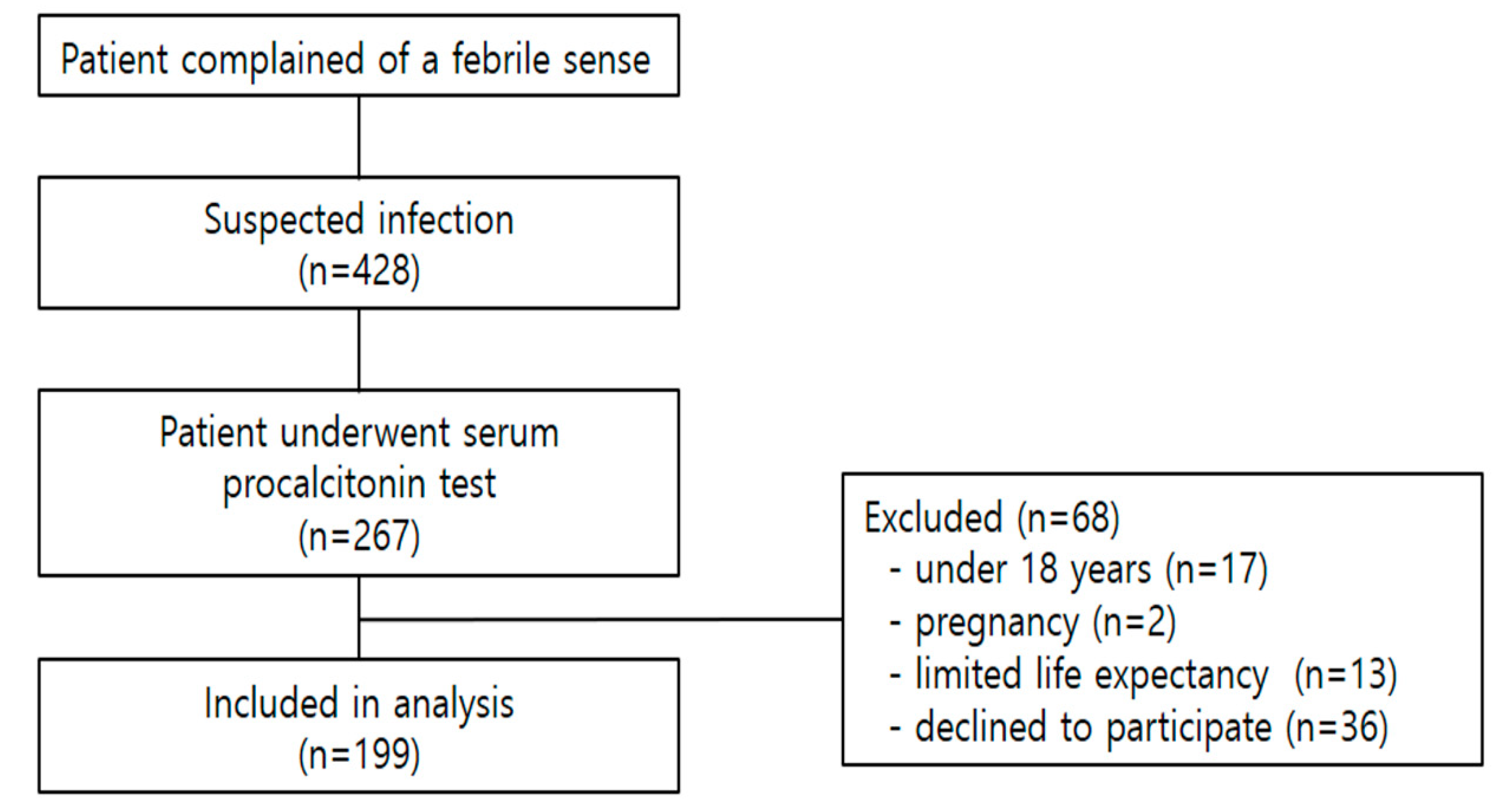
2.2. Study Participants and Inclusion Criteria
2.3. Data Sampling
2.4. Outcome Measure
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Clinical Outcome of Enrolled Patients
3.3. The Comparison of Diagnostic Values in qSOFA Score, Lactate, Serum Procalcitonin and Whole Blood Procalcitonin for Bacteremia
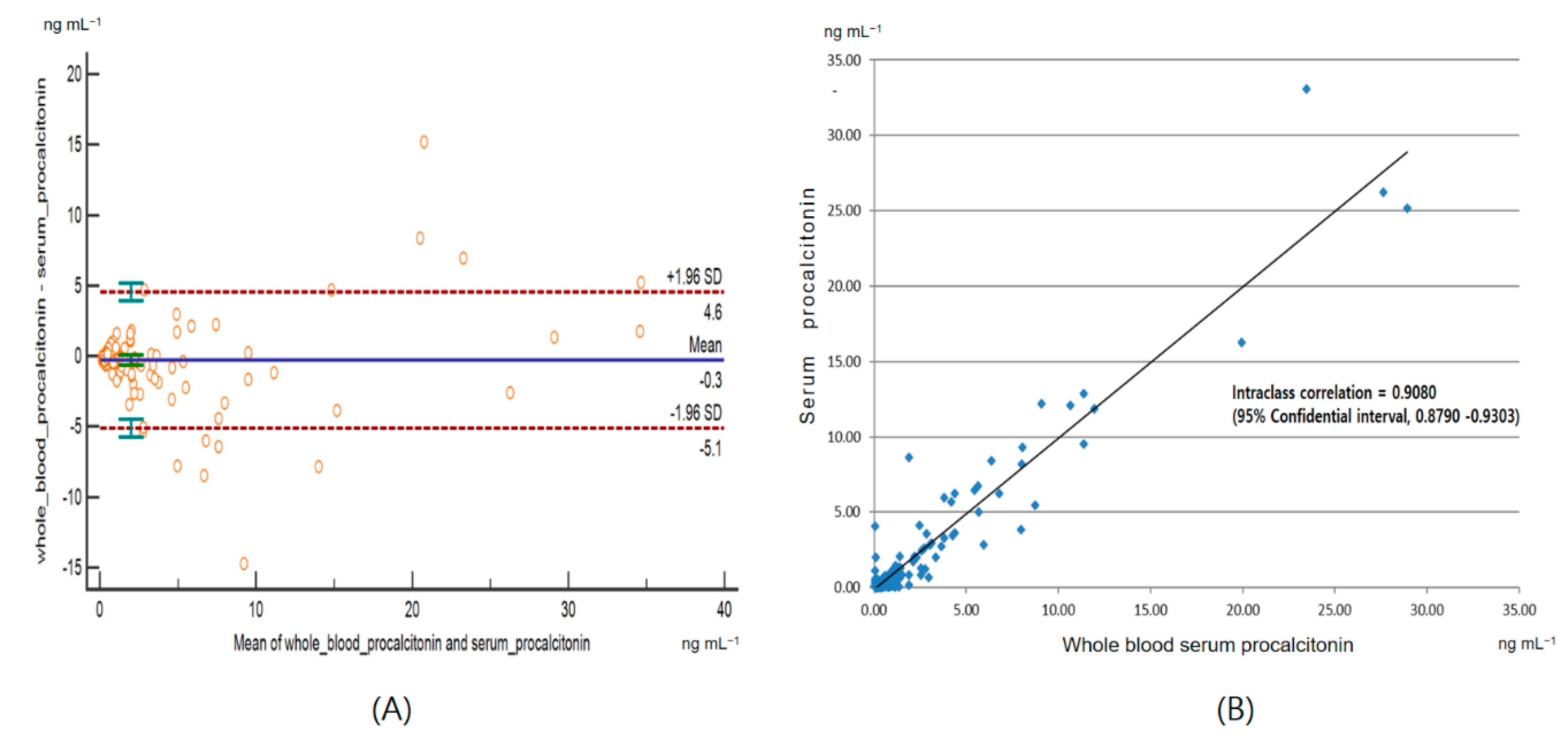
3.4. Agreement Between the Whole Blood Procalcitonin and the Serum Procalcitonin
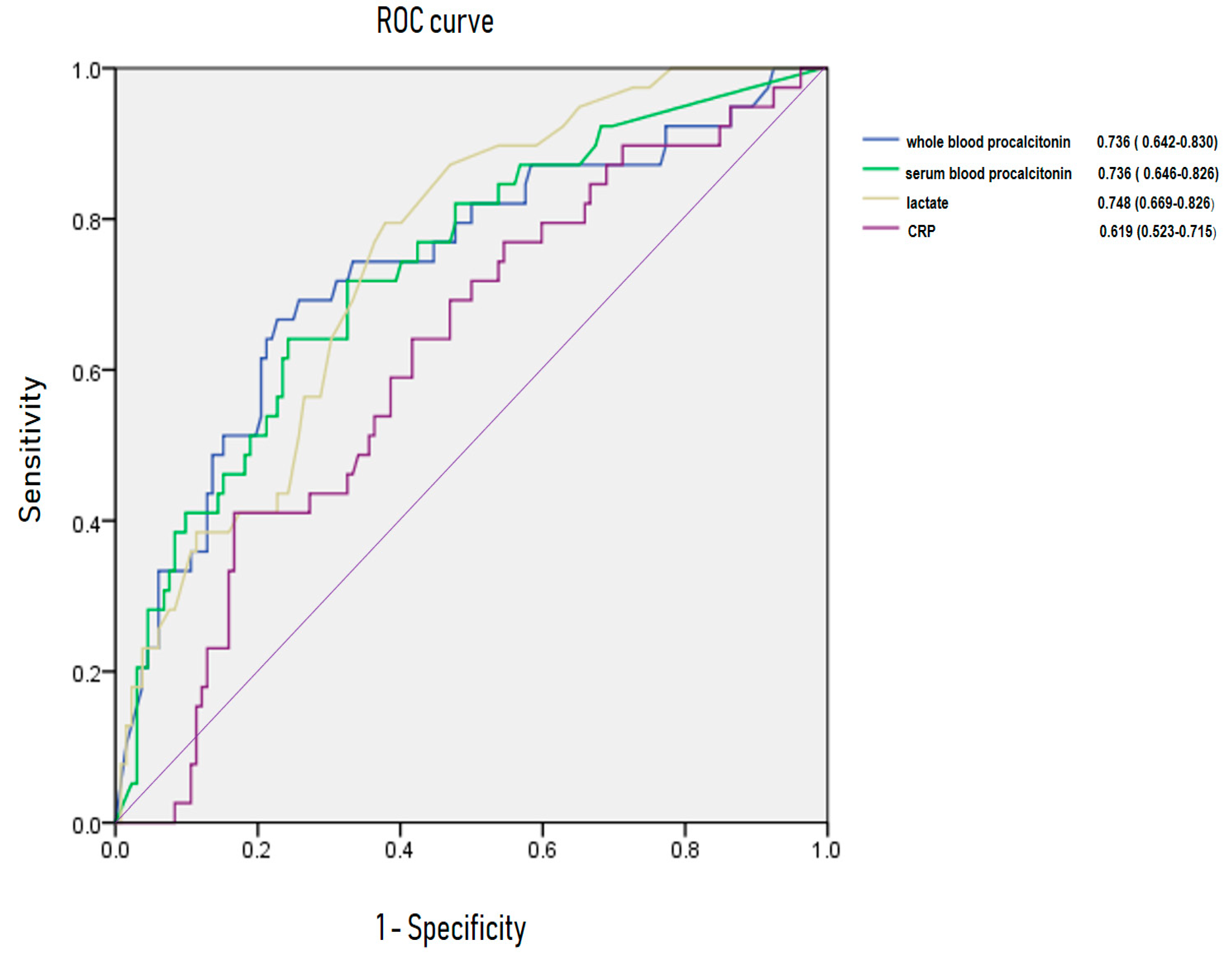
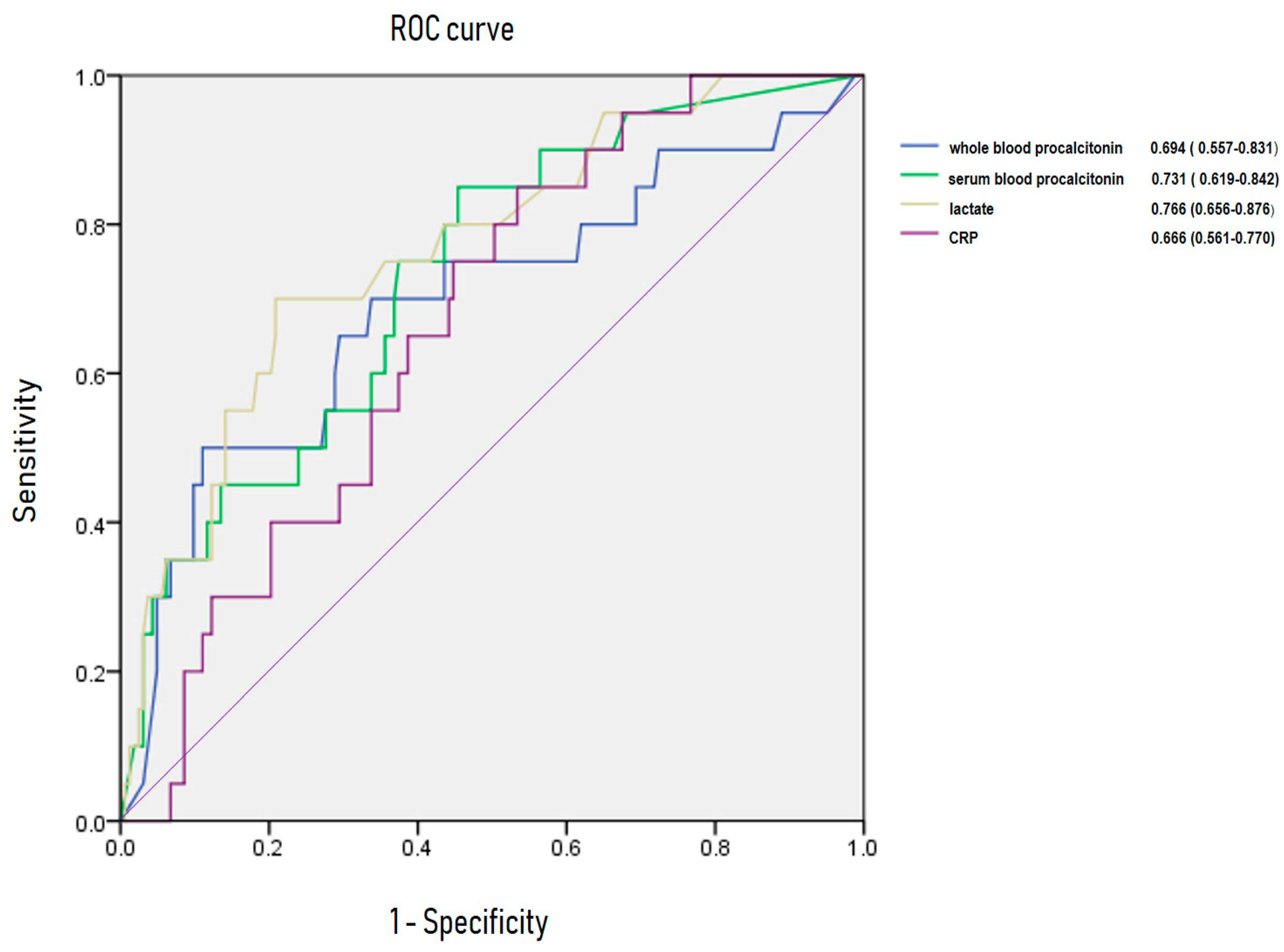
3.5. Area Under Receiving OperatingCurve of C-Reactive Protein, Lactate, Whole Blood Procalcitonin for Predicting Bacteremia and 28-Day Mortality
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; MacDonald, S.P.; Williams, J.M.; van Bockxmeer, J.; de Groot, B.; Esteve Cuevas, L.M.; Ansems, A.; Green, M.; Thompson, K.; Lander, H.; et al. Lactate ≥2 mmol/L plus qSOFA improves utility over qSOFA alone in emergency department patients presenting with suspected sepsis. Emerg. Med. Australas. 2017, 29, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.; Bourbeau, P.; Swartz, B.; Brecher, S.; Carroll, K.C.; Stamper, P.D.; Dunne, W.M.; McCardle, T.; Walk, N.; Fiebelkorn, K.; et al. Timing of specimen collection for blood cultures from febrile patients with bacteremia. J. Clin. Microbiol. 2008, 46, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Johnson, D.W.; Lisco, S.J.; Sun, J. Early goal-directed therapy for sepsis: A novel solution for discordant survival outcomes in clinical trials. Crit. Care Med. 2017, 45, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Biomarkers of sepsis: Clinically useful? Curr. Opin. Crit. Care 2005, 11, 473–480. [Google Scholar] [CrossRef]
- Qu, J.; Lü, X.; Liu, Y.; Wang, X. Evaluation of procalcitonin, C-reactive protein, interleukin-6 & serum amyloid A as diagnostic biomarkers of bacterial infection in febrile patients. Indian J. Med. Res. 2015, 141, 5–21. [Google Scholar]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef]
- Sample Size Calculators for Designing Clinical Research. Available online: http://www.sample-size.net/correlation-sample-size/ (accessed on 8 June 2019).
- Fortunato, A. A new sensitive automated assay for procalcitonin detection: LIAISON® BRAHMS PCT® II GEN. Pract. Lab. Med. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [Google Scholar]
- Singer, A.J.; Ng, J.; Thode, H.C., Jr.; Spiegel, R.; Weingart, S. Quick SOFA scores predict mortality in adult emergency department patients with and without suspected infection. Ann. Emerg. Med. 2017, 69, 475–479. [Google Scholar] [CrossRef]
- Freund, Y.; Lemachatti, N.; Krastinova, E.; Van Laer, M.; Claessens, Y.E.; Avondo, A.; Occelli, C.; Feral-Pierssens, A.L.; Truchot, J.; Ortega, M.; et al. Prognostic accuracy of Sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017, 317, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Chen, Y.X.; Guo, S.B.; Mei, X.; Yang, P. Predictive performance of quick Sepsis-related Organ Failure Assessment for mortality and ICU admission in patients with infection at the ED. Am. J. Emerg. Med. 2016, 34, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- April, M.D.; Aguirre, J.; Tannenbaum, L.I.; Moore, T.; Pingree, A.; Thaxton, R.E.; Sessions, D.J.; Lantry, J.H. Sepsis clinical criteria in emergency department patients admitted to an intensive care unit: An external validation study of quick sequential organ failure assessment. J. Emerg. Med. 2017, 52, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Dorsett, M.; Kroll, M.; Smith, C.S.; Asaro, P.; Liang, S.Y.; Moy, H.P. qSOFA has poor sensitivity for prehospital identification of severe sepsis and septic shock. Prehosp. Emerg. Care 2017, 21, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Moon, H.G.; Kim, S.H. Efficacy of quick Sequential Organ Failure Assessment with lactate concentration for predicting mortality in patients with community-acquired pneumonia in the emergency department. Clin. Exp. Emerg. Med. 2019, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moya, F.; Nieto, A.; Jose, L.R. Calcitonin biosynthesis: Evidence for a precursor. Eur. J. Biochem. 1975, 55, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Albrich, W.; Christ-Crain, M.; Chastre, J.; Mueller, B. Procalcitonin for guidance of antibiotic therapy. Expert Rev. Anti-Infect. Ther. 2010, 8, 575–587. [Google Scholar] [CrossRef]
- Castelli, G.P.; Pognani, C.; Meisner, M.; Stuani, A.; Bellomi, D.; Sgarbi, L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome; sepsis and organ dysfunction. Crit. Care 2004, 8, 234–242. [Google Scholar] [CrossRef]
- Luzzani, A.; Polati, E.; Dorizzi, R.; Rungatscher, A.; Pavan, R.; Merlini, A. Comparison of procalcitonin and Creactive protein as markers of sepsis. Crit. Care Med. 2003, 31, 1737–1741. [Google Scholar] [CrossRef]
- Ventola, C.L. Mobile Devices and Apps for Health Care Professionals: Uses and Benefits. P T 2014, 39, 356–364. [Google Scholar]
- Quesada-González, D.; Merkoçi, A. Mobile phone-based biosensing: An emerging “diagnostic and communication” technology. Biosens. Bioelectron. 2017, 92, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.J.; Lindsell, C.J.; Hollander, J.E.; O’neil, B.; Jackson, R.; Schreiber, D.; Christenson, R.; Gibler, W.B. A multicenter randomized controlled trial comparing central laboratory and point-of-care cardiac marker testing strategies: The disposition impacted by serial point of care markers in acute coronary syndromes (DISPO-ACS) trial. Ann. Emerg. Med. 2009, 53, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lee-Lewandrowski, E.; Corboy, D.; Lewandrowski, K.; Sinclair, J.; McDermot, S.; Benzer, T.I. Implementation of a point-of-care satellite laboratory in the emergency department of an academic medical center. Impact on test turnaround time and patient emergency department length of stay. Arch. Pathol. Lab. Med. 2003, 127, 456–460. [Google Scholar] [PubMed]
- Parvin, C.A.; Lo, S.F.; Deuser, S.M.; Weaver, L.G.; Lewis, L.M.; Scott, M.G. Impact of point-of-care testing on patients’ length of stay in a large emergency department. Clin. Chem. 1996, 42, 711–717. [Google Scholar] [PubMed]
- Singer, A.J.; Viccellio, P.; Thode, H.C., Jr.; Bock, J.L.; Henry, M.C. Introduction of a stat laboratory reduces emergency department length of stay. Acad. Emerg. Med. 2008, 15, 324–328. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Miltiades, A.N.; Gaieski, D.F.; Goyal, M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit. Care Med. 2009, 37, 1670–1677. [Google Scholar] [CrossRef]
| Baseline Characteristic | Value |
|---|---|
| Age (year, mean ± standard deviation) | 67.1 ± 17.1 |
| Male (n, %) | 100 (50.3) |
| Mode of transportation (n, %) | |
| Emergency medical service | 52 (17.4) |
| Self-transport by vehicle | 94 (47.2) |
| Transferred from another hospital | 53 (26.6) |
| Vital sign (mean ± standard deviation) | |
| Systolic blood pressure (mmHg) | 118.5 ± 28.6 |
| Diastolic blood pressure (mmHg) | 72.9 ± 16.8 |
| Heart rate (beats min−1) | 98.0 ± 22.1 |
| Respiratory rate (breaths min−1) | 21.9 ± 4.8 |
| Body temperature (°C) | 37.7 ± 1.1 |
| Mental status (n, %) | |
| Alert | 161 (80.9) |
| Verbal | 14 (7.0) |
| Pain | 23 (11.6) |
| Unresponsive | 1 (0.5) |
| Infection source (n, %) | |
| Lung | 56 (28.1) |
| Urinary tract | 54 (27.1) |
| Gastrointestinal tract | 16 (8.0) |
| Hepatobiliary-pancreas | 26 (13.1) |
| Bone-joint-soft tissue | 15 (7.5) |
| Central nervous system | 6 (3.0) |
| Indwelling catheter | 1 (0.5) |
| Endocarditis | 1 (0.5) |
| Blood stream | 10 (5.0) |
| Unknown | 16 (8.0) |
| Laboratory finding | |
| White blood cell (×103 μL−1) | 11.4 ± 7.6 |
| Platelet (×103 μL−1) | 197.7 ± 96.3 |
| Creatinine (mg dL−1) | 1.3 ± 1.4 |
| Lactate (1.4 mmol L−1) | 2.6 ± 2.2 |
| C-reactive protein (mg L−1) | 102.4 ± 96.7 |
| Serum procalcitonin (ng mL−1) | 2.9 ± 7.4 |
| Whole blood procalcitonin (ng mL−1) | 2.0 ± 4.2 |
| Positive result on the culture test | N/total N (%) |
| Blood | 41/182 (22.5) |
| Sputum | 108/127 (85.0) |
| Urine | 47/112 (42.0) |
| qSOFA score | N (%) |
| 0 | 114 (57.3) |
| 1 | 11 (5.5) |
| 2 | 57 (28.6) |
| 3 | 17 (8.5) |
| Intensive care unit admission (N, %) | 39 (19.6) |
| 28 day mortality (N, %) | 20 (10.1) |
| Positive Blood Culture | qSOFA Score ≥2 (95%CI) | Whole Blood Procalcitonin >0.5 ng mL−1 (95%CI) | Serum Blood Procalcitonin >0.5 ng mL−1 (95%CI) | Serum Lactate >2 mmol L−1 (95%CI) |
|---|---|---|---|---|
| Sensitivity | 34.15 (20.08–50.59) | 75.61 (59.70–87.64) | 82.93 (67.94–92.85) | 79.49 (63.54–90.70) |
| Specificity | 65.25 (56.78–73.06) | 63.83 (55.32–71.75) | 46.10 (37.68–54.69) | 61.94 (53.16–70.18) |
| PPV | 22.22 (15.00–31.62) | 37.80 (31.48–44.57) | 30.91 (26.68–35.48) | 37.80 (31.73–44.29) |
| NPV | 77.31 (72.61–81.41) | 90.00 (83.81–93.99) | 90.28 (82.21–94.91) | 91.21 (84.65–95.13) |
| p > 0.05 | p = 0.000 | p = 0.001 | p = 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, B.-S.; Yoon, Y.-H.; Kim, J.-Y.; Cho, Y.-D.; Park, S.-J.; Lee, E.-S.; Choi, S.-H. Clinical Value of Whole Blood Procalcitonin Using Point of Care Testing, Quick Sequential Organ Failure Assessment Score, C-Reactive Protein and Lactate in Emergency Department Patients with Suspected Infection. J. Clin. Med. 2019, 8, 833. https://doi.org/10.3390/jcm8060833
Shim B-S, Yoon Y-H, Kim J-Y, Cho Y-D, Park S-J, Lee E-S, Choi S-H. Clinical Value of Whole Blood Procalcitonin Using Point of Care Testing, Quick Sequential Organ Failure Assessment Score, C-Reactive Protein and Lactate in Emergency Department Patients with Suspected Infection. Journal of Clinical Medicine. 2019; 8(6):833. https://doi.org/10.3390/jcm8060833
Chicago/Turabian StyleShim, Bo-Sun, Young-Hoon Yoon, Jung-Youn Kim, Young-Duck Cho, Sung-Jun Park, Eu-Sun Lee, and Sung-Hyuk Choi. 2019. "Clinical Value of Whole Blood Procalcitonin Using Point of Care Testing, Quick Sequential Organ Failure Assessment Score, C-Reactive Protein and Lactate in Emergency Department Patients with Suspected Infection" Journal of Clinical Medicine 8, no. 6: 833. https://doi.org/10.3390/jcm8060833
APA StyleShim, B.-S., Yoon, Y.-H., Kim, J.-Y., Cho, Y.-D., Park, S.-J., Lee, E.-S., & Choi, S.-H. (2019). Clinical Value of Whole Blood Procalcitonin Using Point of Care Testing, Quick Sequential Organ Failure Assessment Score, C-Reactive Protein and Lactate in Emergency Department Patients with Suspected Infection. Journal of Clinical Medicine, 8(6), 833. https://doi.org/10.3390/jcm8060833





