Haemotherapy with Fibrinogen for Perioperative Bleeding Prevention—A View on Arterial Thrombogenesis and Myocardial Infarction in the Rat In Vivo
Abstract
1. Introduction
2. Material and Methods
2.1. Pilot Experiments
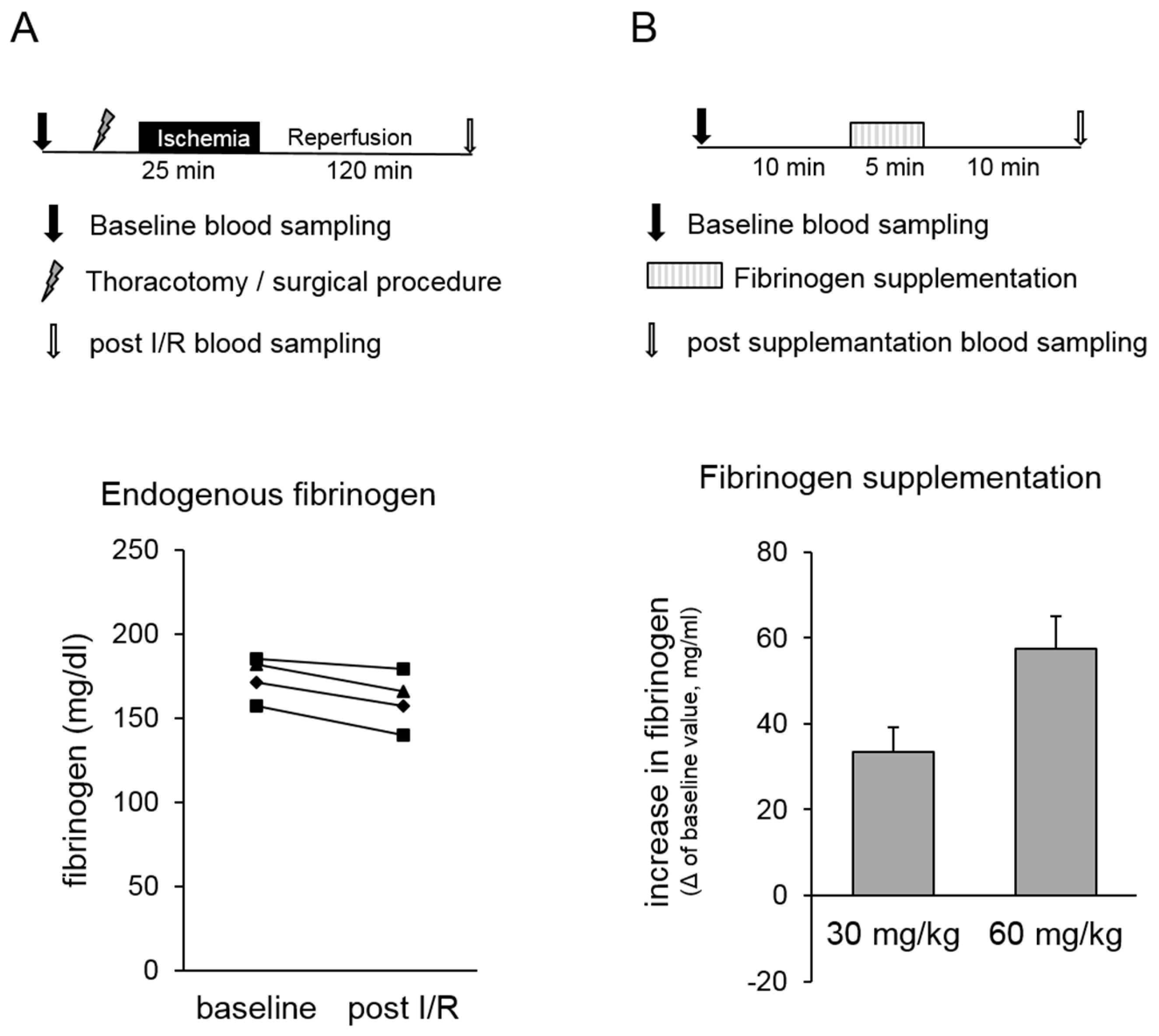
- In our in vivo rat model, both the surgical procedure and myocardial infarction might cause a change in “endogenous” fibrinogen concentration that possibly influenced our study outcome. Therefore, pilot experiments were conducted, and fibrinogen concentrations were analysed before and after surgical procedure, including thoracotomy and myocardial ischaemia/reperfusion (I/R) injury (Figure 1A, upper panel). Methodological details are described below (please see In vivo model of myocardial infarction and ischaemic preconditioning).
- Postoperative blood loss after coronary artery bypass grafting was significantly reduced by preoperative substitution of 2 g fibrinogen, causing an increase in plasma fibrinogen of ~60 mg/dL [9]. Therefore, we conducted pilot experiments to determine the required amount of fibrinogen yielding at an increase in fibrinogen concentration of ~60 mg/dL in our in vivo rat model (Figure 1B, upper panel). For this, pentobarbital anaesthetised rats (bolus 100 mg/kg BW i.p., continuously 40 mg/kg/h i.v.) received 30 mg/kg or 60 mg/kg fibrinogen in 0.9% NaCl as continuous infusion over 5 min via a catheter that was placed in the right jugular vein. Blood was collected 10 min before the start (baseline) and 10 min after the end of fibrinogen infusion via a catheter that was placed in the left carotid artery.
2.2. In Vivo Model of Thrombus Formation
2.3. In Vivo Model for Myocardial Infarction and Ischaemic Preconditioning
2.4. Statistical Analysis
3. Results
3.1. Pilot Experiments
- Fibrinogen levels were determined at baseline and after the surgical procedure, including thoracotomy and myocardial ischaemia/reperfusion injury in rats in vivo. No changes in endogenous fibrinogen concentration were observed within the time window that is relevant for our study protocol (Figure 1A, lower panel).
- The required amount of fibrinogen leading to an increase of fibrinogen in plasma of ~60 mg/dL was determined. This substitution corresponds to an intraoperative substitution of 2 g fibrinogen in humans [9]. Our results show that a substitution of 60 mg/kg body weight was required to increase the fibrinogen concentration by approximately 60 mg/dL in the rat (Figure 1B, lower panel). Therefore, all further experiments were conducted after supplementation of 60 mg/kg body weight fibrinogen (Fiblow). In addition, groups with animals receiving higher fibrinogen doses of 120 mg/kg body weight Fibrinogen (Fibhigh) were performed in the arterial thrombus formation experiments as well as in the myocardial ischaemia and reperfusion experiments to test the potential occurrence of prothrombotic side-effects limiting the therapeutic window of fibrinogen supplementation.
3.2. In Vivo Model of Thrombus Formation
Coagulation Parameter and Haemodynamic Data
3.3. In Vivo Model for Myocardial Infarction and Ischaemic Preconditioning
Haemodynamic Data
4. Discussion
4.1. Fibrinogen, Thrombogenesis and Myocardial Infarction
4.2. Fibrinogen and IPC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moulton, M.J.; Creswell, L.L.; Mackey, M.E.; Cox, J.L.; Rosenbloom, M. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J. Thorac. Cardiovasc. Surg. 1996, 111, 1037–1046. [Google Scholar] [CrossRef]
- Spiess, B.D. Transfusion of blood products affects outcome in cardiac surgery. Semin. Cardiothorac. Vasc. Anesth. 2004, 8, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Aljassim, O.; Karlsson, M.; Wiklund, L.; Jeppsson, A.; Olsson, P.; Berglin, E. Inflammatory response and platelet activation after off-pump coronary artery bypass surgery. Scand. Cardiovasc. J. 2006, 40, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Blome, M.; Isgro, F.; Kiessling, A.H.; Skuras, J.; Haubelt, H.; Hellstern, P.; Saggau, W. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thromb. Haemost. 2005, 93, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Ternstrom, L.; Hyllner, M.; Baghaei, F.; Nilsson, S.; Jeppsson, A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion 2008, 48, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; McNicol, P.L.; McCall, P.R.; Bellomo, R.; Connellan, J.; McInnes, F.; Przybylowski, G.M.; Bowkett, J.; Choo, F.; Thurlow, P.J. Prediction of the mediastinal drainage after coronary artery bypass surgery. Anaesth. Intensive Care 2000, 28, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.; Rothe, G.; Lodes, H.; Barlage, S.; Schmitz, G.; Birnbaum, D.E. Predictors of blood loss after coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 1997, 11, 824–827. [Google Scholar] [CrossRef]
- Gielen, C.; Dekkers, O.; Stijnen, T.; Schoones, J.; Brand, A.; Klautz, R.; Eikenboom, J. The effects of pre- and postoperative fibrinogen levels on blood loss after cardiac surgery: a systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 292–298. [Google Scholar] [CrossRef]
- Karlsson, M.; Ternstrom, L.; Hyllner, M.; Baghaei, F.; Flinck, A.; Skrtic, S.; Jeppsson, A. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb. Haemost. 2009, 102, 137–144. [Google Scholar] [CrossRef]
- Rahe-Meyer, N.; Pichlmaier, M.; Haverich, A.; Solomon, C.; Winterhalter, M.; Piepenbrock, S.; Tanaka, K.A. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br. J. Anaesth. 2009, 102, 785–792. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.D.; Whittaker, P.; Frelinger, A.L., III; Barnard, M.R.; Michelson, A.D.; Przyklenk, K. Preconditioning ischemia attenuates molecular indices of platelet activation-aggregation. J. Thromb. Haemost. 2006, 4, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Kurz, K.D.; Main, B.W.; Sandusky, G.E. Rat model of arterial thrombosis induced by ferric chloride. Thromb. Res. 1990, 60, 269–280. [Google Scholar] [CrossRef]
- Behmenburg, F.; van Caster, P.; Bunte, S.; Brandenburger, T.; Heinen, A.; Hollmann, M.W.; Huhn, R. Impact of Anesthetic Regimen on Remote Ischemic Preconditioning in the Rat Heart In Vivo. Anesth. Analg. 2018, 126, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.; Fries, D.; Velik-Salchner, C.; Oswald, E.; Innerhofer, P. Fibrinogen in craniosynostosis surgery. Anesth. Analg. 2008, 106, 725–731. [Google Scholar] [CrossRef]
- Sorensen, B.; Larsen, O.H.; Rea, C.J.; Tang, M.; Foley, J.H.; Fenger-Eriksen, C. Fibrinogen as a hemostatic agent. Semin. Thromb. Hemost. 2012, 38, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, S.T.; Myllyla, G.J.; Vahtera, E.M. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth. Analg. 1995, 81, 360–365. [Google Scholar] [PubMed]
- Fenger-Eriksen, C.; Anker-Moller, E.; Heslop, J.; Ingerslev, J.; Sorensen, B. Thrombelastographic whole blood clot formation after ex vivo addition of plasma substitutes: improvements of the induced coagulopathy with fibrinogen concentrate. Br. J. Anaesth. 2005, 94, 324–329. [Google Scholar] [CrossRef]
- Fries, D.; Krismer, A.; Klingler, A.; Streif, W.; Klima, G.; Wenzel, V.; Haas, T.; Innerhofer, P. Effect of fibrinogen on reversal of dilutional coagulopathy: a porcine model. Br. J. Anaesth. 2005, 95, 172–177. [Google Scholar] [CrossRef]
- Martini, J.; Maisch, S.; Pilshofer, L.; Streif, W.; Martini, W.; Fries, D. Fibrinogen concentrate in dilutional coagulopathy: a dose study in pigs. Transfusion 2014, 54, 149–157. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef] [PubMed]
- Green, F.R. Fibrinogen polymorphisms and atherothrombotic disease. Ann. New York Acad. Sci. 2001, 93, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.; Lowe, G.D.; Rumley, A.; Tunstall-Pedoe, H. Fibrinogen as a risk factor for coronary heart disease and mortality in middle-aged men and women. The Scottish Heart Health Study. Eur. Heart J. 1998, 19, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Thani, K.B.; Ilapakurti, M.; Lee, M.S.; Palakodeti, V.; Mahmud, E. Elevated plasma fibrinogen rather than residual platelet reactivity after clopidogrel pre-treatment is associated with an increased ischemic risk during elective percutaneous coronary intervention. J. Am. Coll. Cardiol. 2013, 61, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, E.; Ramsis, M.; Behnamfar, O.; Enright, K.; Huynh, A.; Kaushal, K.; Palakodeti, S.; Li, S.; The, P.; Lin, F.; et al. Effect of Serum Fibrinogen, Total Stent Length, and Type of Acute Coronary Syndrome on 6-Month Major Adverse Cardiovascular Events and Bleeding After Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 117, 1575–1581. [Google Scholar] [CrossRef]
- Sun, Y.; Mao, P.; Lu, J.; Li, L.; Lu, W.; Jiang, Q.; Teng, H. Localized lower extremity ischemic preconditioning prevents against local thrombus formation. Vasa 2015, 44, 285–288. [Google Scholar] [CrossRef]
- Hata, K.; Whittaker, P.; Kloner, R.A.; Przyklenk, K. Brief antecedent ischemia attenuates platelet-mediated thrombosis in damaged and stenotic canine coronary arteries: role of adenosine. Circulation 1998, 97, 692–702. [Google Scholar] [CrossRef]



| Group | Fibrinogen (mg/dL) | Platelets (*1000/µL) | INR | PTT (s) |
|---|---|---|---|---|
| Con | 200 ± 25 | 696 ± 100 | 0.91 ± 0.04 | 36 ± 22 |
| Fiblow | 261 ± 33 * | 787 ± 83 | 0.84 ± 0.05 | 34 ± 19 |
| Fibhigh | 279 ± 37 * | 731 ± 83 | 0.90 ± 0.08 | 31 ± 20 |
| Group | Heart Rate (bpm) | AOP Mean (mmHg) | Flow A. Femoralis (mL/min) |
|---|---|---|---|
| Con | 384 ± 43 | 110 ± 17 | 1.26 ± 0.30 |
| Fiblow | 370 ± 37 | 101 ± 17 | 1.25 ± 0.31 |
| Fibhigh | 369 ± 50 | 101 ± 25 | 1.38 ± 0.38 |
| Area at Risk (% of Left Ventricle) | |||||||||||||||
| Con | Fiblow | Fibhigh | Con + IPC | Fiblow + IPC | Fibhigh + IPC | ||||||||||
| 11.3 ± 5.4 | 16.5 ± 6.4 | 13.4 ± 4.6 | 12.0 ± 7.9 | 12.7 ± 5.4 | 16.9 ± 8.4 | ||||||||||
| Baseline | Washout 3 | Ischaemia | Reperfusion | ||||||||||||
| 15 min | 30 min | 120 min | |||||||||||||
| Heart Rate (bpm) | |||||||||||||||
| Con | 358 | ± | 59 | 355 | ± | 40 | 334 | ± | 92 | 392 | ± | 96 | 394 | ± | 95 |
| Fiblow | 364 | ± | 57 | 362 | ± | 54 | 361 | ± | 64 | 353 | ± | 43 | 336 | ± | 53 |
| Fibhigh | 399 | ± | 35 | 360 | ± | 87 | 378 | ± | 42 | 350 | ± | 64 | 333 | ± | 64 |
| Con + IPC | 413 | ± | 44 | 386 | ± | 20 | 391 | ± | 22 | 380 | ± | 28 | 366 | ± | 19 |
| Fiblow + IPC | 410 | ± | 36 | 349 | ± | 27 | 364 | ± | 26 | 376 | ± | 28 | 321 | ± | 44 |
| Fibhigh + IPC | 397 | ± | 21 | 362 | ± | 59 | 352 | ± | 40 | 361 | ± | 68 | 326 | ± | 66 |
| Mean Aortic Pressure (mmHg) | |||||||||||||||
| Con | 95 | ± | 22 | 94 | ± | 17 | 87 | ± | 16 | 78 | ± | 13 | 71 | ± | 17 |
| Fiblow | 105 | ± | 19 | 103 | ± | 23 | 94 | ± | 31 | 86 | ± | 33 | 54 | ± | 29 * |
| Fibhigh | 112 | ± | 19 | 90 | ± | 19 | 89 | ± | 23 | 73 | ± | 26 * | 51 | ± | 26 * |
| Con + IPC | 123 | ± | 8 | 92 | ± | 21 | 90 | ± | 18 | 85 | ± | 13 * | 68 | ± | 18 * |
| Fiblow + IPC | 114 | ± | 19 | 77 | ± | 21 * | 77 | ± | 18 * | 74 | ± | 24 * | 42 | ± | 12 * |
| Fibhigh + IPC | 114 | ± | 24 | 88 | ± | 36 | 95 | ± | 33 | 75 | ± | 34 * | 69 | ± | 25 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinen, A.; Welke, V.; Behmenburg, F.; Stroethoff, M.; Stoldt, V.; Hoffmann, T.; Hollmann, M.W.; Huhn, R. Haemotherapy with Fibrinogen for Perioperative Bleeding Prevention—A View on Arterial Thrombogenesis and Myocardial Infarction in the Rat In Vivo. J. Clin. Med. 2019, 8, 880. https://doi.org/10.3390/jcm8060880
Heinen A, Welke V, Behmenburg F, Stroethoff M, Stoldt V, Hoffmann T, Hollmann MW, Huhn R. Haemotherapy with Fibrinogen for Perioperative Bleeding Prevention—A View on Arterial Thrombogenesis and Myocardial Infarction in the Rat In Vivo. Journal of Clinical Medicine. 2019; 8(6):880. https://doi.org/10.3390/jcm8060880
Chicago/Turabian StyleHeinen, André, Vera Welke, Friederike Behmenburg, Martin Stroethoff, Volker Stoldt, Till Hoffmann, Markus W. Hollmann, and Ragnar Huhn. 2019. "Haemotherapy with Fibrinogen for Perioperative Bleeding Prevention—A View on Arterial Thrombogenesis and Myocardial Infarction in the Rat In Vivo" Journal of Clinical Medicine 8, no. 6: 880. https://doi.org/10.3390/jcm8060880
APA StyleHeinen, A., Welke, V., Behmenburg, F., Stroethoff, M., Stoldt, V., Hoffmann, T., Hollmann, M. W., & Huhn, R. (2019). Haemotherapy with Fibrinogen for Perioperative Bleeding Prevention—A View on Arterial Thrombogenesis and Myocardial Infarction in the Rat In Vivo. Journal of Clinical Medicine, 8(6), 880. https://doi.org/10.3390/jcm8060880




