The Effectiveness of 0.6% Povidone Iodine Eye Drops in Reducing the Conjunctival Bacterial Load and Needle Contamination in Patients Undergoing Anti-VEGF Intravitreal Injection: A Prospective, Randomized Study
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants
2.3. Collection of the Conjunctival Sample
2.4. Injection Procedure and Needle Collection
2.5. Microbiological Determinations
2.6. Adverse Events
2.7. Study Objectives
2.8. Statistical Analysis
3. Results
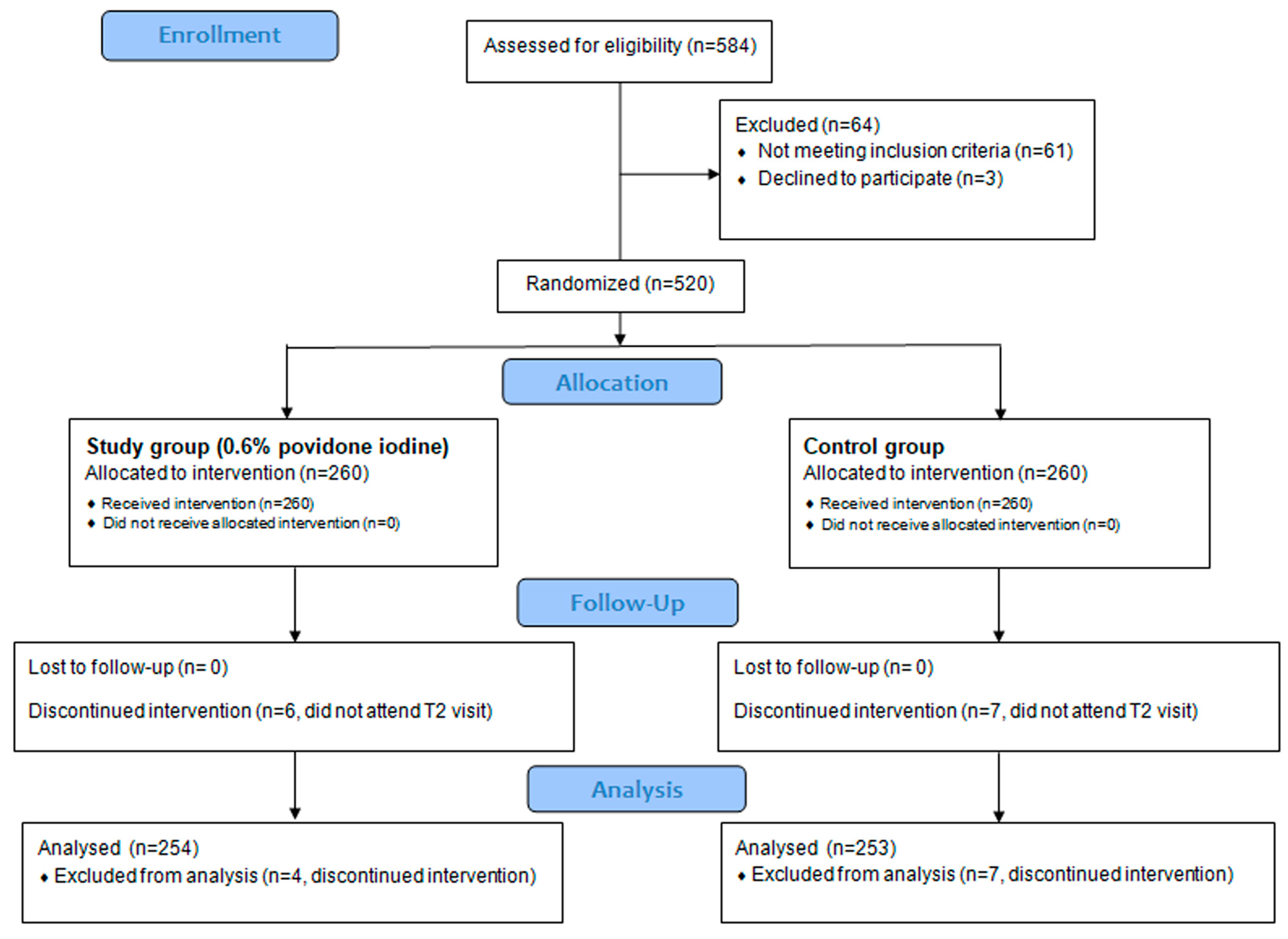
3.1. Patients Disposition and Baseline Characteristics
3.2. Primary Outcome Measures
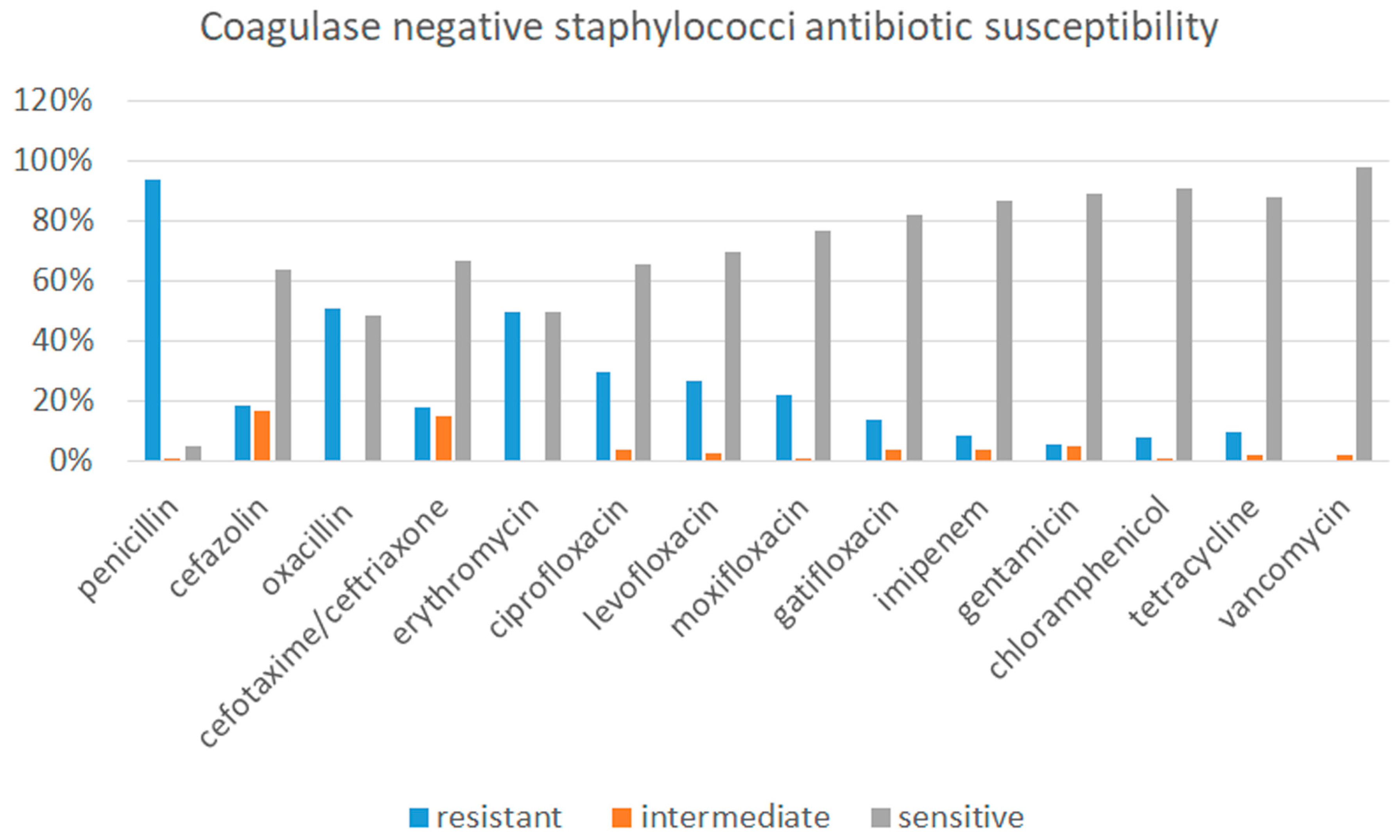
3.2.1. Microbiological Results from Conjunctival Swab
3.2.2. Microbiological Results from Injection Needle
3.3. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Ta, C.N. Minimizing the risk of endophthalmitis following intravitreous injections. Retina 2004, 24, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, A.R.; Glassman, A.R.; Stockdale, C.R.; Jampol, L.M. Diabetic Retinopathy Clinical Research Network. Elimination of Topical Antibiotics for Intravitreous Injections and the Importance of Using Povidone-Iodine: Update from the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2016, 134, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.; Cordovès, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data Dilemmas and Conclusion; European Society of Cataract and Refractive Surgeons: Dublin, Ireland, 2013; Available online: http://www.escrs.org/downloads/Endophthalmitis-Guidelines.pdf (accessed on 16 April 2019).
- Olson, R.J.; Braga-Mele, R.; Chen, S.H.; Miller, K.M.; Pineda, R., 2nd; Tweeten, J.P.; Musch, D.C. Cataract in the Adult Eye Preferred Practice Pattern®. Ophthalmology 2017, 124, P1–P119. [Google Scholar] [CrossRef] [PubMed]
- Ta, C.N.; Singh, K.; Egbert, P.R.; de Kaspar, H.M. Prospective comparative evaluation of povidone-iodine (10% for 5 minutes versus 5% for 1 minute) as prophylaxis for ophthalmic surgery. J. Cataract. Refract. Surg. 2008, 34, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Fileta, J.B.; Scott, I.U.; Flynn, H.W., Jr. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg. Lasers Imaging Retina 2014, 45, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Carrim, Z.I.; Mackie, G.; Gallacher, G.; Wykes, W.N. The efficacy of 5% povidone-iodine for 3 minutes prior to cataract surgery. Eur. J. Ophthalmol. 2009, 19, 560–564. [Google Scholar] [CrossRef]
- Halachmi-Eyal, O.; Lang, Y.; Keness, Y.; Miron, D. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J. Cataract Refract. Surg. 2009, 35, 2109–2114. [Google Scholar] [CrossRef]
- Koerner, J.C.; George, M.J.; Meyer, D.R.; Rosco, M.G.; Habib, M.M. Povidone-iodine concentration and dosing in cataract surgery. Surv. Ophthalmol. 2018, 63, 862–868. [Google Scholar] [CrossRef]
- De Caro, J.J.; Ta, C.N.; Ho, H.K.; Cabael, L.; Hu, N.; Sanislo, S.R.; Blumenkranz, M.S.; Moshfeghi, D.M.; Jack, R.; de Kaspar, H.M. Bacterial contamination of ocular surface and needles in patients undergoing intravitreal injections. Retina 2008, 28, 877–883. [Google Scholar] [CrossRef]
- Stewart, J.M.; Srivastava, S.K.; Fung, A.E.; Mahmoud, T.H.; Telander, D.G.; Hariprasad, S.M.; Ober, M.D.; Mruthyunjaya, P. Bacterial contamination of needles used for intravitreal injections: A prospective, multicenter study. Ocul. Immunol. Inflamm. 2011, 19, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.; Yactayo-Miranda, Y.; Weimann, S.; Froehlich, S.; Wolf, A.; Kampik, A.; Mino De Kaspar, H. Bacterial contamination of needle points after intravitreal injection. Eur. J. Ophthalmol. 2009, 19, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Tufan, H.A.; Vural, A.; Gencer, B.; Kara, S.; Arikan, S.; Yuksel, E. Bacterial contamination of needles used for intravitreal injections: Comparison between 27-gauge and 30-gauge needles. Ocul. Immunol. Inflamm. 2013, 21, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Berkelman, R.L.; Holland, B.W.; Anderson, R.L. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J. Clin. Microbiol. 1982, 15, 635–639. [Google Scholar] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Miño de Kaspar, H.; Kreutzer, T.C.; Aguirre-Romo, I.; Ta, C.N.; Dudichum, J.; Bayrhof, M.; Klauss, V.; Kampik, A. A prospective randomized study to determine the efficacy of preoperative topical levofloxacin in reducing conjunctival bacterial flora. Am. J. Ophthalmol. 2008, 145, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.M.; Sanislo, S.R.; Ta, C.N. Antibiotic susceptibility patterns of ocular bacterial flora in patients undergoing intravitreal injections. Ophthalmology 2010, 117, 2141–2145. [Google Scholar] [CrossRef]
- Martin, D.F. Evolution of Intravitreal Therapy for Retinal Diseases-From CMV to CNV: The LXXIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2018, 191, xli–lviii. [Google Scholar] [CrossRef]
- Reibaldi, M.; Longo, A.; Pulvirenti, A.; Avitabile, T.; Russo, A.; Cillino, S.; Mariotti, C.; Casuccio, A. Geo-Epidemiology of Age-Related Macular Degeneration: New Clues into the Pathogenesis. Am. J. Ophthalmol. 2016, 161, 78–93. [Google Scholar] [CrossRef]
- Reibaldi, M.; Pulvirenti, A.; Avitabile, T.; Bonfiglio, V.; Russo, A.; Mariotti, C.; Bucolo, C.; Mastropasqua, R.; Parisi, G.; Longo, A. Pooled Estimates of Incidence of Endophthalmitis After Intravitreal Injection of Anti-Vascular Endothelial Growth Factor Agents with and without Topical Antibiotic Prophylaxis. Retina 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Milder, E.; Vander, J.; Shah, C.; Garg, S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology 2012, 119, 1420–1424. [Google Scholar] [CrossRef]
- Musumeci, R.; Bandello, F.; Martinelli, M.; Calaresu, E.; Cocuzza, C.E. In Vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur. J. Ophthalmol. 2018, 7, 1120672118802541. [Google Scholar] [CrossRef]
- Pinna, A.; Donadu, M.G.; Usai, D.; D’amico-Ricci, G.; Boscia, F.; Zanetti, S. In Vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6%(IODIM (R)). ACTA Ophthalmol. 2018, 96, 111. [Google Scholar]
- Zamora, J.L. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am. J. Surg. 1986, 151, 400–406. [Google Scholar] [CrossRef]
- Trost, L.W.; Kivilcim, M.; Peyman, G.A.; Aydin, E.; Kazi, A.A. The effect of intravitreally injected povidone-iodine on Staphylococcus epidermidis in rabbit eyes. J. Ocul. Pharmacol. Ther. 2007, 23, 70–77. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, M.; Shen, T. The toxic effect of different concentrations of povidone iodine on the rabbit’s cornea. Cutan. Ocul. Toxicol. 2009, 28, 119–124. [Google Scholar] [CrossRef]
- Apt, L.; Isenberg, S.J.; Yoshimori, R.; Spierer, A. Outpatient topical use of povidone-iodine in preparing the eye for surgery. Ophthalmology 1989, 96, 289–292. [Google Scholar] [CrossRef]
- Hansmann, F.; Below, H.; Kramer, A.; Miiller, G.; Geerling, G. Prospective study to determine the penetration of iodide into the anterior chamber following preoperative application of topical 1.25% povidone–iodine. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 789–793. [Google Scholar] [CrossRef]
- Shimada, H.; Nakashizuka, H.; Hattori, T.; Mori, R.; Mizutani, Y.; Yuzawa, M. Effect of operative field irrigation on intraoperative bacterial contamination and postoperative endophthalmitis rates in 25-gauge vitrectomy. Retina 2010, 30, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Chen, P.; Wu, J.; Hua, H.; Yao, K. The occurrence rate of acute-onset postoperative endophthalmitis after cataract surgery in Chinese small-and medium-scale departments of ophthalmology. Sci. Rep. 2017, 7, 40776. [Google Scholar] [CrossRef]
- Peden, M.C.; Hammer, M.E.; Suñer, I.J. Dilute Povidone-Iodine Prophylaxis Maintains Safety While Improving Patient Comfort after Intravitreal Injections. Retina 2018, 00, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pepose, J.S.; Ahuja, A.; Liu, W.; Narvekar, A.; Haque, R. Randomized, Controlled, Phase 2 Trial of Povidone-Iodine/Dexamethasone Ophthalmic Suspension for Treatment of Adenoviral Conjunctivitis. Am. J. Ophthalmol. 2018, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Nakashizuka, H.; Shoji, J.; Shimada, H.; Yuzawa, M. Experimental Visualization and Quantification of Vitreous Contamination Following Intravitreal Injections. Retina 2016, 36, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Russo, A.; Zagari, M.; Toro, M.; De Grande, V.; Cifalinò, V.; Rametta, S.; Faro, S.; Longo, A. Resolution of Persistent Cystoid Macular Edema due to Central Retinal Vein Occlusion in a Vitrectomized Eye following Intravitreal Implant of Dexamethasone 0.7 mg. Case Rep. Ophthalmol. 2012, 3, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Fallico, M.R.; Russo, A.; De Grande, V.; Longo, A.; Uva, M.G.; Reibaldi, M.; Avitabile, T. Intravitreal dexamethasone implant for cystoid macular edema and inflammation after scleral buckling. Eur. J. Ophthalmol. 2015, 25, e98–e100. [Google Scholar] [CrossRef]
| Study Group (n = 254) | Control Group (n = 253) | |
|---|---|---|
| Male n, % | 114, 45% | 121, 48% |
| Age (mean ± SD) | 72 ± 8 | 74 ± 9 |
| Diagnosis | ||
| AMD | 126 (50%) | 135 (53%) |
| DME | 100 (39%) | 94 (37%) |
| RVO | 12 (5%) | 10 (4%) |
| Myopic CNV | 16 (6%) | 14 (6%) |
| Agent | ||
| Ranibizumab | 121 (48%) | 119 (47%) |
| Aflibercept | 133 (52%) | 134 (53%) |
| Blood Agar | Chocolate Agar | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||||
| N Units | Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | Study Group | Control Group |
| 0 to 10 | 172 | 181 | 240 | 168 | 209 | 203 | 246 | 197 |
| 11 to 100 | 74 | 61 | 14 | 76 | 41 | 48 | 8 | 51 |
| 101 to 1000 | 8 | 11 | 0 | 9 | 4 | 2 | 0 | 5 |
| Mean | 28.3 | 27.8 | 1.5 | 26.7 | 10.3 | 12.1 | 1 | 14.9 |
| SD | 45.4 | 51.6 | 6.4 | 48.9 | 30.6 | 32.9 | 5.8 | 39.8 |
| Tot | 254 | 253 | 254 | 253 | 254 | 253 | 254 | 253 |
| T0 | T1 | |||
|---|---|---|---|---|
| Study Group | Control Group | Study Group | Control Group | |
| Positive broth culture | 186 (73%) | 192 (76%) | 33 (13%) | 187 (74%) |
| Coagulase-negative Staphylococcus | 143 (77%) | 157 (82%) | 27 (82%) | 146 (78%) |
| Staphylococcus aureus | 13 (7%) | 10 (5%) | 2 (6%) | 15 (8%) |
| α-Hemolytic Streptococcus | 5 (3%) | 3 (2%) | 1 (3%) | 5 (3%) |
| Β-Hemolytic Streptococcus | 2 (1%) | 4 (2%) | 1 (3%) | 3 (2%) |
| Streptococcus group D | 6 (3%) | 3 (2%) | 0 | 4 (2%) |
| Corynebacterium species | 6 (3%) | 6 (3%) | 0 | 3 (2%) |
| Propionibacterium acnes | 2 (1%) | 4 (2%) | 1 (3%) | 4 (2%) |
| Micrococcus species | 2 (1%) | 3 (2%) | 0 | 4 (2%) |
| Other gram-negative rods | 5 (3%) | 4 (2%) | 0 | 3 (2%) |
| Bacillus species | 4 (2%) | 2 (1%) | 1 (3%) | 3 (2%) |
| Study Group (n = 254) | Control Group (n = 253) | Kruskall-Wallis | |
|---|---|---|---|
| Conjunctival hyperemia n, (%) | 2 (0.8%) | 2 (0.8%) | p > 0.05 |
| Conjunctival discharge n, (%) | 2 (0.8%) | 3 (1.2%) | p > 0.05 |
| Conjunctival follicles/papillae n, (%) | 1 (0.4%) | 1 (0.4%) | p > 0.05 |
| Eye pain n, (%) | 0 | 0 | p > 0.05 |
| Corneal epithelial erosion n, (%) | 3 (1.2%) | 1 (0.4) | p > 0.05 |
| Keratitis n, (%) | 0 | 0 | p > 0.05 |
| Eyelid edema n, (%) | 0 | 0 | p > 0.05 |
| Study Group (n = 254) | Control Group (n = 253) | Kruskall-Wallis | |
|---|---|---|---|
| No discomfort n, (%) | 241 (95%) | 241 (95%) | p > 0.05 |
| Mild discomfort n, (%) | 12 (5%) | 10 (4%) | p > 0.05 |
| Moderate discomfort n, (%) | 1 (0.4%) | 2 (0.8%) | p > 0.05 |
| Severe discomfort n, (%) | 0 | 0 | p > 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reibaldi, M.; Avitabile, T.; Bandello, F.; Longo, A.; Bonfiglio, V.; Russo, A.; Castellino, N.; Rejdak, R.; Nowomiejska, K.; Toro, M.; et al. The Effectiveness of 0.6% Povidone Iodine Eye Drops in Reducing the Conjunctival Bacterial Load and Needle Contamination in Patients Undergoing Anti-VEGF Intravitreal Injection: A Prospective, Randomized Study. J. Clin. Med. 2019, 8, 1031. https://doi.org/10.3390/jcm8071031
Reibaldi M, Avitabile T, Bandello F, Longo A, Bonfiglio V, Russo A, Castellino N, Rejdak R, Nowomiejska K, Toro M, et al. The Effectiveness of 0.6% Povidone Iodine Eye Drops in Reducing the Conjunctival Bacterial Load and Needle Contamination in Patients Undergoing Anti-VEGF Intravitreal Injection: A Prospective, Randomized Study. Journal of Clinical Medicine. 2019; 8(7):1031. https://doi.org/10.3390/jcm8071031
Chicago/Turabian StyleReibaldi, Michele, Teresio Avitabile, Francesco Bandello, Antonio Longo, Vincenza Bonfiglio, Andrea Russo, Niccolò Castellino, Robert Rejdak, Katarzyna Nowomiejska, Mario Toro, and et al. 2019. "The Effectiveness of 0.6% Povidone Iodine Eye Drops in Reducing the Conjunctival Bacterial Load and Needle Contamination in Patients Undergoing Anti-VEGF Intravitreal Injection: A Prospective, Randomized Study" Journal of Clinical Medicine 8, no. 7: 1031. https://doi.org/10.3390/jcm8071031
APA StyleReibaldi, M., Avitabile, T., Bandello, F., Longo, A., Bonfiglio, V., Russo, A., Castellino, N., Rejdak, R., Nowomiejska, K., Toro, M., Furino, C., Cillino, S., Fiore, T., Cagini, C., Grassi, P., Musumeci, R., Cocuzza, C. E., Martinelli, M., & Fallico, M. (2019). The Effectiveness of 0.6% Povidone Iodine Eye Drops in Reducing the Conjunctival Bacterial Load and Needle Contamination in Patients Undergoing Anti-VEGF Intravitreal Injection: A Prospective, Randomized Study. Journal of Clinical Medicine, 8(7), 1031. https://doi.org/10.3390/jcm8071031










