Clinical Approach to the Patient in Critical State Following Immunotherapy and/or Stem Cell Transplantation: Guideline for the On-Call Physician
Abstract
1. Critically Ill Hematology Patients
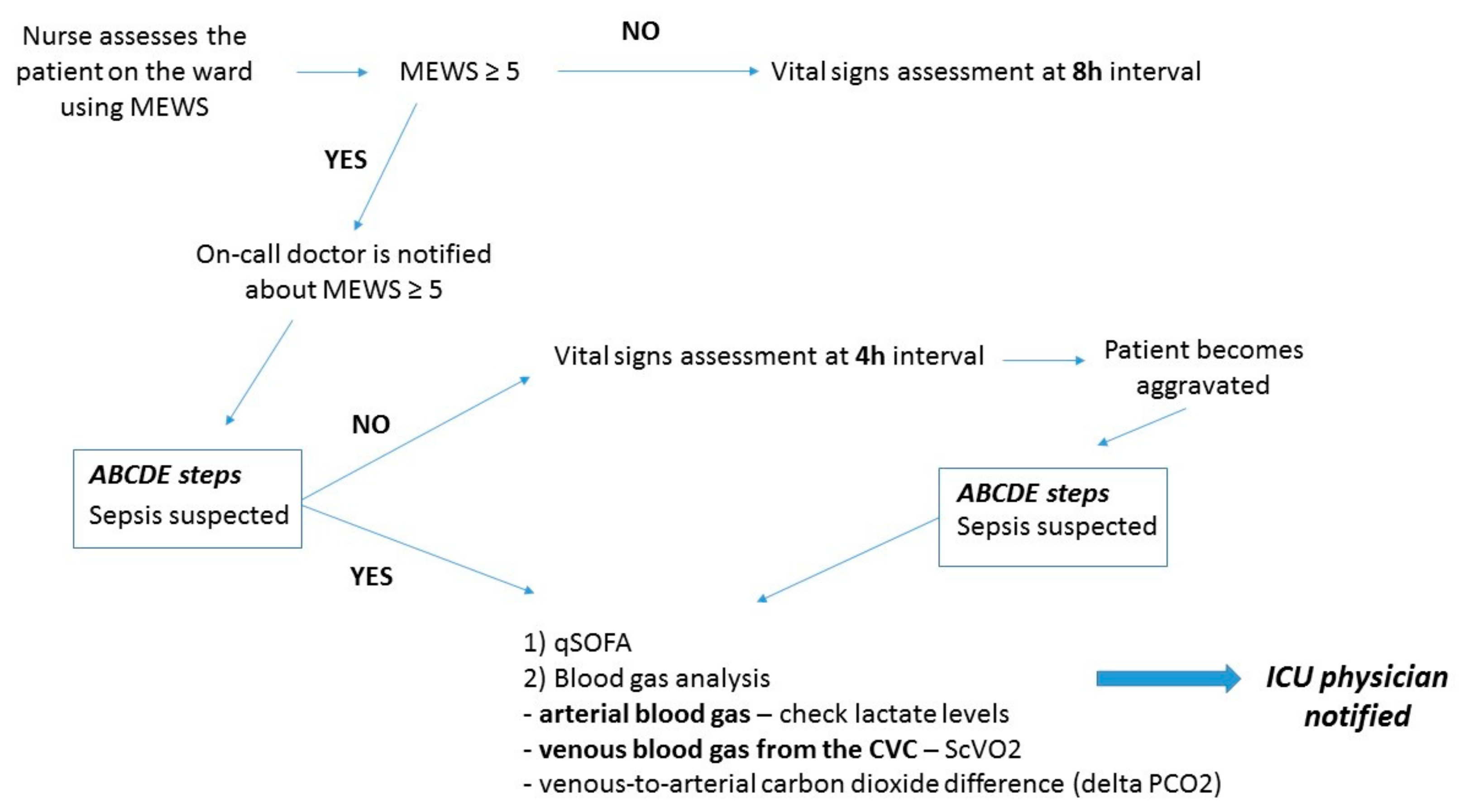
2. Acknowledgment of the Impending Critical Situation and Initial Management of the Critical Patient on the Ward: The Modified Early Warning Score (MEWS)
3. ABCDE (Airway, Breathing, Circulation, Disability, Exposure) of the Potential Critically Ill Patient on the Ward
4. Screening for Sepsis: Quick Sequential Organ Failure (qSOFA) Score
5. Procalcitonin, Presepsin, and C-Reactive Protein in the Immunotherapy and Transplant Setting
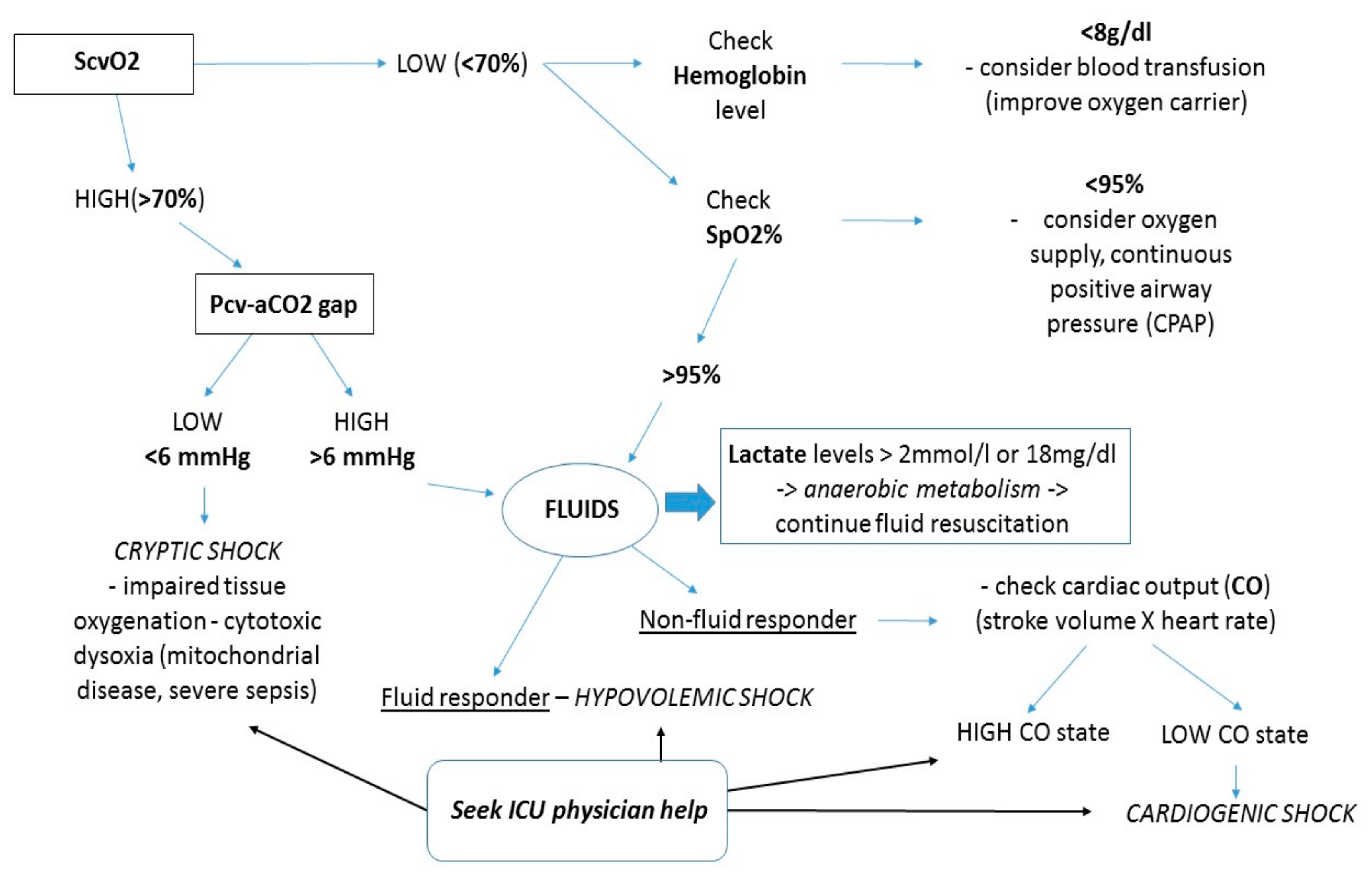
6. Assessment of Microvascular Perfusion with Lactate, ScvO2, and Delta PCO2
7. Management of Cytokine Release Syndrome
8. Ethical Concerns and Communication with Patients and Family Members
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Thanarajasingam, G.; Minasian, L.M.; Baron, F.; Cavalli, F.; De Claro, R.A.; Dueck, A.C.; El-Galaly, T.C.; Everest, N.; Geissler, J.; Gisselbrecht, C.; et al. Beyond maximum grade: Modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol. 2018, 5, e563–e598. [Google Scholar] [CrossRef]
- Chino, F.; Kamal, A.H.; Chino, J.; LeBlanc, T.W. Disparities in place of death for patients with hematological malignancies, 1999 to 2015. Blood Adv. 2019, 3, 333–338. [Google Scholar] [CrossRef] [PubMed]
- El-Jawahri, A.; LeBlanc, T.; VanDusen, H.; Traeger, L.; Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; Spitzer, T.R.; et al. Effect of Inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: A randomized clinical trial. JAMA 2016, 316, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Fojo, T.; Mailankody, S. An appraisal of clinically meaningful outcomes guidelines for oncology clinical trials. JAMA Oncol. 2016, 2, 1238–1240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarantopoulos, S. Allogeneic stem-cell transplantation—A T-cell balancing ACT. New Engl. J. Med. 2018, 378, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Brammer, J.E.; Khouri, I.; Gaballa, S.; Anderlini, P.; Tomuleasa, C.; Ahmed, S.; Ledesma, C.; Hosing, C.; Champlin, R.E.; Ciurea, S.O.; et al. Outcomes of haploidentical stem cell transplantation for lymphoma with melphalan-based conditioning. Boil. Blood Marrow Transplant. 2016, 22, 493–498. [Google Scholar] [CrossRef]
- Bardas, A.; Colita, A.; Orban, C.; Ciurea, S.O.; Tanase, A.; Tomuleasa, C.; Marculescu, A. Haploidentical donors: Can faster transplantation be life-saving for patients with advanced disease? Acta Haematol. 2016, 135, 211–216. [Google Scholar]
- Gaballa, S.; Ge, I.; El Fakih, R.; Brammer, J.E.; Kongtim, P.; Tomuleasa, C.; Wang, S.A.; Lee, D.; Petropoulos, D.; Cao, K.; et al. Results of a 2-Arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer 2016, 122, 3316–3326. [Google Scholar] [CrossRef]
- Dima, D.; Oprita, L.; Rosu, A.-M.; Trifa, A.; Selicean, C.; Moisoiu, V.; Frinc, I.; Zdrenghea, M.; Tomuleasa, C. Adult acute megakaryoblastic leukemia: Rare association with cytopenias of undetermined significance and p210 and p190 BCR–ABL transcripts. OncoTargets Ther. 2017, 10, 5047–5051. [Google Scholar] [CrossRef]
- Azoulay, E.; Mokart, D.; Pene, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.-M.; et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium—A Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef]
- Azoulay, E.; Pène, F.; Darmon, M.; Lengliné, E.; Benoit, D.; Soares, M.; Vincent, F.; Bruneel, F.; Perez, P.; Lemiale, V.; et al. Managing critically Ill hematology patients: Time to think differently. Blood Rev. 2015, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ñamendys-Silva, S.A.; García-Guillén, F.J.; Herrera-Gómez, A. Opening the doors of the intensive care unit to patients with hematologic malignancies. J. Clin. Oncol. 2014, 32, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Balit, C.R.; Horan, R.; Dorofaeff, T.; Frndova, H.; Doyle, J.; Cox, P.N. Pediatric hematopoietic stem cell transplant and intensive care: Have things changed? Pediatr. Crit. Care Med. 2016, 17, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Kamio, T.; Sanui, M.; Horikita, S.; Fujii, T.; Kawagishi, T.; Lefor, A.K. Hematopoietic stem cell transplant in facilities without intensive care units in Japan. Exp. Clin. Transplant. 2018, 16, 116–118. [Google Scholar] [PubMed]
- Nakamura, M.; Fujii, N.; Shimizu, K.; Ikegawa, S.; Seike, K.; Inomata, T.; Sando, Y.; Fujii, K.; Nishimori, H.; Matsuoka, K.-I.; et al. Long-term outcomes in patients treated in the intensive care unit after hematopoietic stem cell transplantation. Int. J. Hematol. 2018, 108, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.; Wise, M.P. Recognition and early management of the critically ill ward patient. Br. J. Hosp. Med. 2007, 68, M180–M183. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Green, S.M. Cheerio, Laddie! Bidding farewell to the Glasgow Coma Scale. Ann. Emerg. Med. 2011, 58, 427–430. [Google Scholar] [CrossRef]
- Qian, L.; Tomuleasa, C.; Florian, I.-A.; Shen, J.; Zdrenghea, M.; Dima, D. Advances in the treatment of newly diagnosed primary central nervous system lymphomas. Blood Res. 2017, 52, 159. [Google Scholar] [CrossRef]
- Iyer, V.N.; Mandrekar, J.N.; Danielson, R.D.; Zubkov, A.Y.; Elmer, J.L.; Wijdicks, E.F. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin. Proc. 2009, 84, 694–701. [Google Scholar] [CrossRef]
- Fischer, M.; Rüegg, S.; Czaplinski, A.; Strohmeier, M.; Lehmann, A.; Tschan, F.; Hunziker, P.R.; Marsch, S.C. Inter-rater reliability of the Full Outline of UnResponsiveness score and the Glasgow Coma Scale in critically ill patients: A prospective observational study. Crit. Care 2010, 14, R64. [Google Scholar] [CrossRef] [PubMed]
- Kause, J.; Smith, G.; Prytherch, D.; Parr, M.; Flabouris, A.; Hillman, K.; Intensive Care Society (UK); Australian and New Zealand Intensive Care Society Clinical Trials Group. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—The ACADEMIA study. Resuscitation 2004, 62, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Crandall, M.; West, M.A. Evaluation of the abdomen in the critically ill patient: opening the black box. Curr. Opin. Intern. Med. 2006, 5, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Goldhill, D.R.; Sumner, A. Outcome of intensive care patients in a group of British intensive care units. Crit. Care Med. 1998, 26, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Young, R.S.; Gobel, B.H.; Schumacher, M.; Lee, J.; Weaver, C.; Weitzman, S. Use of the modified early warning score and serum lactate to prevent cardiopulmonary arrest in hematology-oncology patients: A quality improvement study. Am. J. Med. Qual. 2014, 29, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, I.; Oyadomari, S.; Maedomari, S.; Toma, R.; Igei, C.; Kobata, S.; Koyama, J.; Tomori, R.; Kawamitsu, N.; Yamamoto, Y.; et al. Use of a modified early warning score system to reduce the rate of in-hospital cardiac arrest. J. Intensive Care 2016, 4, 244. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, K.; Mitchell, M.L.; White, H.; Morrison, L.; Sutton, J.; Sharratt, J.; Lin, F. A feasibility study of a randomised controlled trial to examine the impact of the ABCDE bundle on quality of life in ICU survivors. Pilot Feasibility Stud. 2018, 4, 32. [Google Scholar] [CrossRef]
- Costa, D.K.; Valley, T.S.; Miller, M.A.; Manojlovich, M.; Watson, S.R.; McLellan, P.; Pope, C.; Hyzy, R.C.; Iwashyna, T.J. ICU team composition and its association with ABCDE implementation in a quality collaborative. J. Crit. Care 2018, 44, 1–6. [Google Scholar] [CrossRef]
- Ren, X.-L.; Li, J.-H.; Peng, C.; Chen, H.; Wang, H.-X.; Wei, X.-L.; Cheng, Q.-H. Effects of ABCDE bundle on hemodynamics in patients on mechanical ventilation. Med. Sci. Monit. 2017, 23, 4650–4656. [Google Scholar] [CrossRef]
- Cook, F.; Lobo, D.; Martin, M.; Imbert, N.; Grati, H.; Daami, N.; Cherait, C.; Saïdi, N.-E.; Abbay, K.; Jaubert, J.; et al. Prospective validation of a new airway management algorithm and predictive features of intubation difficulty. Br. J. Anaesth. 2019, 122, 245–254. [Google Scholar] [CrossRef]
- Vats, A.; Hopkins, C.; Hatfield, K.M.; Yan, J.; Palmer, R.; Keskinocak, P. An airway risk assessment score for unplanned extubation in intensive care pediatric patients. Pediatr. Crit. Care Med. 2017, 18, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit. Care 2016, 20, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Bakker, J. Clinical assessment of peripheral circulation. Curr. Opin. Crit. Care 2015, 21, 1–231. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, H.; Gómez, H. The crashing patient: Hemodynamic collapse. Curr. Opin. Crit. Care 2017, 23, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Guerin, L.; Monnet, X.; Teboul, J.-L. Monitoring volume and fluid responsiveness: From static to dynamic indicators. Best Pr. Res. Clin. Anaesthesiol. 2013, 27, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Thim, T.; Krarup, N.H.V.; Grove, E.L.; Rohde, C.V.; Løfgren, B. Initial assessment and treatment with the Airway, Breathing, Circulation, Disability, Exposure (ABCDE) approach. Int. J. Gen. Med. 2012, 5, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hachimi-Idrissi, S.; Watelet, J.B. First-line attitudes in acute medicine. B-ENT 2016, 1 (Suppl. 26), 31–39. [Google Scholar]
- Martínez-Balzano, C.D.; Oliveira, P.; O’Rourke, M.; Hills, L.; Sosa, A.F. Critical Care Operations Committee of the UMass Memorial Healthcare Center. An educational intervention optimizes the use of arterial blood gas determinations across ICUs from different specialties: A quality-improvement study. Chest 2017, 151, 579–585. [Google Scholar] [CrossRef]
- Martin, C.M.; Priestap, F. Agreement between venous and arterial blood gas analysis of acid-base status in critical care and ward patients: a retrospective cohort study. Can. J. Anaesth. 2017, 64, 1138–1143. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Bennett, V.; Board, J.; McCracken, P.; McGloughlin, S.; Nanjayya, V.; Teo, A.; et al. Potential Impact of the 2016 Consensus Definitions of Sepsis and Septic Shock on Future Sepsis Research. Ann. Emerg. Med. 2017, 70, 553–561.e1. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-U.; Sin, C.K.; Park, H.K.; Shim, S.R.; Lee, J. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: A systematic review and meta-analysis. Crit. Care 2018, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Edelson, D.P.; Churpek, M.M. Identifying Patients With Sepsis on the Hospital Wards. Chest 2017, 151, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, M.; Gustad, L.T.; Mehl, A.; Bangstad, I.L.; Vinje, L.J.; Damås, J.K.; Solligård, E. Early identification of sepsis in hospital inpatients by ward nurses increases 30-day survival. Crit. Care 2016, 20, 435. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Oda, S.; Hirasawa, H.; Sugai, T.; Shiga, H.; Nakanishi, K.; Kitamura, N.; Sadahiro, T.; Hirano, T. Comparison of Sepsis-related Organ Failure Assessment (SOFA) score and CIS (cellular injury score) for scoring of severity for patients with multiple organ dysfunction syndrome (MODS). Intensiv. Care Med. 2000, 26, 1786–1793. [Google Scholar] [CrossRef]
- DeWitt, S.; Chavez, S.A.; Perkins, J.; Long, B.; Koyfman, A. Evaluation of fever in the emergency department. Am. J. Emerg. Med. 2017, 35, 1755–1758. [Google Scholar] [CrossRef]
- Perrone, J.; E Hollander, J.; Datner, E.M. Emergency Department evaluation of patients with fever and chemotherapy-induced neutropenia. J. Emerg. Med. 2004, 27, 115–119. [Google Scholar] [CrossRef]
- De Kruif, M.D.; Limper, M.; Gerritsen, H.; Spek, C.A.; Brandjes, D.P.M.; Cate, H.T.; Bossuyt, P.M.; Reitsma, P.H.; Van Gorp, E.C.M. Additional value of procalcitonin for diagnosis of infection in patients with fever at the emergency department. Crit. Care Med. 2010, 38, 457–463. [Google Scholar] [CrossRef]
- Pasikhova, Y.; Ludlow, S.; Baluch, A. Fever in Patients With Cancer. Cancer Control 2017, 24, 193–197. [Google Scholar] [CrossRef]
- Patel, D.M.; Riedel, D.J. Fever in Immunocompromised Hosts. Emerg. Med. Clin. North. Am. 2013, 31, 1059–1071. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primer 2016, 2, 16045. [Google Scholar] [CrossRef]
- Trippella, G.; Galli, L.; De Martino, M.; Lisi, C.; Chiappini, E. Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2017, 15, 1041–1057. [Google Scholar] [CrossRef]
- Su, L.; Han, B.; Liu, C.; Liang, L.; Jiang, Z.; Deng, J.; Yan, P.; Jia, Y.; Feng, D.; Xie, L. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: A prospective cohort study. BMC Infect. Dis. 2012, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.; Docherty, P.; Ouellette, S.; Cartier, L.J. Causes and outcomes of markedly elevated C-reactive protein levels. Can. Fam. Physician 2017, 63, e316–e323. [Google Scholar] [PubMed]
- Aygun, F. Procalcitonin Value Is an Early Prognostic Factor Related to Mortality in Admission to Pediatric Intensive Care Unit. Crit. Care Res. Pr. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; McCarthy, M.W. Novel applications for serum procalcitonin testing in clinical practice. Expert Rev. Mol. Diagn. 2018, 18, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Davison, B.; Givertz, M.M.; Bloomfield, D.M.; Dittrich, H.; et al. Procalcitonin-based indication of bacterial infection identifies high risk acute heart failure patients. Int. J. Cardiol. 2016, 204, 164–171. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensiv. Care 2017, 5, 51. [Google Scholar] [CrossRef]
- Pugni, L.; Pietrasanta, C.; Milani, S.; Vener, C.; Ronchi, A.; Falbo, M.; Arghittu, M.; Mosca, F. Presepsin (Soluble CD14 Subtype): Reference Ranges of a New Sepsis Marker in Term and Preterm Neonates. PLoS ONE 2015, 10, e0146020. [Google Scholar] [CrossRef]
- Behnes, M.; Bertsch, T.; Lepiorz, D.; Lang, S.; Trinkmann, F.; Brueckmann, M.; Borggrefe, M.; Hoffmann, U. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit. Care 2014, 18, 580. [Google Scholar] [CrossRef]
- Mussap, M.; Puxeddu, E.; Puddu, M.; Ottonello, G.; Coghe, F.; Comite, P.; Cibecchini, F.; Fanos, V. Soluble CD14 subtype (sCD14-ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin. Chim. Acta 2015, 451, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Wątek, M.; Wollny, T.; Niemirowicz, K.; Marzec, M.; Bucki, R.; Góźdź, S. Utility of blood procalcitonin concentration in the management of cancer patients with infections. OncoTargets Ther. 2016, 9, 469–475. [Google Scholar]
- Robinson, J.O.; Lamoth, F.; Bally, F.; Knaup, M.; Calandra, T.; Marchetti, O. Monitoring Procalcitonin in Febrile Neutropenia: What Is Its Utility for Initial Diagnosis of Infection and Reassessment in Persistent Fever? PLoS ONE 2011, 6, 18886. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, Y.; Kobayashi, K.; Ishida, A.; Maeda, T.; Takahashi, N.; Taji, Y.; Asou, N.; Ikebuchi, K. Diagnostic performance of procalcitonin, presepsin, and C-reactive protein in patients with hematological malignancies. J. Clin. Lab. Anal. 2017, 31, e22147. [Google Scholar] [CrossRef]
- Baraka, A.; Zakaria, M. Presepsin as a diagnostic marker of bacterial infections in febrile neutropenic pediatric patients with hematological malignancies. Int. J. Hematol. 2018, 108, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.C.; Orloski, C.J. End Points of Sepsis Resuscitation. Emerg Med. Clin. N. Am. 2017, 35, 93–107. [Google Scholar] [CrossRef]
- Saleh, A.S. Early, Goal-Directed Therapy for Septic Shock—A Patient-Level Meta-Analysis. N. Engl. J. Med. 2017, 377, 994. [Google Scholar]
- Jiang, L.-B.; Zhang, M.; Jiang, S.-Y.; Ma, Y.-F. Early goal-directed resuscitation for patients with severe sepsis and septic shock: A meta-analysis and trial sequential analysis. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 10404. [Google Scholar] [CrossRef]
- Leach, R.M.; Treacher, D.F. The relationship between oxygen delivery and consumption. Dis. 1994, 40, 301–368. [Google Scholar]
- Ducrocq, N.; Kimmoun, A.; Levy, B. LACTATE or ScVO2 as an endpoint in resuscitation of shock states? Minerva Anestesiol 2013, 79, 1049–1058. [Google Scholar] [PubMed]
- Arnold, R.C.; Shapiro, N.I.; Jones, A.E.; Schorr, C.; Pope, J.; Casner, E.; Parrillo, E.; Dellinger, JR.P.; Trzeciak, S.; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009, 32, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Vallet, B.; Robin, E.; Lebuffe, G. Venous Oxygen Saturation as a Physiologic Transfusion Trigger. Intensive Care Med. 2010, 14, 313–320. [Google Scholar]
- Zeroual, N.; Samarani, G.; Gallais, J.; Culas, G.; Saour, M.; Mourad, M.; Parrillo, J.E.; Dellinger, R.P.; Trzeciak, S.; Emergency Medicine Shock Research Network (EMShockNet) Investigators. ScVO2 changes after red-blood-cell transfusion for anaemia in cardiothoracic and vascular ICU patients: An observational study. Vox Sang. 2018, 113, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Surve, R.M.; Muthuchellappan, R.; Rao, G.; Philip, M. The effect of blood transfusion on central venous oxygen saturation in critically ill patients admitted to a neurointensive care unit. Transfus. Med. 2016, 26, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; An, W.S. New clinical criteria for septic shock: serum lactate level as new emerging vital sign. J. Thorac. Dis. 2016, 8, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Shaban, M.; Salahuddin, N. Clarification on the Method of Calculating Central Venous-to-Arterial CO2 Difference/Arterial-Central Venous O2 Difference Ratio. Shock 2017, 48, 690–691. [Google Scholar] [CrossRef]
- Ospina-Tascón, G.A.; Umaña, M.; Bermúdez, W.F.; Bautista-Rincón, D.F.; Valencia, J.D.; Madriñán, H.J.; Hernandez, G.; Bruhn, A.; Arango-Dávila, C.; De Backer, D.; et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med. 2016, 42, 211–221. [Google Scholar] [CrossRef]
- Mallat, J.; Pepy, F.; Lemyze, M.; Gasan, G.; Vangrunderbeeck, N.; Tronchon, L.; Vallet, B.; Thevenin, D. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock: A prospective observational study. Eur J. Anaesthesiol. 2014, 31, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mallat, J.; Lemyze, M.; Tronchon, L.; Vallet, B.; Thevenin, D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J. Crit. Care Med. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tat, T.; Li, H.; Constantinescu, C.-S.; Onaciu, A.; Chira, S.; Osan, C.; Pasca, S.; Petrushev, B.; Moisoiu, V.; Micu, W.-T.; et al. Correction: Genetically enhanced T lymphocytes and the intensive care unit. Oncotarget 2018, 9, 32097. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Dima, D.; Berce, C.; Liu, Y.; Rus, I.; Raduly, L.-Z.; Liu, Y.; Petrushev, B.; Berindan-Neagoe, I.; Irimie, A.; et al. Protein dysregulation in graft versus host disease. Oncotarget 2018, 9, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Fuji, S.; Berce, C.; Onaciu, A.; Chira, S.; Petrushev, B.; Micu, W.-T.; Moisoiu, V.; Osan, C.; Constantinescu, C.; et al. Chimeric Antigen Receptor T-Cells for the Treatment of B-Cell Acute Lymphoblastic Leukemia. Front. Immunol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S. Cytokine-Release Syndrome: Overview and Nursing Implications. Clin. J. Oncol. Nurs. 2007, 11, 37–41. [Google Scholar] [CrossRef]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing Cytokine Release Syndrome Associated With Novel T Cell-Engaging Therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
- Frey, N.V.; Porter, D.L. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology 2016, 2016, 567–572. [Google Scholar] [CrossRef]
- Frey, N.V.; Levine, B.L.; Lacey, S.F.; Grupp, S.A.; Maude, S.L.; Schuster, S.J. Refractory Cytokine Release Syndrome in Recipients of Chimeric Antigen Receptor (CAR) T Cells. Blood 2014, 124, 2296. [Google Scholar]
- De Vriese, A.S.; Colardyn, F.A.; Philippé, J.J.; Vanholder, R.C.; De Sutter, J.H.; Lameire, N.H. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol. 1999, 10, 846–853. [Google Scholar] [PubMed]
- Ronco, C. Evolution of Technology for Continuous Renal Replacement Therapy: Forty Years of Improvements. Contrib. Nephrol. 2017, 189, 114–123. [Google Scholar] [PubMed]
- Ricci, Z.; Ronco, C.; Bachetoni, A.; D’Amico, G.; Rossi, S.; Alessandri, E.; Rocco, M.; Pietropaoli, P. Solute removal during continuous renal replacement therapy in critically ill patients: Convection versus diffusion. Crit. Care 2006, 10, R67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Wang, D.; Li, H.; Huang, J.; Zhang, Z.; Qiao, Y.; Zhang, H.; Zeng, Y.; Tang, C.; et al. Hemofiltration Successfully Eliminates Severe Cytokine Release Syndrome Following CD19 CAR-T-Cell Therapy. J. Immunother. 2018, 41, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Selicean, C.; Cismas, S.; Jurj, A.; Marian, M.; Dima, D.; Paşca, S.; Petrushev, B.; Moisoiu, V.; Micu, W.-T.; et al. Minimal residual disease in chronic lymphocytic leukemia: A consensus paper that presents the clinical impact of the presently available laboratory approaches. Crit. Rev. Clin. Lab. Sci. 2018, 55, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Fuji, S.; Cucuianu, A.; Kapp, M.; Pileczki, V.; Petrushev, B.; Selicean, S.; Tănase, A.; Dima, D.; Berindan-Neagoe, I.; et al. MicroRNAs as biomarkers for graft-versus-host disease following allogeneic stem cell transplantation. Ann. Hematol. 2015, 94, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Wang, Y.; Mead, E.; Brentjens, R.J.; Sadelain, M.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. New Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016, 126, 2123–2138. [Google Scholar] [CrossRef]
- Gökbuget, N.; Zugmaier, G.; Klinger, M.; Kufer, P.; Stelljes, M.; Viardot, A.; Horst, H.A.; Neumann, S.; Brüggemann, M.; Ottmann, O.G.; et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica 2017, 102, e132–e135. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. New Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, J.; Wei, G.; Yu, J.; Luo, Y.; Shi, J.; Wu, W.; Zhao, K.; Xiao, L.; Zhang, Y.; et al. A retrospective comparison of allogenic and autologous chimeric antigen receptor T cell therapy targeting CD19 in patients with relapsed/refractory acute lymphoblastic leukemia. Bone Marrow Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 2018, 183, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Taraseviciute, A.; Turtle, C.J. Neurotoxicity Associated with CD19-Targeted CAR-T Cell Therapies. CNS Drugs 2018, 32, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Turtle, C.J. Toxicities of CD19 CAR-T cell immunotherapy. Am. J. Hematol. 2019, 94, S42–S49. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Jordan, J.T.; Forst, D.A.; Arrillaga-Romany, I.C.; Batchelor, T.T.; Baehring, J.M.; Clement, N.F.; Castro, L.N.G.; Herlopian, A.; Maus, M.V.; et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 2019, 133, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.D.; A Jacobson, C. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J. Natl. Cancer Inst. 2019. [Google Scholar] [CrossRef]
- Ñamendys-Silva, S.A.; González-Herrera, M.O.; García-Guillén, F.J.; Texcocano-Becerra, J.; Herrera-Gómez, A. Outcome of critically ill patients with hematological malignancies. Ann. Hematol. 2013, 92, 699–705. [Google Scholar] [CrossRef]
- Larché, J.; Azoulay, É.; Fieux, F.; Mesnard, L.; Moreau, D.; Thiery, G.; Darmon, M.; Le Gall, J.-R.; Schlemmer, B. Improved survival of critically ill cancer patients with septic shock. Intensiv. Care Med. 2003, 29, 1688–1695. [Google Scholar]
- Azoulay, É.; Thiéry, G.; Chevret, S.; Moreau, D.; Darmon, M.; Bergeron, A.; Yang, K.; Meignin, V.; Ciroldi, M.; Le Gall, J.-R.; et al. The Prognosis of Acute Respiratory Failure in Critically Ill Cancer Patients. Med. 2004, 83, 360–370. [Google Scholar] [CrossRef]
- Hampshire, P.A.; Welch, C.A.; McCrossan, L.A.; Francis, K.; Harrison, D.A. Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: A secondary analysis of the ICNARC Case Mix Programme Database. Crit. Care 2009, 13, R137. [Google Scholar]
- Townsend, W.M.; Holroyd, A.; Pearce, R.; MacKinnon, S.; Naik, P.; Goldstone, A.H.; Linch, D.C.; Peggs, K.S.; Thomson, K.J.; Singer, M.; et al. Improved intensive care unit survival for critically ill allogeneic haematopoietic stem cell transplant recipients following reduced intensity conditioning. Br. J. Haematol. 2013, 161, 578–586. [Google Scholar]
- Yeo, C.D.; Kim, J.W.; Kim, S.C.; Kim, Y.K.; Kim, K.H.; Kim, H.J.; Lee, S.; Rhee, C.K. Prognostic factors in critically ill patients with hematologic malignancies admitted to the intensive care unit. J. Crit. Care 2012, 27, 739.e1–739.e6. [Google Scholar]
- Hill, Q.A. Intensify, resuscitate or palliate: Decision making in the critically ill patient with haematological malignancy. Blood Rev. 2010, 24, 17–25. [Google Scholar]
- Khassawneh, B.Y.; White, P.; Jr Anaissie, E.J.; Barlogie, B.; Charles Hiller, F. Outcome from mechanical ventilation after autologous peripheral blood stem cell transplantation. Chest 2002, 121, 185–188. [Google Scholar]
- Peigne, V.; Rusinová, K.; Karlin, L.; Darmon, M.; Fermand, J.P.; Schlemmer, B.; Azoulay, E. Continued survival gains in recent years among critically ill myeloma patients. Intensive Care Med. 2009, 35, 512–518. [Google Scholar]
- Pène, F.; Percheron, S.; Lemiale, V.; Viallon, V.; Claessens, Y.-E.; Marqué, S.; Charpentier, J.; Angus, D.C.; Cariou, A.; Chiche, J.-D.; et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit*. Crit. Care Med. 2008, 36, 690–696. [Google Scholar]
- Bird, G.; Farquhar-Smith, P.; Wigmore, T.; Potter, M.; Gruber, P. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: A 5 yr study. Br. J. Anaesth. 2012, 108, 452–459. [Google Scholar]
- Geerse, D.A.; Span, L.F.; Pinto-Sietsma, S.-J.; Van Mook, W.N. Prognosis of patients with haematological malignancies admitted to the intensive care unit: Sequential Organ Failure Assessment (SOFA) trend is a powerful predictor of mortality. Eur. J. Intern. Med. 2011, 22, 57–61. [Google Scholar]
- Bokhari, S.W.I.; Munir, T.; Memon, S.; Byrne, J.L.; Russell, N.H.; Beed, M. Impact of critical care reconfiguration and track-and-trigger outreach team intervention on outcomes of haematology patients requiring intensive care admission. Ann. Hematol. 2010, 89, 505–512. [Google Scholar]
- Cooksley, T.; Kitlowski, E.; Haji-Michael, P. Effectiveness of Modified Early Warning Score in predicting outcomes in oncology patients. QJM 2012, 105, 1083–1088. [Google Scholar]
- Von Lilienfeld-Toal, M.; Midgley, K.; Lieberbach, S.; Barnard, L.; Glasmacher, A.; Gilleece, M.; Cook, G. Observation-Based Early Warning Scores to Detect Impending Critical Illness Predict In-Hospital and Overall Survival in Patients Undergoing Allogeneic Stem Cell Transplantation. Boil. Blood Marrow Transplant. 2007, 13, 568–576. [Google Scholar]
- Malak, S.; Sotto, J.-J.; Ceccaldi, J.; Colombat, P.; Casassus, P.; Jaulmes, D.; Rochant, H.; Cheminant, M.; Beaussant, Y.; Zittoun, R.; et al. Ethical and Clinical Aspects of Intensive Care Unit Admission in Patients with Hematological Malignancies: Guidelines of the Ethics Commission of the French Society of Hematology. Adv. Hematol. 2014, 2014, 1–8. [Google Scholar]
- Wright, A.A.; Zhang, B.; Ray, A.; Mitchell, S.L.; Jackson, V.A.; Block, S.D.; Maciejewski, P.K.; Prigerson, H.G. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. J. Am. Med. Assoc. 2008, 300, 1665–1673. [Google Scholar]
- Mack, J.W.; Cronin, A.; Keating, N.L.; Taback, N.; Huskamp, H.A.; Malin, J.L.; Earle, C.C.; Weeks, J.C. Associations Between End-of-Life Discussion Characteristics and Care Received Near Death: A Prospective Cohort Study. J. Clin. Oncol. 2012, 30, 4387–4395. [Google Scholar]
- Lecuyer, L.; Chevret, S.; Thiery, G.; Darmon, M.; Schlemmer, B.; Azoulay, É. The ICU Trial: A new admission policy for cancer patients requiring mechanical ventilation. Crit. Care Med. 2007, 35, 808–814. [Google Scholar]
- Cesta, M.A.; Cárdenas-Turanzas, M.; Wakefield, C.; Price, K.J.; Nates, J.L. Life-Supportive Therapy Withdrawal and Length of Stay in a Large Oncologic Intensive Care Unit at the End of Life. J. Palliat. Med. 2009, 12, 713–718. [Google Scholar]


| Best Eye Response | Best Verbal Response | Best Motor Response |
|---|---|---|
| To pain (+2) | Oriented (+5) | Obeys commands (+6) |
| To verbal command (+3) | Confused (+4) | Localizes pain (+5) |
| Spontaneously (+4) | Inappropriate words (+3) | Withdrawal from pain (+4) |
| No eye opening (+1) | Incomprehensible sounds (+2) | Flexion to pain (+3) |
| Not assessable (trauma, edema) (+1c) | No verbal response (+1) | Extension to pain (+2) |
| Intubated (+1t) | No motor response (+1) |
| Vital Parameters | 3 | 2 | 1 | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|
| Respiratory rate | ≤8 | 9–14 | 15–20 | 21–19 | >30 | ||
| SpO2 | ≤91 | 92–93 | 94–95 | ≥96 | |||
| Temperature | ≤35.0 | 35.1–36.0 | 36.1–38.0 | 38.1–38.5 | >38.6 | ||
| Systolic blood pressure | <70 | 71–80 | 81–100 | 101–199 | >200 | ||
| Heart rate | <40 | 40–50 | 51–100 | 101–110 | 111–129 | >129 | |
| Level of consciousness | U = GCS 6 | P = GCS 8 | V = GCS 13 | A = GCS 15 | |||
| Urine output (measured hourly) | <10 mL/h | <30 mLl/h | <45 mL/h |
| Quick SOFA Score (qSOFA) |
|---|
| Altered mental status GCS < 15 |
| Respiratory rate ≥ 22 |
| Systolic BP ≤ 100 mmHg |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu, C.; Bodolea, C.; Pasca, S.; Teodorescu, P.; Dima, D.; Rus, I.; Tat, T.; Achimas-Cadariu, P.; Tanase, A.; Tomuleasa, C.; et al. Clinical Approach to the Patient in Critical State Following Immunotherapy and/or Stem Cell Transplantation: Guideline for the On-Call Physician. J. Clin. Med. 2019, 8, 884. https://doi.org/10.3390/jcm8060884
Constantinescu C, Bodolea C, Pasca S, Teodorescu P, Dima D, Rus I, Tat T, Achimas-Cadariu P, Tanase A, Tomuleasa C, et al. Clinical Approach to the Patient in Critical State Following Immunotherapy and/or Stem Cell Transplantation: Guideline for the On-Call Physician. Journal of Clinical Medicine. 2019; 8(6):884. https://doi.org/10.3390/jcm8060884
Chicago/Turabian StyleConstantinescu, Catalin, Constantin Bodolea, Sergiu Pasca, Patric Teodorescu, Delia Dima, Ioana Rus, Tiberiu Tat, Patriciu Achimas-Cadariu, Alina Tanase, Ciprian Tomuleasa, and et al. 2019. "Clinical Approach to the Patient in Critical State Following Immunotherapy and/or Stem Cell Transplantation: Guideline for the On-Call Physician" Journal of Clinical Medicine 8, no. 6: 884. https://doi.org/10.3390/jcm8060884
APA StyleConstantinescu, C., Bodolea, C., Pasca, S., Teodorescu, P., Dima, D., Rus, I., Tat, T., Achimas-Cadariu, P., Tanase, A., Tomuleasa, C., & Einsele, H. (2019). Clinical Approach to the Patient in Critical State Following Immunotherapy and/or Stem Cell Transplantation: Guideline for the On-Call Physician. Journal of Clinical Medicine, 8(6), 884. https://doi.org/10.3390/jcm8060884






