Efficacy of the Small Step Program in a Randomized Controlled Trial for Infants under 12 Months Old at Risk of Cerebral Palsy (CP) and Other Neurological Disorders
Abstract
:1. Introduction
2. Methods and Design
2.1. Participants
2.2. Randomization, Blinding, and Sample Size
2.3. Intervention
2.4. Data Collection Procedure
2.5. Primary Outcome Measure
2.6. Secondary Outcome Measure
2.7. Follow-up Outcomes at Two Years of Age
2.8. Diagnosis and Brain Pathology
2.9. Statistics
3. Results
3.1. Participants
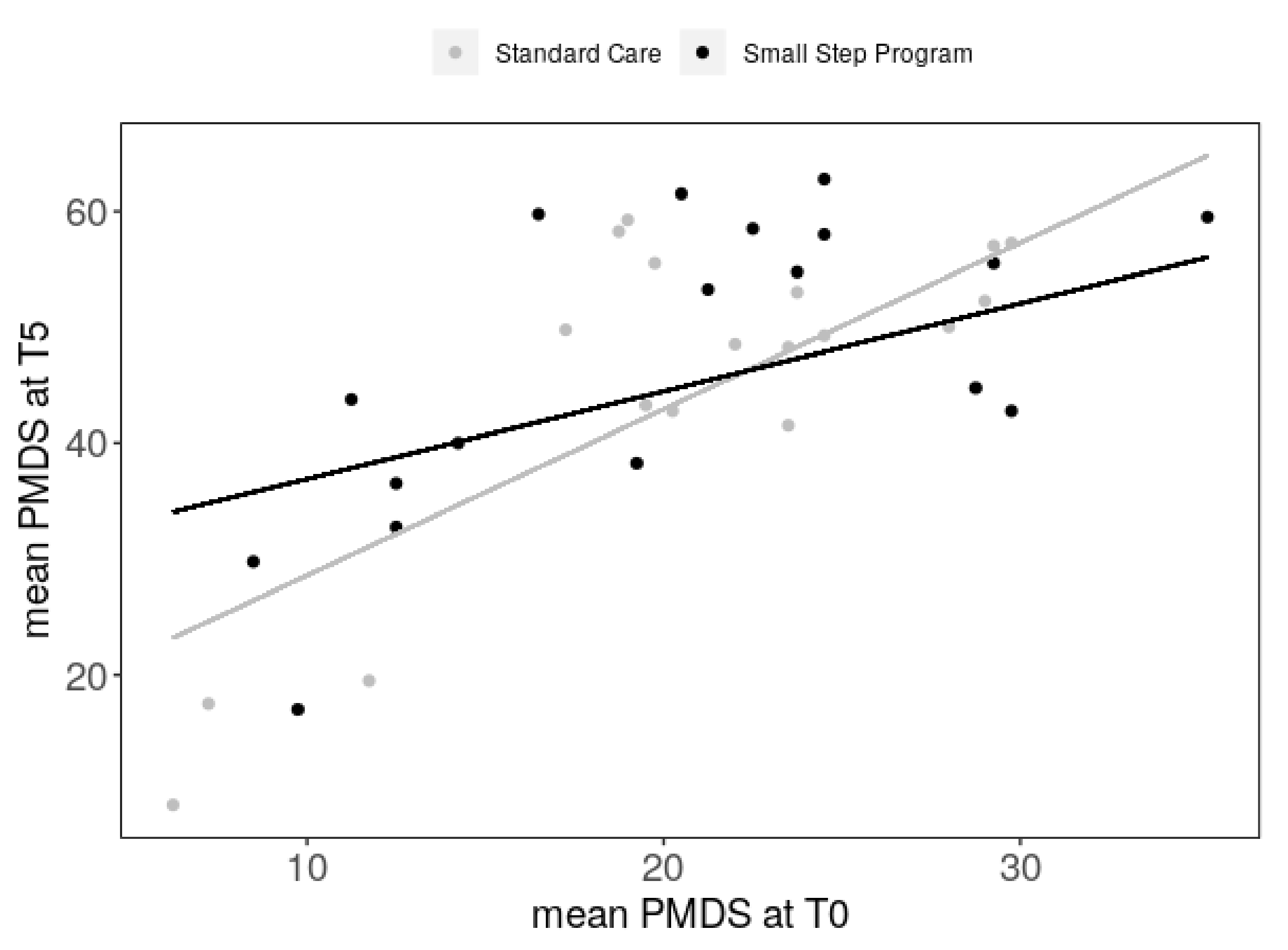
3.2. Primary Outcome Investigated after Intervention, T5
3.3. Secondary Outcomes after Intervention, T5
3.4. Follow-up at Two Years of Age, T6
3.5. Child Diagnostic Outcomes
3.6. Well-being of Mothers
3.7. Parental Experience of the Project
4. Discussion
4.1. The Unique Concept and Feasibility of the Small Step Program
4.2. Effect of Small Step in Relation to Previous Evidence
4.5. Strengths and Limitations
5. Clinical Implications and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McIntyre, S.; Morgan, C.; Walker, K.; Novak, I. Cerebral palsy-don’t delay. Dev. Disabil. Res. Rev. 2011, 17, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hubermann, L.; Boychuck, Z.; Shevell, M.; Majnemer, A. Age at Referral of Children for Initial Diagnosis of Cerebral Palsy and Rehabilitation: Current Practices. J. Child Neurol. 2016, 31, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev. Med. Child Neurol. 2000, 42, 816–824.
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Rogers, E.E.; Hintz, S.R. Early neurodevelopmental outcomes of extremely preterm infants. Semin. Perinatol. 2016, 40, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Serenius, F.; Ewald, U.; Farooqi, A.; Fellman, V.; Hafstrom, M.; Hellgren, K.; Marsal, K.; Ohlin, A.; Olhager, E.; Stjernqvist, K.; et al. Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA Pediatr. 2016, 170, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Hadders-Algra, M.; Boxum, A.G.; Hielkema, T.; Hamer, E.G. Effect of early intervention in infants at very high risk of cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2017, 59, 246–258. [Google Scholar] [CrossRef]
- Morgan, C.; Darrah, J.; Gordon, A.M.; Harbourne, R.; Spittle, A.; Johnson, R.; Fetters, L. Effectiveness of motor interventions in infants with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2016. [Google Scholar] [CrossRef]
- Chorna, O.; Hamm, E.; Cummings, C.; Fetters, A.; Maitre, N.L. Speech and language interventions for infants aged 0 to 2 years at high risk for cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2017, 59, 355–360. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Nordstrand, L.; Ek, L.; Lennartsson, F.; Sjostrand, L.; Tedroff, K.; Krumlinde-Sundholm, L. The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral-cerebral palsy; an explorative study with randomized design. Res. Dev. Disabil. 2018, 72, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hielkema, T.; Blauw-Hospers, C.H.; Dirks, T.; Drijver-Messelink, M.; Bos, A.F.; Hadders-Algra, M. Does physiotherapeutic intervention affect motor outcome in high-risk infants? An approach combining a randomized controlled trial and process evaluation. Dev. Med. Child Neurol. 2011, 53, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Novak, I.; Dale, R.C.; Guzzetta, A.; Badawi, N. Single blind randomised controlled trial of GAME (Goals-Activity-Motor Enrichment) in infants at high risk of cerebral palsy. Res. Dev. Disabil. 2016, 55, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- van Wassenaer-Leemhuis, A.G.; Jeukens-Visser, M.; van Hus, J.W.; Meijssen, D.; Wolf, M.J.; Kok, J.H.; Nollet, F.; Koldewijn, K. Rethinking preventive post-discharge intervention programmes for very preterm infants and their parents. Dev. Med. Child Neurol. 2016, 58 (Suppl. 4), 67–73. [Google Scholar] [CrossRef]
- Whittingham, K.; Wee, D.; Boyd, R. Systematic review of the efficacy of parenting interventions for children with cerebral palsy. Child Care Health Dev. 2011, 37, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C.; Holmstrom, L.; Aarne, P.; Nakeva von Mentzer, C.; Weiland, A.L.; Sjostrand, L.; Forssberg, H.; Tedroff, K.; Lowing, K. Efficacy of the small step program in a randomised controlled trial for infants below age 12 months with clinical signs of CP; a study protocol. BMC Pediatr. 2016, 16, 175. [Google Scholar] [CrossRef]
- Dubowitz, L.M.; Levene, M.I.; Morante, A.; Palmer, P.; Dubowitz, V. Neurologic signs in neonatal intraventricular hemorrhage: A correlation with real-time ultrasound. J. Pediatr. 1981, 99, 127–133. [Google Scholar] [CrossRef]
- Darrah, J.; Redfern, L.; Maguire, T.O.; Beaulne, A.P.; Watt, J. Intra-individual stability of rate of gross motor development in full-term infants. Early Hum. Dev. 1998, 52, 169–179. [Google Scholar] [CrossRef]
- Darrah, J.; Piper, M.; Watt, M.J. Assessment of gross motor skills of at-risk infants: Predictive validity of the Alberta Infant Motor Scale. Dev. Med. Child Neurol. 1998, 40, 485–491. [Google Scholar] [CrossRef]
- Morgan, C.; Novak, I.; Dale, R.C.; Badawi, N. Optimising motor learning in infants at high risk of cerebral palsy: A pilot study. BMC Pediatr. 2015, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Folio, M.R.; Fewell, R.R. Peabody Developmental Motor Scales, PDMS-2; PRO-ED, Inc.: Austin, TX, USA, 2000; Volume 2. [Google Scholar]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [PubMed]
- Russell, D.J.; Rosenbaum, P.; Avery, L.; Lane, M. Gross Motor Function Measure (GMFM-66 & GMFM 88); Users Manual; Cambridge University Press: London, UK, 2002. [Google Scholar]
- Krumlinde-Sundholm, L.; Ek, L.; Sicola, E.; Sjostrand, L.; Guzzetta, A.; Sgandurra, G.; Cioni, G.; Eliasson, A.C. Development of the Hand Assessment for Infants: Evidence of internal scale validity. Dev. Med. Child Neurol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bayley, N. Bayley Scale of Infant and Toddler Development, 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Haley, S.M.; Coster, W.L.; Ludlow, L.H.; Haltiwanger, J.T.; Andrellos, P.J. Pediatric Evaluation of Disability Inventory (PEDI). Development, Standardization and Administration Manual; New England Medical Center Hospital Inc. and PEDI Research Group: Boston, MA, USA, 1992. [Google Scholar]
- Nordmark, E.; Orban, K.; Hagglund, G.; Jarnlo, G.B. The American Paediatric Evaluation of Disability Inventory (PEDI). Applicability of PEDI in Sweden for children aged 2.0–6.9 years. Scand. J. Rehabil. Med. 1999, 31, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Cameron, D.; Rosenbaum, P.L.; Walter, S.D.; Russell, D. Stability of the gross motor function classification system. Dev. Med. Child Neurol. 2006, 48, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C.; Ullenhag, A.; Wahlstrom, U.; Krumlinde-Sundholm, L. Mini-MACS: Development of the Manual Ability Classification System for children younger than 4 years of age with signs of cerebral palsy. Dev. Med. Child Neurol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shevell, M.I.; Dagenais, L.; Hall, N. Comorbidities in cerebral palsy and their relationship to neurologic subtype and GMFCS level. Neurology 2009, 72, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Blauw-Hospers, C.H.; Dirks, T.; Hulshof, L.J.; Bos, A.F.; Hadders-Algra, M. Pediatric physical therapy in infancy: From nightmare to dream? A two-arm randomized trial. Phys. Ther. 2011, 91, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Sakzewski, L.; Gordon, A.; Eliasson, A.C. The State of the Evidence for Intensive Upper Limb Therapy Approaches for Children with Unilateral Cerebral Palsy. J. Child Neurol. 2014, 29, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Tsorlakis, N.; Evaggelinou, C.; Grouios, G.; Tsorbatzoudis, C. Effect of intensive neurodevelopmental treatment in gross motor function of children with cerebral palsy. Dev. Med. Child Neurol. 2004, 46, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.R.; Malamiri, R.A. Effect of treatment environment on modified constraint-induced movement therapy results in children with spastic hemiplegic cerebral palsy: A randomized controlled trial. Disabil. Rehabil. 2012, 34, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Schuengel, C.; Rentinck, I.C.; Stolk, J.; Voorman, J.M.; Loots, G.M.; Ketelaar, M.; Gorter, J.W.; Becher, J.G. Parents’ reactions to the diagnosis of cerebral palsy: Associations between resolution, age and severity of disability. Child Care Health Dev. 2009, 35, 673–680. [Google Scholar] [CrossRef] [PubMed]

| Small Step (n = 19) | Standard Care (n = 20) | P Value | |
|---|---|---|---|
| Child Characteristics | |||
| Gestational age, weeks, mean (SD) | 33 (6.5) | 33 (6.95) | p > 0.05 a |
| Inclusion CA age (T0), months, mean (SD) CA age at end of treatment (T5), mean (SD) | 6.3 (1.62) 16.7 (2.23) | 6.7 (1.96) 16.5 (1.96) | p > 0.05 a |
| Gender, male/female | 12/7 | 14/6 | p > 0.059 b |
| CP risk factors | |||
| Extreme premature/preterm/term | 6/5/8 | 8/3/9 | p > 0.059 b |
| AIMS (raw score) (T0) | 18.0 (6.87) | 19.52 (7,58) | p > 0.05 c |
| HINE (T0) Neurological signs Behavior | 49.0 (11.03) 13.8 (0.91) | 48.27 (12.76) 14.3 (1.80) | p > 0.05 c |
| Twins, n (families) | 4 (3) | 3 (2) | |
| MRI, available | 13 | 16 | |
| Treatment | |||
| Therapist-led treatment hours, mean | 28 | 16 range 0–58 | |
| Weeks included in study (T0–T5), mean (SD) | 43.5 (5.88) | 41.7 (5.23) | p > 0.05 c |
| Mother’s characteristics Age, years | 32.7 (4.49) | 34.7 (5.72) | p > 0.05 c |
| First-born child | 10 | 10 | |
| HADS, T0, frequency, d depression/no depression (missing) | 7/11 | 3/12 (4) | |
| HADS, T0, frequency, anxiety/no anxiety (missing) | 8/10 | 3/12 (4) | |
| HADS, total mean, SD | 13.5 (8,53) | 10.4 (7.0) | |
| Family socioeconomic factor Education of 1 parent beyond ≥12 years | 14 | 14 |
| Assessment | Small Step, n = 19 | Standard Care, n = 19 | ||||
|---|---|---|---|---|---|---|
| T0 | T5 | 2 Years | T0 | T5 | 2 Years | |
| PDMS, Stat | 21.9 (18.8–25.0) | 35 (32.7–37.3) | 39.22 (31.1–47.4) | 23.5 (20–26) | 33.8 (31–36.6) | 34.9 (32–37.9) |
| PDMS, Loc | 19.7 (15.8–23.6) | 58 (45.1–70.9) | 70.83 (56.1–85.6) | 20.4 (16.3–24.4) | 58.8 (46.7–71.0) | 71.7 (58.34–85.12) |
| PDMS, Gr | 17.5 (13.3–21.7) | 36 (32.6–39.4) | 38.94 (35.6–42.25) | 17.89 (13.9–21.9) | 34.78 (30.2–39.4) | 37.47 (32.6–42.3) |
| PDMS, Vm | 20 (14.4–25.6) | 59.6 (50.5–68.2) | 72 (62.7–81.3) | 20.68 (16.7–24.7) | 53.89 (43.2–64.6) | 71.15 (58.1–84.2) |
| GMFM-66 | 27.7 (24.8–30.5) | 48.4 (43–54) | 52.9 (45.4–60.3) | 29.2 (26.5–31.8) | 48.6 (43.6–53.7) | 54.0 (47.5–60.5) |
| PEDI, SC | 36.4 (32.7–40.2) | 32.2 (27.3–37.1) | 37.7 (34.9–40.6) | 27.2 (19.8–34.6) | ||
| PEDI, Mob | 43.2 (41.2–45.2) | 47.9 (40.9–54.9) | 37.7 (34.9–40.6) | 44.5 (33.7–55.2) | ||
| PEDI, Soc | 24.67 (19.24–30.1) | 43.2 (41.2–45.1) | 21.11 (13.2–29) | 41.2 (35.9–46.8) | ||
| BSID, Cog | 84 (73–96) | 81 (70–92) | ||||
| BSID, Lang | 82 (75–89) | 82 (73–91) | ||||
| BSID, Mot | 74 (61–87) | 75 (65–83) | ||||
| T5, After Intervention | T6, 2-Year Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean PDMS-2 | GMFM-66 * | Mean PDMS-2 | PEDI Mobility | |||||
| Reg. Coeff. | P-Value | Reg. Coeff. | P-Value | Reg. Coeff. | P-Value | Reg. Coeff. | P-Value | |
| Small Step/Standard Care | 18.271 | 0.02 | 18.977 | 0.05 | 21.497 | 0.02 | 15.666 | 0.03 |
| PDMS-2, T0 | 1.525 | <0.001 | 0.929 | 0.01 | 1.403 | 0.016 | 1.284 | <0.001 |
| HINE, T0 | 0.289 | 0.04 | 0.208 | 0.04 | 0.297 | 0.09 | 0.231 | 0.07 |
| Time, T0–T5 | 0.416 | 0.07 | 0.251 | 0.13 | 0.159 | 0.576 | 0.0297 | 0.89 |
| Inclusion age | –1.532 | 0.16 | –0.830 | 0.33 | –1.954 | 0.167 | –2.420 | 0.02 |
| GMFCS/2 groups | −9.522 | 0.26 | −13.305 | <0.001 | −15.540 | 0.01 | −17.482 | <0.001 |
| Interaction: Small Step/Standard Care and PDMS-2 T0 | −0.839 | 0.02 | −0.621 | 0.06 | −1.095 | 0.02 | −0.750 | 0.02 |
| Small Step (n = 19) | Standard Care (n = 19) | |
|---|---|---|
| Diagnosis | ||
| Bilateral CP | 5 | 6 |
| Unilateral CP | 0 | 1 |
| Dyskinetic CP | 3 | 2 |
| Ataxic CP | 1 | 0 |
| Unspecific CP | 1 | 1 |
| Malformation syndrome | 1 | 0 |
| Autism spectrum disorder | 1 | 0 |
| Other neurodevelopmental disorder | 6 | 8 |
| Delayed development | 1 | 0 |
| Typical development | 1 | 1 |
| Comorbidity | ||
| Epilepsy | 6 | 2 |
| Visual impairment | 3 | 4 |
| Hearing impairment (cochlea, n = 1) | 1 | 2 |
| Hydrocephalus, shunt | 3 | 3 |
| High comorbidity * | 6 | 3 |
| Neuroradiological findings | ||
| Grey matter injury | 1 | 2 |
| White matter damage of immaturity | 5 | 7 |
| Maldevelopment | 2 | 2 |
| Miscellaneous | 4 | 0 |
| No visual deviation | 1 | 5 |
| Missing | 6 | 3 |
| Mini-MACS | ||
| Level 1:2 | 8/2 | 12/3 |
| Level 3:4:5 | 6/0/3 | 2/1/2 |
| GMFCS | ||
| Level 1:2 | 5/7 | 13/1 |
| Level 3:4:5 | 4/1/2 | 2/1/2 |
| Questions (Scored 1–9) * | Small Step (n = 17) | Standard Care (n = 8) |
|---|---|---|
| Based on your experience of the intervention, how useful do you find this kind of treatment? | 8.6 | 6.4 |
| To what extent do you think the intervention/follow-up has affected the pace of your infant’s development? | 8.2 | 6.5 |
| What is your overall experience of the attitude and approach of the staff towards you and your family? | 8.6 | 9 |
| How important was answering the questions on parental wellbeing? | 6.7 | 6 |
| How useful were the hospital assessments of your child? | 8.3 | 7.5 |
| Do you feel you received sufficient feedback from us on the assessments of your child’s development? | 7.6 | 7.5 |
| How likely is it that you would recommend this treatment to a friend with an infant with developmental delay? | 8.8 | |
| Rate your motivation to participate in this training programme. | 8.3 | |
| During treatment, training was conducted for one developmental domain at a time: gross motor skills, communication, and fine motor skills. To what extent do you think that training each domain separately was an advantage (high value) or a disadvantage (low value)? | 8.1 | |
| You met several staff with different backgrounds, knowledge, and expertise. To what degree do you rate this as an advantage (high value) or a disadvantage (low value)? | 8.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmström, L.; Eliasson, A.-C.; Almeida, R.; Furmark, C.; Weiland, A.-L.; Tedroff, K.; Löwing, K. Efficacy of the Small Step Program in a Randomized Controlled Trial for Infants under 12 Months Old at Risk of Cerebral Palsy (CP) and Other Neurological Disorders. J. Clin. Med. 2019, 8, 1016. https://doi.org/10.3390/jcm8071016
Holmström L, Eliasson A-C, Almeida R, Furmark C, Weiland A-L, Tedroff K, Löwing K. Efficacy of the Small Step Program in a Randomized Controlled Trial for Infants under 12 Months Old at Risk of Cerebral Palsy (CP) and Other Neurological Disorders. Journal of Clinical Medicine. 2019; 8(7):1016. https://doi.org/10.3390/jcm8071016
Chicago/Turabian StyleHolmström, Linda, Ann-Christin Eliasson, Rita Almeida, Catarina Furmark, Ann-Louise Weiland, Kristina Tedroff, and Kristina Löwing. 2019. "Efficacy of the Small Step Program in a Randomized Controlled Trial for Infants under 12 Months Old at Risk of Cerebral Palsy (CP) and Other Neurological Disorders" Journal of Clinical Medicine 8, no. 7: 1016. https://doi.org/10.3390/jcm8071016





