Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
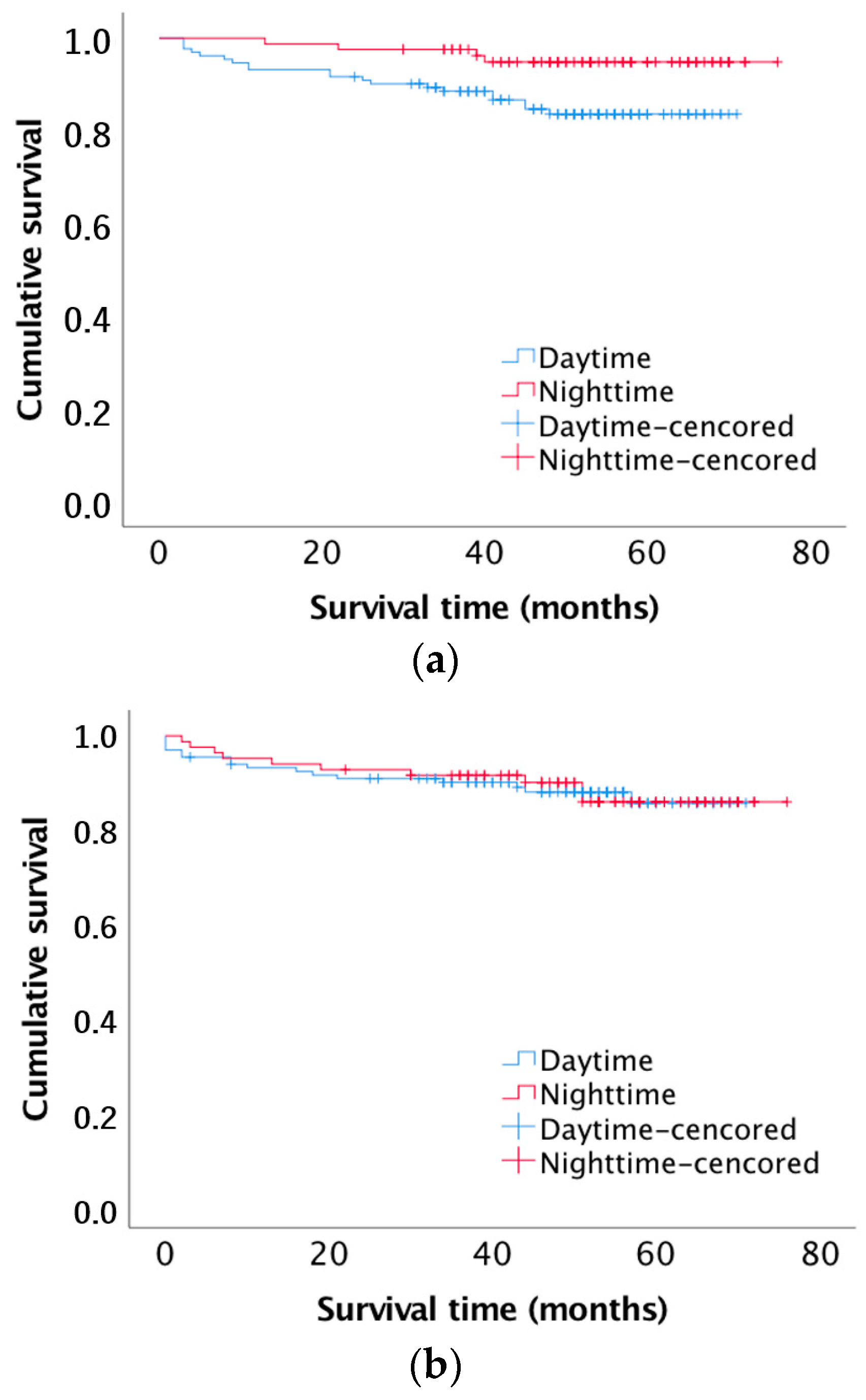
3.1. Patient and Graft Survival
3.2. Early Graft Failure
3.3. Postoperative Complications
4. Discussion
5. Concluding Remarks and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hariharan, S.; Johnson, C.P.; Bresnahan, B.A.; Taranto, S.E.; McIntosh, M.J.; Stablein, D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N. Engl. J. Med. 2000, 342, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef] [PubMed]
- Brockschmidt, C.; Huber, N.; Paschke, S.; Hartmann, B.; Henne-Bruns, D.; Wittau, M. Minimal access kidney transplant: A novel technique to reduce surgical tissue trauma. Exp. Clin. Transplant. 2012, 10, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Bessede, T.; Droupy, S.; Hammoudi, Y.; Bedretdinova, D.; Durrbach, A.; Charpentier, B.; Benoit, G. Surgical prevention and management of vascular complications of kidney transplantation. Transpl. Int. 2012, 25, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Osman, Y.; Shokeir, A.; Ali-el-Dein, B.; Tantawy, M.; Wafa, E.W.; el-Dein, A.B.; Ghoneim, M.A. Vascular complications after live donor renal transplantation: Study of risk factors and effects on graft and patient survival. J. Urol. 2003, 169, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Phelan, P.J.; O’Kelly, P.; Tarazi, M.; Tarazi, N.; Salehmohamed, M.R.; Little, D.M.; Magee, C.; Conlon, P.J. Renal allograft loss in the first post-operative month: Causes and consequences. Clin. Transplant. 2012, 26, 544–549. [Google Scholar] [CrossRef]
- Humar, A.; Matas, A.J. Surgical complications after kidney transplantation. Semin. Dial. 2005, 18, 505–510. [Google Scholar] [CrossRef]
- Debout, A.; Foucher, Y.; Trébern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015, 87, 343–349. [Google Scholar] [CrossRef]
- Olson, E.J.; Drage, L.A.; Auger, R.R. Sleep deprivation, physician performance, and patient safety. Chest 2009, 136, 1389–1396. [Google Scholar] [CrossRef]
- Lockley, S.W.; Barger, L.K.; Ayas, N.T.; Rothschild, J.M.; Czeisler, C.A.; Landrigan, C.P.; Harvard Work Hours, Health and Safety Group. Effects of health care provider work hours and sleep deprivation on safety and performance. Jt. Comm. J. Qual. Patient Saf. 2007, 33 (Suppl. 11), 7–18. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Hamilton, E.C.; O’Keefe, G.E.; Rege, R.V.; Valentine, R.J.; Jones, D.J.; Tesfay, S.; Thal, E.R. Effect of sleep deprivation on the performance of simulated laparoscopic surgical skill. Am. J. Surg. 2003, 186, 169–174. [Google Scholar] [CrossRef]
- Gerdes, J.; Kahol, K.; Smith, M.; Leyba, M.J.; Ferrara, J.J. Jack Barney award: The effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons. Am. J. Surg. 2008, 196, 813–819, discussion 819–820. [Google Scholar] [CrossRef]
- Peskun, C.; Walmsley, D.; Waddell, J.; Schemitsch, E. Effect of surgeon fatigue on hip and knee arthroplasty. Can. J. Surg. 2012, 55, 81–86. [Google Scholar] [CrossRef]
- Kelz, R.R.; Freeman, K.M.; Hosokawa, P.W.; Asch, D.A.; Spitz, F.R.; Moskowitz, M.; Henderson, W.G.; Mitchell, M.E.; Itani, K.M. Time of day is associated with postoperative morbidity: An analysis of the national surgical quality improvement program data. Ann. Surg. 2008, 247, 544–552. [Google Scholar] [CrossRef]
- Egol, K.A.; Tolisano, A.M.; Spratt, K.F.; Koval, K.J. Mortality rates following trauma: The difference is night and day. J. Emerg. Trauma Shock 2011, 4, 178–183. [Google Scholar]
- Schieman, C.; MacLean, A.R.; Buie, W.D.; Rudmik, L.R.; Ghali, W.A.; Dixon, E. Does surgeon fatigue influence outcomes after anterior resection for rectal cancer? Am. J. Surg. 2008, 195, 684–687, discussion 687–688. [Google Scholar] [CrossRef]
- Yaghoubian, A.; Kaji, A.H.; Ishaque, B.; Park, J.; Rosing, D.K.; Lee, S.; Stabile, B.E.; de Virgilio, C. Acute care surgery performed by sleep deprived residents: Are outcomes affected? J. Surg. Res. 2010, 163, 192–196. [Google Scholar] [CrossRef]
- Ellman, P.I.; Kron, I.L.; Alvis, J.S.; Tache-Leon, C.; Maxey, T.S.; Reece, T.B.; Peeler, B.B.; Kern, J.A.; Tribble, C.G. Acute sleep deprivation in the thoracic surgical resident does not affect operative outcomes. Ann. Thorac. Surg. 2005, 80, 60–64, discussion 64–65. [Google Scholar] [CrossRef]
- Rothschild, J.M.; Keohane, C.A.; Rogers, S.; Gardner, R.; Lipsitz, S.R.; Salzberg, C.A.; Yu, T.; Yoon, C.S.; Williams, D.H.; Wien, M.F.; et al. Risks of complications by attending physicians after performing nighttime procedures. JAMA 2009, 302, 1565–1572. [Google Scholar] [CrossRef]
- Lonze, B.E.; Parsikia, A.; Feyssa, E.L.; Khanmoradi, K.; Araya, V.R.; Zaki, R.F.; Segev, D.L.; Ortiz, J.A. Operative start times and complications after liver transplantation. Am. J. Transplant. 2010, 10, 1842–1849. [Google Scholar] [CrossRef]
- Fechner, G.; Pezold, C.; Hauser, S.; Gerhardt, T.; Muller, S.C. Kidney’s nightshift, kidney’s nightmare? Comparison of daylight and nighttime kidney transplantation: Impact on complications and graft survival. Transplant. Proc. 2008, 40, 1341–1344. [Google Scholar] [CrossRef]
- Kienzl-Wagner, K.; Schneiderbauer, S.; Bosmuller, C.; Schneeberger, S.; Pratschke, J.; Ollinger, R. Nighttime procedures are not associated with adverse outcomes in kidney transplantation. Transpl. Int. 2013, 26, 879–885. [Google Scholar] [CrossRef]
- Seow, Y.Y.; Alkari, B.; Dyer, P.; Riad, H. Cold ischemia time, surgeon, time of day, and surgical complications. Transplantation 2004, 77, 1386–1389. [Google Scholar] [CrossRef]
- Brunschot, D.M.; Hoitsma, A.J.; van der Jagt, M.F.; d’Ancona, F.C.; Donders, R.A.; van Laarhoven, C.J.; Hilbrands, L.B.; Warle, M.C. Nighttime kidney transplantation is associated with less pure technical graft failure. World J. Urol. 2016, 34, 955–961. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Taffinder, N.J.; McManus, I.C.; Gul, Y.; Russell, R.C.; Darzi, A. Effect of sleep deprivation on surgeons’ dexterity on laparoscopy simulator. Lancet 1998, 352, 1191. [Google Scholar] [CrossRef]
- Zuber, A.M.; Centeno, G.; Pradervand, S.; Nikolaeva, S.; Maquelin, L.; Cardinaux, L.; Bonny, O.; Firsov, D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. USA 2009, 106, 16523–16528. [Google Scholar] [CrossRef]
- Myung, J.; Wu, M.Y.; Lee, C.Y.; Rahim, A.R.; Truong, V.H.; Wu, D.; Piggins, H.D.; Wu, M.S. The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock. Int. J. Mol. Sci. 2019, 20, 2765. [Google Scholar] [CrossRef]
- Tokonami, N.; Mordasini, D.; Pradervand, S.; Centeno, G.; Jouffe, C.; Maillard, M.; Bonny, O.; Gachon, F.; Gomez, R.A.; Sequeira-Lopez, M.L.; et al. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J. Am. Soc. Nephrol. 2014, 25, 1430–1439. [Google Scholar] [CrossRef]
- Gumz, M.L. Tick tock: Time to recognize the kidney clock. J. Am. Soc. Nephrol. 2014, 25, 1369–1371. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Sayk, F.; Teckentrup, C.; Becker, C.; Heutling, D.; Wellhöner, P.; Lehnert, H.; Dodt, C. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R191–R197. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Leproult, R.; Van Cauter, E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat. Rev. Endocrinol. 2009, 5, 253–261. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Bender, A.M.; Dinges, D.F. Systematic individual differences in sleep homeostatic and circadian rhythm contributions to neurobehavioral impairment during sleep deprivation. Accid. Anal. Prev. 2012, 45, 11–16. [Google Scholar] [CrossRef]
- Chee, M.W.; Tan, J.C. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. Neuroimage 2010, 51, 835–843. [Google Scholar] [CrossRef]
- Van Dongen, H.P. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol. Int. 2006, 23, 1139–1147. [Google Scholar] [CrossRef]
- Leff, D.R.; Aggarwal, R.; Rana, M.; Nakhjavani, B.; Purkayastha, S.; Khullar, V.; Darzi, A.W. Laparoscopic skills suffer on the first shift of sequential night shifts: Program directors beware and residents prepare. Ann. Surg. 2008, 247, 530–539. [Google Scholar] [CrossRef]
- Lamond, N.; Dorrian, J.; Roach, G.D.; McCulloch, K.; Holmes, A.L.; Burgess, H.J.; Fletcher, A.; Dawson, D. The impact of a week of simulated night work on sleep, circadian phase, and performance. Occup. Environ. Med. 2003, 60, e13. [Google Scholar] [CrossRef]
- Katz, J.D. Noise in the operating room. Anesthesiology 2014, 121, 894–898. [Google Scholar] [CrossRef]
- Moorthy, K.; Munz, Y.; Forrest, D.; Pandey, V.; Undre, S.; Vincent, C.; Darzi, A. Surgical crisis management skills training and assessment: A simulation[corrected]-based approach to enhancing operating room performance. Ann. Surg. 2006, 244, 139–147. [Google Scholar] [CrossRef]
- Aggarwal, R.; Mishra, A.; Crochet, P.; Sirimanna, P.; Darzi, A. Effect of caffeine and taurine on simulated laparoscopy performed following sleep deprivation. Br. J. Surg. 2011, 98, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Yoon, P.D.; Chalasani, V.; Woo, H.H. Use of Clavien-Dindo classification in reporting and grading complications after urological surgical procedures: Analysis of 2010 to 2012. J. Urol. 2013, 190, 1271–1274. [Google Scholar] [CrossRef]
| Donor Characteristics | All (n = 215) | 8:00 a.m.–8:00 p.m. (n = 132) | 8:00 p.m.–8:00 a.m. (n = 83) | p-Value |
|---|---|---|---|---|
| Age (years) | 54.2 ± 14.8 | 55.2 ± 15.1 | 52.5 ± 14.3 | 0.19 |
| Male gender | 114 (53.0%) | 77 (58.3%) | 37 (44.6%) | 0.05 |
| BMI (kg/m2) | 25.8 ± 4.4 | 25.8 ± 4.2 | 25.8 ± 4.8 | 0.88 |
| Right kidney side | 107 (49.8%) | 66 (50.0%) | 41 (49.4%) | 0.93 |
| Multiple renal arteries (%) | 45 (20.9%) | 28 (21.2%) | 17 (20.5%) | 0.90 |
| Atherosclerosis of graft vessels | 128 (59.5%) | 77 (58.3%) | 51 (61.4%) | 0.65 |
| Recipient Characteristics | ||||
| Age (years) | 53.3 ± 14.7 | 54.6 ± 14.6 | 51.1 ±14.7 | 0.12 |
| Age > 65 years | 64 (29.8%) | 44 (33.3%) | 20 (24.1%) | 0.15 |
| Male gender | 120 (55.8%) | 78 (59.1%) | 42 (50.6%) | 0.22 |
| BMI (kg/m2) | 25.8 ± 4.4 | 26.2 ± 4.4 | 25.2 ± 4.5 | 0.12 |
| Cause of ESRD | ||||
| Glomerulonephritis | 85 (39.5%) | 52 (39.4%) | 33 (39.8%) | |
| Hypertension/renovascular | 41 (19.1%) | 25 (18.9%) | 16 (19.3%) | |
| Polycystic kidney disease | 31 (14.4%) | 20 (15.2%) | 11 (13.3%) | |
| Diabetes mellitus | 16 (7.4%) | 10 (7.6%) | 6 (7.2%) | |
| Interstitial nephritis | 8 (3.7%) | 5 (3.8%) | 3 (3.6%) | |
| System diseases | 8 (3.7%) | 4 (3.0%) | 4 (4.8%) | |
| Reflux nephropathy | 6 (2.8%) | 3 (2.3%) | 3 (3.6%) | |
| Congenital uropathy | 4 (1.9%) | 2 (1.5%) | 2 (2.4%) | |
| Other | 12 (5.6%) | 8 (6.1%) | 4 (4.8%) | |
| Unknown | 4 (1.9%) | 3 (2.3%) | 1 (1.2%) | |
| Re-transplantation | 29 (13.5%) | 17 (12.9%) | 12 (14.5%) | 0.74 |
| Duration on dialysis (days) | 2304 ± 1155 | 2331 ± 1145.5 | 2261 ± 1174.6 | 0.67 |
| Mean HLA-mismatches | 2.5 ± 1.5 | 2.5 ± 1.5 | 2.5 ± 1.5 | 0.95 |
| Co-Morbidities | ||||
| Diabetes mellitus | 44 (20.5%) | 29 (22.0%) | 15 (18.1%) | 0.49 |
| Hypertension | 184 (85.6%) | 110 (83.3%) | 74 (89.2%) | 0.24 * |
| Pre-transplant cardiovascular disease | 47 (21.9%) | 33 (25.0%) | 14 (16.9%) | 0.16 |
| Stroke | 18 (8.4%) | 11 (8.3%) | 7 (8.4%) | 0.98 |
| Peripheral vascular disease | 21 (9.8%) | 7 (5.3%) | 14 (16.9%) | 0.05 |
| Pre-transplant abdominal surgery | 84 (39.1%) | 56 (42.4%) | 28 (33.7%) | 0.20 |
| Operation Characteristics | ||||
| Total operative time (min) | 203 ± 46.3 | 203.5 ± 44.4 | 202.3 ± 49.6 | 0.85 |
| Warm ischemia time (min) | 51.2 ± 12.3 | 51.4 ± 12.1 | 50.8 ± 12.6 | 0.74 |
| Cold ischemia time (h) | 11.4 ± 4.5 | 10.7 ± 3.6 | 12.4 ± 5.3 | 0.01 * |
| Consultant | 161 (74.9%) | 101 (76.5%) | 60 (72.3%) | 0.49 |
| Intraoperative complication | 6 (2.8%) | 5 (3.8%) | 1 (1.2%) | 0.34 |
| All (n = 215) | 8:00 a.m.–8:00 p.m. (n = 132) | 8:00 p.m.–8:00 a.m. (n = 83) | p-Value | |
|---|---|---|---|---|
| Overall patient survival | 0.017 * | |||
| At 3 months | 212 (98.6%) | 129 (97.7%) | 83 (100%) | |
| At 1 year | 206 (95.8%) | 123 (93.2%) | 83 (100%) | |
| Death censored graft survival | 0.907 | |||
| At 3 months | 207 (96.3%) | 126 (95.5%) | 81 (97.6%) | |
| At 1 year | 202 (93.9%) | 123 (93.2%) | 79 (95.2%) | |
| Delayed graft function | 108 (50.2%) | 63 (47.7%) | 45 (54.2%) | 0.350 |
| Acute rejection rate | 50 (23.3%) | 29 (22%) | 21 (25.3%) | 0.570 |
| Serum creatinine (mg/dL) after transplantation median (range) | ||||
| 1 week (n = 213) | 4.4 (0.93–14.51) | 4.4 (0.93–12.99) | 4.4 (1.06–14.51) | 0.730 |
| 4 weeks (n = 212) | 1.72 (0.68–17.0) | 1.74 (0.68–17.0) | 1.71 (0.80–7.16) | 0.710 |
| 24 weeks (n = 205) | 1.45 (0.59–4.42) | 1.45 (0.59–4.42) | 1.45 (0.71–3.35) | 0.660 |
| 60 weeks (n = 200) | 1.38 (0.46–4.71) | 1.39 (0.46–4.71) | 1.34 (0.67–2.49) | 0.270 |
| Follow-up (months) | 49.2 ± 14.6 | 47.1 ± 15.6 | 52.50 ± 12.4 | 0.008 * |
| All (n = 215) | 8:00 a.m.–8:00 p.m. (n = 132) | 8:00 p.m.–8:00 a.m. (n = 83) | p-Value | |
|---|---|---|---|---|
| Complications (all grades) | 115 (46.5%) | 58 (43.9%) | 42 (50.6%) | 0.340 |
| Grade of complication * | ||||
| I | 25 (11.6%) | 17 (12.9%) | 8 (9.6%) | 0.470 |
| II | 39 (18.1%) | 24 (18.2%) | 15 (18.1%) | 0.984 |
| IIIa | 15 (7.0%) | 11 (8.3%) | 4 (4.8%) | 0.325 |
| IIIb | 28 (13.0%) | 16 (12.0%) | 12 (14.5%) | 0.620 |
| IVa | 3 (1.4%) | 3 (2.3%) | 0 (0%) | 0.286 |
| IVb | 4 1.9%) | 2 (1.5%) | 2 (2.4%) | 0.640 |
| V | 1 (0.5%) | 1 (0.8%) | 0 (0%) | 1.000 |
| Surgical Complications | All (n = 215) | 8:00 a.m.–8:00 p.m. (n = 132) | 8:00 p.m.–8:00 a.m. (n = 83) | p-Value |
|---|---|---|---|---|
| Vascular | ||||
| Renal artery stenosis | 2 (0.9%) | 1 (0.8%) | 1 (1.2%) | 1.0 |
| Renal vein thrombosis | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Iliac artery thrombosis | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Renal artery aneurysm | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Renal anastomotic leak | 1 (0.5%) | 0 | 1 (1.2%) | 0.39 |
| Renal pole infarct | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Coeliac Trunk stenosis | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Haemorrhagic | ||||
| Haematoma | 31 (14%) | 20 (15.2%) | 11 (13.3%) | 0.70 |
| Haemorrhage | 6 (2.8%) | 3 (2.3%) | 3 (3.6%) | 0.56 |
| Urological | ||||
| Urinary leak | 3 (1.4%) | 2 (1.5%) | 1 (1.2%) | 1.0 |
| Urethral necrosis | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Urethral stent complication | 1 (0.5%) | 1 (0.8%) | 0 | 1.0 |
| Urethral stricture | 2 (0.9%) | 1 (0.8%) | 1 (1.2%) | 1.0 |
| Bladder outflow obstruction/Blood clot retention | 4 (1.9%) | 4 (3.0%) | 0 | 0.16 |
| Wound related | ||||
| Lymphocele | 23 (10.7%) | 13 (9.8%) | 10 (12%) | 0.53 |
| Seroma | 17 (7.9%) | 9 (6.8%) | 8 (9.6%) | 0.46 |
| Wound dehiscence | 15 (7%) | 9 (6.8%) | 6 (7.3%) | 0.89 |
| Impaired wound healing | 3 (1.4%) | 0 | 3 (3.6%) | 0.06 |
| Wound infection | 3 (1.4%) | 3 (2.3%) | 0 | 0.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugünes, N.; Bichmann, A.; Biernath, N.; Peters, R.; Budde, K.; Liefeldt, L.; Schlomm, T.; Friedersdorff, F. Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes. J. Clin. Med. 2019, 8, 1051. https://doi.org/10.3390/jcm8071051
Sugünes N, Bichmann A, Biernath N, Peters R, Budde K, Liefeldt L, Schlomm T, Friedersdorff F. Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes. Journal of Clinical Medicine. 2019; 8(7):1051. https://doi.org/10.3390/jcm8071051
Chicago/Turabian StyleSugünes, Nesrin, Anna Bichmann, Nadine Biernath, Robert Peters, Klemens Budde, Lutz Liefeldt, Thorsten Schlomm, and Frank Friedersdorff. 2019. "Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes" Journal of Clinical Medicine 8, no. 7: 1051. https://doi.org/10.3390/jcm8071051
APA StyleSugünes, N., Bichmann, A., Biernath, N., Peters, R., Budde, K., Liefeldt, L., Schlomm, T., & Friedersdorff, F. (2019). Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes. Journal of Clinical Medicine, 8(7), 1051. https://doi.org/10.3390/jcm8071051




