Pulmonary Embolism and Coexisting Deep Vein Thrombosis: A Detrimental Association?
Abstract
:1. Introduction
2. Experimental Section
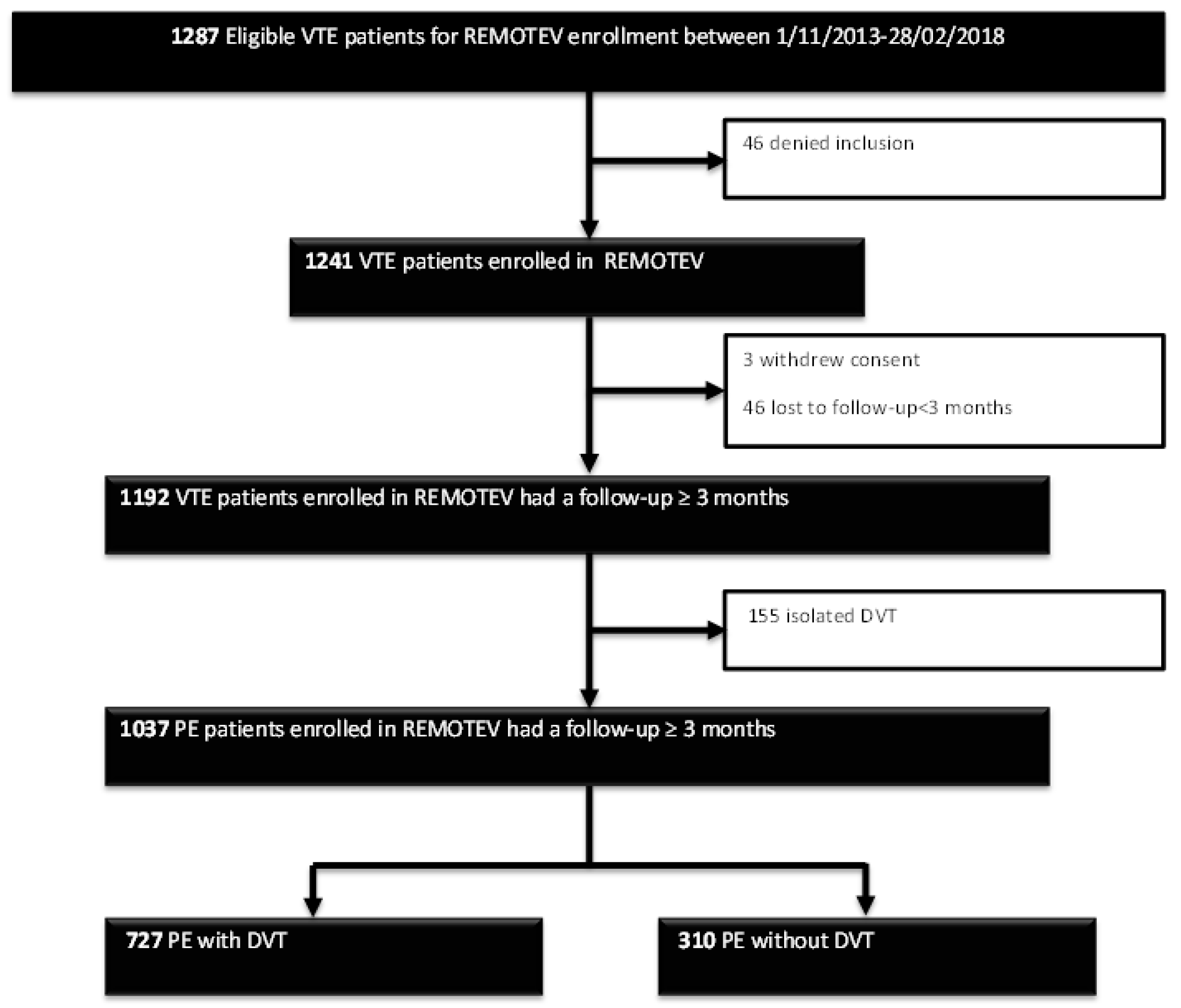
2.1. Study Design and Patient Selection
2.2. Requirements for VTE Diagnosis
2.3. Baseline Variables
2.4. Treatment Regimens
2.5. Follow-Up and Outcome Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Deep Vein Thrombosis Prevalence
3.2. DVT and PE Severity Assessment
3.3. Risk Factors Associated with PE Severity
3.4. Concomitant DVT and Risk of Poor Outcomes after a 3-Month Follow-Up
4. Discussion
Author Contributions
Conflicts of Interest
References
- White, R.H. The epidemiology of venous thromboembolism. Circulation 2003, 107, I4–I8. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z.; Visani, L.; De Rosa, M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999, 353, 1386–1389. [Google Scholar] [CrossRef]
- Stein, P.D.; Matta, F.; Alrifai, A.; Rahman, A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb. Res. 2012, 130, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Donzé, J.; Le Gal, G.; Fine, M.J.; Roy, P.-M.; Sanchez, O.; Verschuren, F.; Cornuz, J.; Meyer, G.; Perrier, A.; Righini, M.; et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb. Haemost. 2008, 100, 943–948. [Google Scholar] [PubMed]
- Righini, M.; Roy, P.-M.; Meyer, G.; Verschuren, F.; Aujesky, D.; Le Gal, G. The Simplified Pulmonary Embolism Severity Index (PESI): Validation of a clinical prognostic model for pulmonary embolism: Letter to the Editor. J. Thromb. Haemost. 2011, 9, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069. [Google Scholar] [PubMed]
- Monreal, M.; Barba, R.; Tolosa, C.; Tiberio, G.; Todolí, J.; Samperiz, A.L.; RIETE Investigators. Deep vein thrombosis and pulmonary embolism: The same disease? Pathophysiol. Haemost. Thromb. 2006, 35, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Huang, W.; Ren, W.; Cheng, J.; Zhang, M.; Zhao, Y. Risk factors associated with the occurrence of silent pulmonary embolism in patients with deep venous thrombosis of the lower limb. Phlebology 2014, 29, 442–446. [Google Scholar] [CrossRef]
- Stein, P.D.; Matta, F.; Musani, M.H.; Diaczok, B. Silent pulmonary embolism in patients with deep venous thrombosis: A systematic review. Am. J. Med. 2010, 123, 426–431. [Google Scholar] [CrossRef]
- Becattini, C.; Cohen, A.T.; Agnelli, G.; Howard, L.; Castejón, B.; Trujillo-Santos, J.; Monreal, M.; Perrier, A.; Yusen, R.D.; Jiménez, D. Risk Stratification of Patients with Acute Symptomatic Pulmonary Embolism Based on Presence or Absence of Lower Extremity DVT: Systematic Review and Meta-analysis. Chest 2016, 149, 192–200. [Google Scholar] [CrossRef]
- Bradley, M.J.; Alexander, L. The role of venous colour flow Doppler to aid the non-diagnostic lung scintigram for pulmonary embolism. Clin. Radiol. 1995, 50, 232–234. [Google Scholar] [CrossRef]
- van Rossum, A.B.; van Houwelingen, H.C.; Kieft, G.J.; Pattynama, P.M. Prevalence of deep vein thrombosis in suspected and proven pulmonary embolism: A meta-analysis. Br. J. Radiol. 1998, 71, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Wicki, J.; Perrier, A.; Perneger, T.V.; Bounameaux, H.; Junod, A.F. Predicting adverse outcome in patients with acute pulmonary embolism: A risk score. Thromb. Haemost. 2000, 84, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Sanchez, O.; Leroyer, C.; Musset, D.; Meyer, G.; Stern, J.-B.; Parent, F. Evaulation du Scanner Spiralé dans l’Embolie Pulmonaire Study Group. Deep venous thrombosis in patients with acute pulmonary embolism: Prevalence, risk factors, and clinical significance. Chest 2005, 128, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Cordeanu, M.; Gaertner, S.; Nouri, S.; Alt, M.; Stephan, D. Rivaroxaban-induced liver injury: Results from a venous thromboembolism registry. Int. J. Cardiol. 2015, 191, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Cordeanu, M.; Gaertner, S.; Faller, A.; Mirea, C.; Le Ray, I.; Stephan, D. Prognostic value of the simplified PESI score in comparison with the 2014 ESC risk model in pulmonary embolism. Int. J. Cardiol. 2016, 220, 623–624. [Google Scholar] [CrossRef]
- Gaertner, S.; Cordeanu, E.-M.; Nouri, S.; Faller, A.-M.; Frantz, A.-S.; Mirea, C.; Bilbault, P.; Ohlmann, P.; Le Ray, I.; Stephan, D. Rivaroxaban versus standard anticoagulation for symptomatic venous thromboembolism (REMOTEV observational study): Analysis of 6-month outcomes. Int. J. Cardiol. 2017, 226, 103–109. [Google Scholar] [CrossRef]
- Gaertner, S.; Cordeanu, E.-M.; Mirea, C.; Frantz, A.-S.; Auger, C.; Bilbault, P.; Ohlmann, P.; Schini-Kerth, V.; Stephan, D. Increased risk and severity of unprovoked venous thromboembolism with clustering cardiovascular risk factors for atherosclerosis: Results of the REMOTEV registry. Int. J. Cardiol. 2018, 252, 169–174. [Google Scholar] [CrossRef]
- Cordeanu, E.-M.; Younes, W.; Canuet, M.; Mirea, C.; Faller, A.-M.; Frantz, A.-S.; Daglayan, A.; Gaertner, S.; Stephan, D. Real-life practices of chronic thromboembolic pulmonary hypertension screening: Results from the REMOTEV observational study. Clin. Respir. J. 2018, 12, 2303–2306. [Google Scholar] [CrossRef]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; Janssens, U.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e419S–e496S. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. JTH 2005, 3, 692–694. [Google Scholar] [PubMed]
- Kasper, W.; Konstantinides, S.; Geibel, A.; Olschewski, M.; Heinrich, F.; Grosser, K.D.; Rauber, K.; Iversen, S.; Redecker, M.; Kienast, J. Management strategies and determinants of outcome in acute major pulmonary embolism: Results of a multicenter registry. J. Am. Coll. Cardiol. 1997, 30, 1165–1171. [Google Scholar] [CrossRef]
- Jiménez, D.; Aujesky, D.; Díaz, G.; Monreal, M.; Otero, R.; Martí, D.; Marín, E.; Aracil, E.; Sueiro, A.; Yusen, R.D.; et al. Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am. J. Respir. Crit. Care Med. 2010, 181, 983–991. [Google Scholar] [CrossRef]
- Quezada, C.A.; Bikdeli, B.; Barrios, D.; Morillo, R.; Nieto, R.; Chiluiza, D.; Barbero, E.; Guerassimova, I.; García, A.; Yusen, R.D.; et al. Assessment of coexisting deep vein thrombosis for risk stratification of acute pulmonary embolism. Thromb. Res. 2018, 164, 40–44. [Google Scholar] [CrossRef]
- Lee, J.S.; Moon, T.; Kim, T.H.; Kim, S.Y.; Choi, J.Y.; Lee, K.B.; Kwon, Y.J.; Song, S.H.; Kim, S.H.; Kim, H.O.; et al. Deep Vein Thrombosis in Patients with Pulmonary Embolism: Prevalance, Clinical Significance and Outcome. Vasc. Spec. Int. 2016, 32, 166–174. [Google Scholar] [CrossRef]
- Hirmerova, J.; Seidlerova, J.; Chudacek, Z. The Prevalence of Concomitant Deep Vein Thrombosis, Symptomatic or Asymptomatic, Proximal or Distal, in Patients with Symptomatic Pulmonary Embolism. Clin. Appl. Thromb. Hemost. 2018, 24, 1352–1357. [Google Scholar] [CrossRef]



| Total N (%)/M ± SD/ M (Q1–Q3) | PE with DVT N (%)/M ± SD/ M (Q1–Q3) | PE without DVT N (%)/M ± SD/ M (Q1–Q3) | p-Value | |
|---|---|---|---|---|
| N | 1037 | 727 (70.1) | 310 (29.9) | |
| Age (years) | 69 (55–80) | 71 (57–80) | 67 (50–78) | 0.002 |
| Age ≥ 70 years old | 499 (48.1) | 368 (50.6) | 131 (42.3) | 0.008 |
| Male | 493 (47.5) | 361 (49.7) | 132 (42.6) | 0.04 |
| Weight (kg) N = 1018 | 77.5 (67–188) | 78 (67–167) | 77 (68–188) | 0.47 |
| Weight ≤ 50 kg | 31 (3) | 18 (2.5) | 13 (4.3) | 0.19 |
| BMI (kg/m2) N = 987 | 27 (24–31) | 27 (24–31) | 27 (24–31) | 0.91 |
| BMI ≥ 30 kg/m2 | 324 (32.8) | 230 | 94 | 0.69 |
| eGFR (mL/min/1.73 m2) on admission | 85.5 (67.2–104.3) | 83 (65.1–100.7) | 91.9 (70.8–112.2) | 0.0001 |
| eGFR ≥ 90 | 460 (44.4) | 294 (40.4) | 166 (53.7) | |
| 60 ≤ eGFR < 90 | 381 (37.8) | 285 (39.2) | 96 (31.1) | |
| 30 ≤ eGFR < 60 | 166 (16) | 125 (17.2) | 41 (13.3) | |
| eGFR < 30 | 29 (2.8) | 23 (3.2) | 6 (1.9) | |
| CrCl Cockcroft (mL/min) on admission | 85.2 (58.7–119.6) | 81.7 (55.5–114.1) | 90.9 (64.7–128.4) | 0.001 |
| CrCl < 50 mL/min | 177 (17.4) | 137 (19.2) | 40 (13.2) | 0.02 |
| Cardiovascular risk factors | ||||
| Hypertension | 563 (54.3) | 418 (57.5) | 145 (46.8) | 0.001 |
| Diabetes | 165 (15.9) | 115 (15.8) | 50 (16.1) | 0.97 |
| Dyslipidemia | 344 (33.2) | 251 (34.5) | 93 (30) | 0.17 |
| Smoking (history or current) | 417 (41.2) | 287 (40.3) | 130 (43.3) | 0.40 |
| Medical history | ||||
| Previous thromboembolism | 332 (32) | 230 (31.6) | 102 (33) | 0.71 |
| PAD | 32 (3.1) | 20 (2.8) | 12 (3.9) | 0.71 |
| CAD | 62 (5.9) | 41 (5.6) | 21 (6.8) | 0.57 |
| COPD | 56 (5.4) | 32 (4.4) | 24 (7.7) | 0.04 |
| Active cancer. total | 89 (8.6) | 68 (9.4) | 21 (6.8) | 0.21 |
| Known thrombophilia | 41 (3.9) | 24 (3.3) | 17 (5.5) | 0.13 |
| Antithrombotic treatment on admission | ||||
| Antiplatelet | 202 (19.6) | 131 (18.1) | 71 (22.9) | 0.08 |
| Anticoagulation | 66 (6.4) | 40 (5.5) | 26 (8.4) | 0.11 |
| PE severity | ||||
| Low risk | 364 (35.1) | 220 (30.3) | 144 (46.5) | <0.0001 |
| Intermediate low | 397 (38.3) | 279 (38.4) | 118 (38.1) | |
| Intermediate high | 249 (24) | 202 (27.8) | 47 (15.1) | |
| High risk | 27 (2.6) | 26 (3.5) | 1 (0.3) | |
| DVT location | ||||
| Lower limbs | 717 (69.1) | 717 (98.6) | - | |
| Bilateral | 127 (12.2) | 127 (17.5) | - | |
| Proximal | 454 (43.8) | 454 (62.4) | - | |
| Distal | 263 (25.3) | 263 (36.2) | - | |
| Unusual site | 25 (2.4) | 25 (3.4) | - | |
| Isolated | 10 (1) | 10 (1.4) | - | |
| Type of VTE | ||||
| Unprovoked | 613 (59.1) | 430 (59.1) | 183 (59) | 1 |
| IVC filter | 13 (1.2) | 12 (1.6) | 1 (0.3) | 0.12 |
| PE Thrombolysis/-ectomy/-aspiration | 18 (1.7) | 17 (2.4) | 1 (0.3) | 0.02 |
| Anticoagulant treatment at discharge | ||||
| DOAC | 756 (72.9) | 528 (72.6) | 228 (73.5) | 0.81 |
| VKA | 135 (13) | 92 (12.6) | 43 (13.9) | 0.66 |
| LMWH/Fondaparinux | 145 (14) | 106 (14.6) | 39 (12.6) | 0.44 |
| No anticoagulant | 1 (0.1) | 1 (1.4) | 0 | 1 |
| Anticoagulant treatment duration | ||||
| 3 months | 93 (8.9) | 53 (7.2) | 40 (12.9) | 0.007 |
| 6 months | 474 (45.7) | 342 (47) | 132 (42.6) | 0.21 |
| Indefinite | 469 (45.2) | 331 (45.6) | 138 (44.5) | 0.81 |
| Risk Factor | Unadjusted HR (95% CI) | p–Value | Adjusted HR (95% CI) | p–Value |
|---|---|---|---|---|
| Age > 70 years old | 7.02 (5.15–9.65) | <0.001 | 4.85 (3.46–6.87) | <0.001 |
| Female sex | 1.47 (1.13–1.92) | <0.01 | 1.41 (1.04–1.91) | <0.05 |
| High blood pressure | 3.42 (2.60–4.52) | <0.001 | 1.56 (1.12–2.17) | <0.01 |
| Diabetes | 1.84 (1.24–2.77) | <0.01 | 0.95(0.60–1.52) | NS |
| eGFR < 60 mL/min/1.73 m2 | 5.35 (3.34–8.95) | <0.001 | 2.70 (1.64–4.61) | <0.001 |
| Cancer | 26.82 (7.12–225.30) | <0.001 | 30.64 (9.30–189.37) | <0.001 |
| COPD | 3.41 (1.57–8.45) | <0.001 | 4.00 (1.82–9.83) | <0.01 |
| Ischemic heart disease | 3.36 (1.62–7.86) | <0.001 | 1.50 (0.69–3.56) | NS |
| Presence of DVT | 1.99 (1.50–2.65) | <0.001 | 2.03 (1.47–2.82) | <0.001 |
| Known thrombophilia | 0.36 (0.18–0.72) | <0.01 | 0.88 (0.42–1.77) | NS |
| Outcome | Total n = 1037 | DVT n = 727 | DVT-Free n = 310 | HR (CI 95%) | p-Value |
|---|---|---|---|---|---|
| All-cause death | 50 | 38 | 12 | 1.36 (0.69–2.92) | 0.42 |
| VTE recurrence | 21 | 15 | 6 | 1.28 (0.44–4.55) | 0.80 |
| Major bleeding | 34 | 23 | 11 | 0.88 (0.40–20.4) | 0.70 |
| Clinically relevant non-major bleeding | 59 | 40 | 19 | 0.89 (0.49–1.65) | 0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeanu, E.-M.; Lambach, H.; Heitz, M.; Di Cesare, J.; Mirea, C.; Faller, A.-M.; Cavaro, A.-C.; Frantz, A.-S.; Gaertner, S.; Schini-Kerth, V.; et al. Pulmonary Embolism and Coexisting Deep Vein Thrombosis: A Detrimental Association? J. Clin. Med. 2019, 8, 899. https://doi.org/10.3390/jcm8060899
Cordeanu E-M, Lambach H, Heitz M, Di Cesare J, Mirea C, Faller A-M, Cavaro A-C, Frantz A-S, Gaertner S, Schini-Kerth V, et al. Pulmonary Embolism and Coexisting Deep Vein Thrombosis: A Detrimental Association? Journal of Clinical Medicine. 2019; 8(6):899. https://doi.org/10.3390/jcm8060899
Chicago/Turabian StyleCordeanu, Elena-Mihaela, Hélène Lambach, Marie Heitz, Julie Di Cesare, Corina Mirea, Alix-Marie Faller, Anne-Cécile Cavaro, Anne-Sophie Frantz, Sebastien Gaertner, Valérie Schini-Kerth, and et al. 2019. "Pulmonary Embolism and Coexisting Deep Vein Thrombosis: A Detrimental Association?" Journal of Clinical Medicine 8, no. 6: 899. https://doi.org/10.3390/jcm8060899




