Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application
Abstract
:1. Introduction
2. Characteristics of ASCs
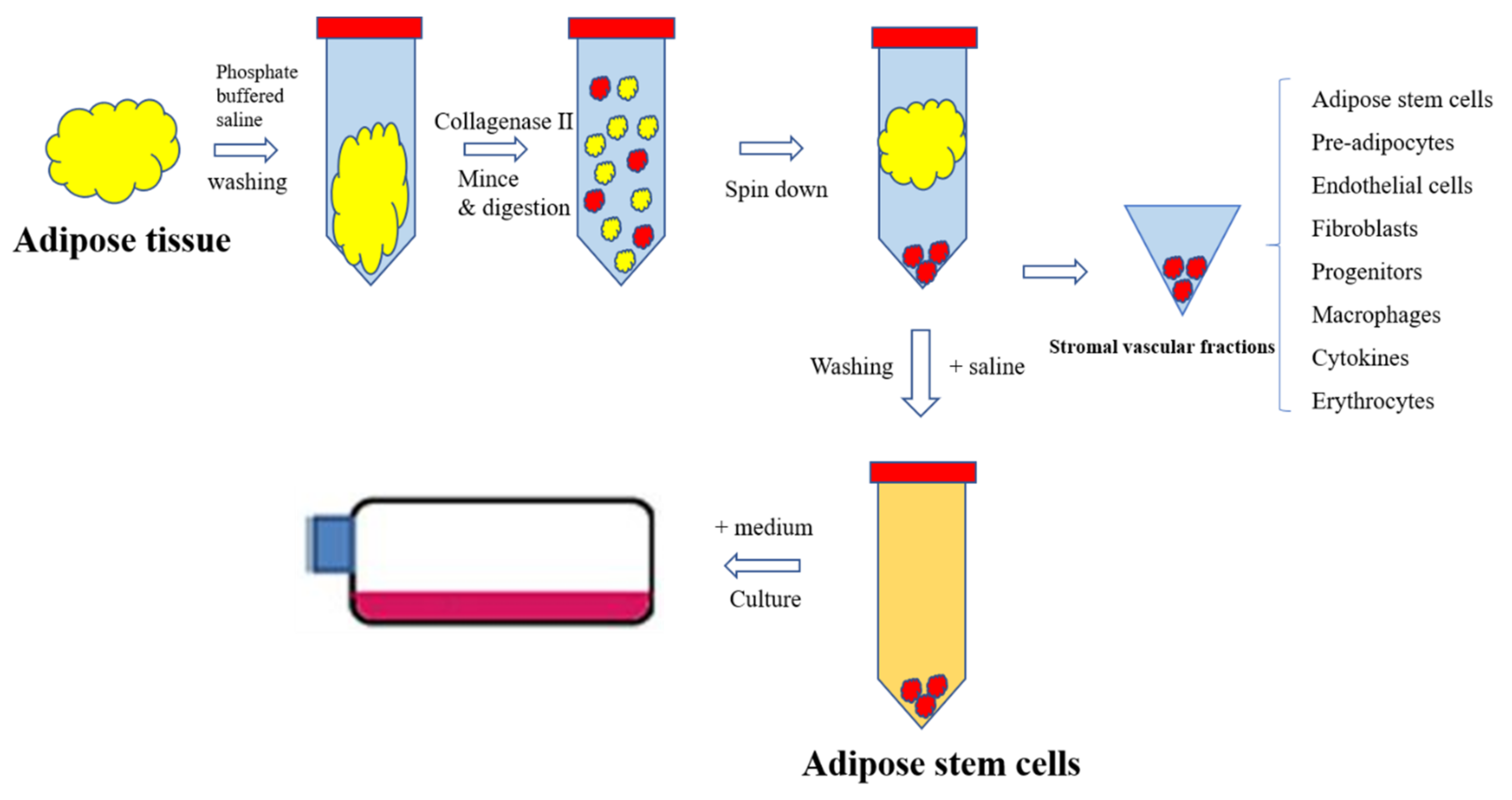
3. Processes in Isolation of ASCs
4. Processes in the Culture of ASCs
5. Processes in the Differentiation of ASCs
6. Processes in the Storage of ASCs
7. Processes in the Clinical Application of ASCs
8. Conclusions
9. Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rogne, M.; Chu, D.-T.; Küntziger, T.M.; Mylonakou, M.-N.; Collas, P.; Tasken, K.; Parton, R.G. OPA1-anchored PKA phosphorylates perilipin 1 on S522 and S497 in adipocytes differentiated from human adipose stem cells. Mol. Biol. Cell 2018, 29, 1487–1501. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Tao, Y. Human thermogenic adipocytes: A reflection on types of adipocyte, developmental origin, and potential application. J. Physiol. Biochem. 2017, 73, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Tao, Y.; Son, L.H.; Le, D.-H. Cell source, differentiation, functional stimulation, and potential application of human thermogenic adipocytes in vitro. J. Physiol. Biochem. 2016, 73, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.U.; Greiser, U.; Wang, W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014, 22, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Shingyochi, Y.; Orbay, H.; Mizuno, H. Adipose-derived stem cells for wound repair and regeneration. Expert Opin. Biol. Ther. 2015, 15, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Holm, J.S.; Toyserkani, N.M.; Sorensen, J.A. Adipose-derived stem cells for treatment of chronic ulcers: Current status. Stem Cell Res. Ther. 2018, 9, 142. [Google Scholar] [CrossRef]
- Sabol, R.A.; Bowles, A.C.; Côté, A.; Wise, R.; Pashos, N.; Bunnell, B.A. Therapeutic Potential of Adipose Stem Cells. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jeong, J.-H. Clinical application of adipose stem cells in plastic surgery. J. Korean Med. Sci. 2014, 29, 462–467. [Google Scholar] [CrossRef]
- The Number of Trials on Human by Year. Available online: https://www.clinicaltrials.gov (accessed on 10 April 2019).
- Phase of Adipose Stem Cell Trials from 2007 to 2019. Available online: https://www.clinicaltrials.gov (accessed on 10 April 2019).
- Map of Clinical Trials on Adipose Stem Cells in the WorLd. Available online: https://www.clinicaltrials.gov (accessed on 10 April 2019).
- Zhao, L.; Johnson, T.; Liu, D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res. Ther. 2017, 8, 125. [Google Scholar] [CrossRef]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.M.; Seifalian, A.M.; et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koh, Y.G.; Choi, Y.-J.; Kim, S.-H.; Yoon, D.S.; Lee, M.; Lee, J.W. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Patrikoski, M.; Mannerstr, B.; Mannerström, S. Perspectives for Clinical Translation of Adipose Stromal/Stem Cells. Stem Cells Int. 2019, 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Bowles, A.C.; Wise, R.M.; Gerstein, B.Y.; Thomas, R.C.; Ogelman, R.; Febbo, I.; Bunnell, B.A. Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells 2017, 35, 2198–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Berman, M.; Lander, E. A Prospective Safety Study of Autologous Adipose-Derived Stromal Vascular Fraction Using a Specialized Surgical Processing System. Am. J. Cosmet. Surg. 2017, 34, 129–142. [Google Scholar] [CrossRef]
- Riester, S.M.; Denbeigh, J.M.; Lin, Y.; Jones, D.L.; De Mooij, T.; Lewallen, E.A.; Nie, H.; Paradise, C.R.; Radel, D.J.; Dudakovic, A. Safety studies for use of adipose tissue-derived mesenchymal stromal/stem cells in a rabbit model for osteoarthritis to support a phase i clinical trial. Stem Cells Transl. Med. 2017, 6, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Sano, M.; Urushihata, N.; Tanemura, H.; Oki, K.; Suzaki, E. Assessment of safety and feasibility of human allogeneic adipose-derived mesenchymal stem cells in a pediatric patient. Pediatr. Res. 2018, 84, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V. Adipose stem cells in the clinic. Biomed. Res. Ther. 2014, 1, 57–70. [Google Scholar]
- Raposio, E.; Simonacci, F.; Perrotta, R.E. Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann. Med. Surg. 2017, 20, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Caruana, G.; Petrella, M.; Bonomini, S.; Grieco, M.P. A standardized method of isolating adipose-derived stem cells for clinical applications. Ann. Plast. Surg. 2016, 76, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Roxburgh, J.; Metcalfe, A.D.; Martin, Y.H. The effect of medium selection on adipose-derived stem cell expansion and differentiation: Implications for application in regenerative medicine. Cytotechnology 2016, 68, 957–967. [Google Scholar] [CrossRef]
- Legzdina, D.; Romanauska, A.; Nikulshin, S.; Kozlovska, T.; Berzins, U. Characterization of senescence of culture-expanded human adipose-derived mesenchymal stem cells. Int. J. Stem Cells 2016, 9, 124. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Kuo, T.-L.; Kuo, K.-K.; Hsiao, C.-C. Human adipose-derived stem cells: Isolation, characterization and current application in regeneration medicine. Genom. Med. Biomark. Health Sci. 2011, 3, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.-T.; Tao, Y.; Taskén, K. OPA1 in Lipid Metabolism: Function of OPA1 in Lipolysis and Thermogenesis of Adipocytes. Horm. Metab. Res. 2017, 49, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, D.-T.; Tao, Y. A homologous stem cell therapy for obesity and its related metabolic disorders. Med. Hypotheses 2017, 103, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Malinowska, E.; Gawronska-Kozak, B.; Kozak, L.P. Expression of Adipocyte Biomarkers in a Primary Cell Culture Models Reflects Preweaning Adipobiology. J. Biol. Chem. 2014, 289, 18478–18488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, D.-T.; Gawronska-Kozak, B. Brown and brite adipocytes: Same function, but different origin and response. Biochimie 2017, 138, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.I.N.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Bertozzi, N. Isolation of Ready-to-Use Adipose-Derived Stem Cell (ASC) Pellet for Clinical Applications and a Comparative Overview of Alternate Methods for ASC Isolation. Curr. Protoc. Stem Cell Biol. 2017, 41, 1F–17. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Picardo, M. Maximizing non-enzymatic methods for harvesting adipose-derived stem from lipoaspirate: Technical considerations and clinical implications for regenerative surgery. Sci. Rep. 2017, 7, 10015. [Google Scholar] [CrossRef]
- Raposio, E.; Caruana, G.; Bonomini, S.; Libondi, G. A novel and effective strategy for the isolation of adipose-derived stem cells: Minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2014, 133, 1406–1409. [Google Scholar]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef]
- Ong, W.K.; Tan, C.S.; Chan, K.L.; Goesantoso, G.G.; Chan, X.H.D.; Chan, E.; Yin, J.; Yeo, C.R.; Khoo, C.M.; So, J.B.Y.; et al. Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Rep. 2014, 2, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Ellenhorn, J.D.I. Adipose stromal vascular fraction isolation: A head-to-head comparison of four commercial cell separation systems. Plast. Reconstr. Surg. 2013, 132, 932e–939e. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Pratta, A.-S.; Abbassi, N.; Fabre, H.; Rodriguez, F.; Debard, C.; Adobati, J.; Boucher, F.; Mallein-Gerin, F.; Auxenfans, C. Evaluation of three devices for the isolation of the stromal vascular fraction from adipose tissue and for ASC culture: a comparative study. Stem Cells Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, R.A.; Aronowitz, J.A.; Dos-Anjos Vilaboa, S. Use of Freshly Isolated Human Adipose Stromal Cells for Clinical Applications. Aesthet. Surg. J. 2017, 37, S4–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Song, K.; Xu, L.; Zhao, F.; Tian, H.; Zhou, C.; Xu, Z.; Ge, Y.; Wu, R.; Jia, R. Protective Effects of Uncultured Adipose-Derived Stromal Vascular Fraction on Testicular Injury Induced by Torsion-Detorsion in Rats. Stem Cells Transl. Med. 2018, 8, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, L.; Shen, B.; Ling, P.; Liu, S.; Xue, J.; Liu, F.; Shao, H.; Chen, J.; Ma, A.; Liu, X. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS ONE 2017, 12, e0176107. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Zachar, V.; Boucher, S.; Vemuri, M.C.; Pennisi, C.P.; Fink, T. Critical steps in the isolation and expansion of adipose-derived stem cells for translational therapy. Expert Rev. Mol. Med. 2015, 17, e11. [Google Scholar] [CrossRef]
- He, Q.; Ye, Z.; Zhou, Y.; Tan, W.-S. Comparative study of mesenchymal stem cells from rat bone marrow and adipose tissue. Turk. J. Biol. 2018, 42, 477–489. [Google Scholar] [CrossRef]
- Aghayan, H.-R.; Goodarzi, P.; Arjmand, B. GMP-compliant human adipose tissue-derived mesenchymal stem cells for cellular therapy. In Stem Cells and Good Manufacturing Practices; Humana Press: New York, NY, USA, 2014; pp. 93–107. [Google Scholar]
- Van Pham, P.; Vu, N.B. In Vitro expansion of mesenchymal stem cells for clinical use. Prog. Stem Cell 2016, 3, 87–96. [Google Scholar] [CrossRef]
- Agostini, F.; Rossi, F.M.; Aldinucci, D.; Battiston, M.; Lombardi, E.; Zanolin, S.; Massarut, S.; Parodi, P.C.; Da Ponte, A.; Tessitori, G. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res. Ther. 2018, 9, 130. [Google Scholar] [CrossRef]
- Haack-Sørensen, M.; Juhl, M.; Follin, B.; Harary Søndergaard, R.; Kirchhoff, M.; Kastrup, J.; Ekblond, A. Development of large-scale manufacturing of adipose-derived stromal cells for clinical applications using bioreactors and human platelet lysate. Scan. J. Clin. Lab. Investig. 2018, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Phetfong, J.; Tawonsawatruk, T.; Seenprachawong, K.; Srisarin, A.; Isarankura-Na-Ayudhya, C.; Supokawej, A. Re-using blood products as an alternative supplement in the optimisation of clinical-grade adipose-derived mesenchymal stem cell culture. Bone Jt. Res. 2017, 6, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, P.; Bui, K.H.-T.; Ngo, D.Q.; Vu, N.B.; Truong, N.H.; Phan, N.L.-C.; Le, D.M.; Duong, T.D.; Nguyen, T.D.; Le, V.T. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res. Ther. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Youn, C.; Kim, J.H.; Park, B.J.; Ahn, J.; Hong, S.; Kim, Y.-D.; Shin, Y.K.; Park, S.G. Enhanced cell growth of adipocyte-derived mesenchymal stem cells using chemically-defined serum-free media. Int. J. Mol. Sci. 2017, 18, 1779. [Google Scholar] [CrossRef] [PubMed]
- Patrikoski, M.; Juntunen, M.; Boucher, S.; Campbell, A.; Vemuri, M.C.; Mannerström, B.; Miettinen, S. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res. Ther. 2013, 4, 27–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmaier, C.; Jossen, V.; Kaiser, S.C.; Jüngerkes, F.; Brill, S.; Safavi-Nab, A.; Siehoff, A.; van den Bos, C.; Eibl, D.; Eibl, R. Scale-up of adipose tissue-derived mesenchymal stem cell production in stirred single-use bioreactors under low-serum conditions. Eng. Life Sci. 2014, 14, 292–303. [Google Scholar] [CrossRef]
- Dufey, V.; Tacheny, A.; Art, M.; Becken, U.; De Longueville, F. Expansion of human bone marrow-derived mesenchymal stem cells in BioBLU 0.3 c single-use bioreactors. Appl. Note 2016, 305, 1–8. [Google Scholar]
- Lipsitz, Y.Y.; Timmins, N.E.; Zandstra, P.W. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016, 34, 393. [Google Scholar] [CrossRef]
- Lawson, T.; Kehoe, D.E.; Schnitzler, A.C.; Rapiejko, P.J.; Der, K.A.; Philbrick, K.; Punreddy, S.; Rigby, S.; Smith, R.; Feng, Q. Process development for expansion of human mesenchymal stromal cells in a 50 L single-use stirred tank bioreactor. Biochem. Eng. J. 2017, 120, 49–62. [Google Scholar] [CrossRef]
- Sart, S.; Agathos, S.N.; Li, Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol. Prog. 2013, 29, 1354–1366. [Google Scholar] [CrossRef]
- Lauvrud, A.T.; Kelk, P.; Wiberg, M.; Kingham, P.J. Characterization of human adipose tissue-derived stem cells with enhanced angiogenic and adipogenic properties. J. Tissue Eng. Regen. Med. 2017, 11, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Shabani Azandaryani, Z.; Davoodian, N.; Samiei, A.; Rouzbehan, S. Insulin-like growth factor-I promotes hepatic differentiation of human adipose tissue-derived stem cells. Cell Biol. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Grottkau, B.E.; Lin, Y. Osteogenesis of Adipose-Derived Stem Cells. Bone Res. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.; Farahzadi, R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/β-catenin signaling pathways. PLoS ONE 2017, 12, e0173877. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, J.-S.; Jeong, H.-S. Neural differentiation of human adipose tissue-derived stem cells involves activation of the Wnt5a/JNK signalling. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Cho, H.-H.; Cho, Y.-B.; Park, J.-S.; Jeong, H.-S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.D.; Li, M.; Liao, X.; Li, S.H.; Yan, J.X.; Fan, L.; She, W.L.; Song, J.X.; Liu, H.W. Effects of storage culture media, temperature and duration on human adipose-derived stem cell viability for clinical use. Mol. Med. Rep. 2019, 19, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Nofianti, C.E.; Sari, I.N.; Marlina, N.; Pawitan, J.A. Temporary storage solution for adipose derived mesenchymal stem cells. Stem Cell Investig. 2018, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Wu, X.; Gimble, J.; Devireddy, R. Effects of Decade Long Freezing Storage on Adipose Derived Stem Cells Functionality. Sci. Rep. 2018, 8, 8162. [Google Scholar] [CrossRef]
- Badowski, M.S.; Muise, A.; Harris, D.T. Patient use of autologous cryopreserved intact adipose tissue from lipoaspirate. AIMS Cell Tissue Eng. 2018, 1, 224–235. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.J.; Kim, J.-H.; Hong, S.J. Adipose Tissue-Derived Stem Cells for Myocardial Regeneration. Korean Circ. J. 2017, 47, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Yan, Y.; Song, Y.-H.; Seidensticker, M.; Rabinovich, B.; Metzele, R.; Bankson, J.A.; Vykoukal, D.; Alt, E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 2009, 31, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haenel, A.; Ghosn, M.; Karimi, T.; Vykoukal, J.; Kettlun, C.; Shah, D.; Dave, A.; Valderrabano, M.; Schulz, D.; Azares, A. Unmodified, autologous adipose-derived regenerative cells improve cardiac function, structure and revascularization in a porcine model of chronic myocardial infarction. bioRxiv 2018. [Google Scholar] [CrossRef]
- Woudstra, L.; Krijnen, P.A.J.; Bogaards, S.J.P.; Meinster, E.; Emmens, R.W.; Kokhuis, T.J.A.; Bollen, I.A.E.; Baltzer, H.; Baart, S.M.T.; Parbhudayal, R.; et al. Development of a new therapeutic technique to direct stem cells to the infarcted heart using targeted microbubbles: StemBells. Stem Cell Res. 2016, 17, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-K.; Park, J.-K.; Kim, J.-H.; Lee, K.-M.; Kim, E.; Jeong, K.-S.; Jeon, W.B. Integrin-binding elastin-like polypeptide as an in situ gelling delivery matrix enhances the therapeutic efficacy of adipose stem cells in healing full-thickness cutaneous wounds. J. Control. Release 2016, 237, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Björninen, M.; Gilmore, K.; Pelto, J.; Seppänen-Kaijansinkko, R.; Kellomäki, M.; Miettinen, S.; Wallace, G.; Grijpma, D.; Haimi, S. Electrically stimulated adipose stem cells on polypyrrole-coated scaffolds for smooth muscle tissue engineering. Ann. Biomed. Eng. 2017, 45, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neoh, K.G.; Kang, E.T. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J. Tissue Eng. Regen. Med. 2018, 12, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Kwok, S.-K.; Lee, S.-H.; Kim, E.-K.; Park, S.-H.; Cho, M.-L. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015, 24, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oca, C.; Breda, S.; Elena, N.; Valentini, R.; Samaila, E.M.; Magnan, B. Mesenchymal Stem Cells injection in hip osteoarthritis: Preliminary results. Acta Bio-Med. Atenei Parm. 2019, 90, 75–80. [Google Scholar]
- Jones, I.A.; Wilson, M.; Togashi, R.; Han, B.; Mircheff, A.K.; Vangsness, C.T., Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: A study protocol. BMC Musculoskelet. Disord. 2018, 19, 383. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2019, 43, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 43. [Google Scholar] [CrossRef]
- Kappy, N.S.; Chang, S.; Harris, W.M.; Plastini, M.; Ortiz, T.; Zhang, P.; Hazelton, J.P.; Carpenter, J.P.; Brown, S.A. Human adipose-derived stem cell treatment modulates cellular protection in both In Vitro and In Vivo traumatic brain injury models. J. Trauma Acute Care Surg. 2018, 84, 745–751. [Google Scholar] [CrossRef]
- Tajiri, N.; Acosta, S.A.; Shahaduzzaman, M.; Ishikawa, H.; Shinozuka, K.; Pabon, M.; Hernandez-Ontiveros, D.; Kim, D.W.; Metcalf, C.; Staples, M. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: Cell graft biodistribution and soluble factors in young and aged rats. J. Neurosci. 2014, 34, 313–326. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal Stem Cells: A Potential Therapeutic Approach for Amyotrophic Lateral Sclerosis? Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Staff, N.P.; Madigan, N.N.; Morris, J.; Jentoft, M.; Sorenson, E.J.; Butler, G.; Gastineau, D.; Dietz, A.; Windebank, A.J. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 2016, 87, 2230–2234. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Kim, H.J.; Oh, J.-H.; Park, H.-G.; Ra, J.C.; Chang, K.-A.; Suh, Y.-H. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson’s disease. Neurobiol. Aging 2015, 36, 2885–2892. [Google Scholar] [CrossRef]
- Ciervo, Y.; Ning, K.; Jun, X.; Shaw, P.J.; Mead, R.J. Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol. Neurodegener. 2017, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Staff, N.P.; Knight, A.M.; Nesbitt, J.J.; Butler, G.W.; Padley, D.J.; Parisi, J.E.; Dietz, A.B.; Windebank, A.J. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting Phase I human trials. Transfusion 2015, 55, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Milanese, M.; Principato, M.C.; Morando, S.; Bonifacino, T.; Vergani, L.; Giunti, D.; Voci, A.; Carminati, E.; Giribaldi, F.; et al. Intravenous Mesenchymal Stem Cells Improve Survival and Motor Function in Experimental Amyotrophic Lateral Sclerosis. Mol. Med. 2012, 18, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Bonaconsa, M.; Scambi, I.; Squintani, G.M.; Rui, W.; Turano, E.; Ungaro, D.; D’agostino, S.; Barbieri, F.; Angiari, S. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience 2013, 248, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.; Fu, R.-H.; Huang, Y.-C.; Chen, S.-Y.; Hsu, C.-J.; Lin, S.-Z.; Tu, C.-T.; Chang, L.-H.; Wu, P.-A.; Liu, S.-P. Adipose-Derived Stem Cells Stimulated with n-Butylidenephthalide Exhibit Therapeutic Effects in a Mouse Model of Parkinson’s Disease. Cell Transplant. 2018, 27, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous infusion of adipose-derived stem/stromal cells improve functional recovery of rats with spinal cord injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Douma, L.S.; Caprez, T.S.; Janki, M.; Benneker, L.M.; Alini, M.; Grad, S. Intervertebral disc response to stem cell treatment is conditioned by disc state and cell carrier: An ex vivo study. J. Orthop. Transl. 2017, 9, 43–51. [Google Scholar] [CrossRef]
- Song, K.; Gu, T.; Shuang, F.; Tang, J.; Ren, D.; Qin, J.; Hou, S. Adipose-derived stem cells improve the viability of nucleus pulposus cells in degenerated intervertebral discs. Mol. Med. Rep. 2015, 12, 4664–4668. [Google Scholar] [CrossRef] [Green Version]
- Comella, K.; Silbert, R.; Parlo, M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J. Transl. Med. 2017, 15, 12. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2016, 39, 655–664. [Google Scholar] [CrossRef]
- Koliakos, G. Treatment with adipose stem cells in a patient with moderate Alzheimer’s disease: Case report. J. Neurorestor. 2015, 3, 115–120. [Google Scholar]
- Kwak, K.-A.; Lee, S.-P.; Yang, J.-Y.; Park, Y.-S. Current Perspectives regarding Stem Cell-Based Therapy for Alzheimer’s Disease. Stem Cells Int. 2018, 2018, 6392986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, W.; Zhang, C.; Sheng, C.; Huang, W.; Wang, Y.; Fan, R. Effects of stem cell transplantation on cognitive decline in animal models of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 12134. [Google Scholar] [CrossRef] [PubMed]
- Eagan, M.J.; Zuk, P.A.; Zhao, K.W.; Bluth, B.E.; Brinkmann, E.J.; Wu, B.M.; McAllister, D.R. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J. Tissue Eng. Regen. Med. 2012, 6, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-M.; Lim, H.C.; Hoon Bae, J. Mesenchymal stem cells for enhancing biologic healing after anterior cruciate ligament injuries. Curr. Stem Cell Res. Ther. 2015, 10, 535–547. [Google Scholar] [CrossRef] [PubMed]
- De Aro, A.A.; Carneiro, G.D.; Teodoro, L.F.R.; da Veiga, F.C.; Ferrucci, D.L.; Simões, G.F.; Simões, P.W.; Alvares, L.E.; de Oliveira, A.L.R.; Vicente, C.P.; et al. Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5. Cells 2018, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kwon, B.; Lee, K.; Son, Y.H.; Chung, S.G. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am. J. Sports Med. 2017, 45, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Pas, H.I.; Moen, M.H.; Haisma, H.J.; Winters, M. No evidence for the use of stem cell therapy for tendon disorders: A systematic review. Br. J. Sports Med. 2017, 51, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: A phase I dose-escalation trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Cuervo, B.; Rubio, M.; Sopena, J.; Dominguez, M.J.; Vilar, J.; Morales, M.; Cugat, R.; Carrillo, M.J. Hip Osteoarthritis in Dogs: A Randomized Study Using Mesenchymal Stem Cells from Adipose Tissue and Plasma Rich in Growth Factors. Int. J. Mol. Sci. 2014, 15, 13437–13460. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Park, K.S.; Lee, S.H. Efficacy of autologous adipose tissue-derived stem cells with extracellular matrix and hyaluronic acid on human hip osteoarthritis. Biomed. Res. 2017. Available online: http://www.biomedres.info/biomedical-research/efficacy-of-autologous-adipose-tissuederived-stem-cells-with-extracellular-matrix-and-hyaluronic-acid-on-human-hip-osteoarthritis.html (accessed on 24 June 2019).
- Valina, C.; Pinkernell, K.; Song, Y.-H.; Bai, X.; Sadat, S.; Campeau, R.J.; Le Jemtel, T.H.; Alt, E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 2007, 28, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kühl, J.T.; Jørgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-Derived Stromal Cells for Treatment of Patients with Chronic Ischemic Heart Disease (MyStromalCell Trial): A Randomized Placebo-Controlled Study. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, H.J.; An, J.; Kim, Y.B.; Ra, J.C.; Lim, I.; Kim, S.U. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant. 2014, 23, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Dietz, A.; Zeller, A.; Gehrking, T.; Schmelzer, J.; Sletten, D.; Gehrking, J.; Coon, E.; Sandroni, P.; Benarroch, E.; et al. Intrathecal Administration of Autologous Mesenchymal Stem Cells in Multiple System Atrophy—A Phase I/II Dose-Escalation Trial (S11.002). Neurology 2017, 88. Available online: https://n.neurology.org/content/88/16_Supplement/S11.002.short (accessed on 24 June 2019).
- Singer, W.; Dietz, A.; Zeller, A.; Gehrking, T.; Schmelzer, J.; Sletten, D.; Gehrking, J.; Coon, E.; Sandroni, P.; Benarroch, E.; et al. Long-Term Administration of Intrathecal Mesenchymal Stem Cells in Multiple System Atrophy—A Compassionate Use Experience (S18.004). Neurology 2019, 92. Available online: https://n.neurology.org/content/92/15_Supplement/S18.004.abstract (accessed on 24 June 2019).
- Lopez-Santalla, M.; Mancheño-Corvo, P.; Menta, R.; Lopez-Belmonte, J.; DelaRosa, O.; Bueren, J.A.; Dalemans, W.; Lombardo, E.; Garin, M.I. Human Adipose-Derived Mesenchymal Stem Cells Modulate Experimental Autoimmune Arthritis by Modifying Early Adaptive T Cell Responses. Stem Cells 2015, 33, 3493–3503. [Google Scholar] [CrossRef]
- Baharlou, R.; Ahmadi-Vasmehjani, A.; Faraji, F.; Atashzar, M.R.; Khoubyari, M.; Ahi, S.; Erfanian, S.; Navabi, S.-S. Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: Regulatory effects on peripheral blood mononuclear cells activation. Int. Immunopharmacol. 2017, 47, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharlou, R.; Ahmadi-Vasmehjani, A.; Rashidi, N.; Khoubyari, M.; Sheikh, M.; Erfanian, S. Immunomodulatory Effects of Human Adipose Tissue-Derived Mesenchymal Stem Cells on T Cell Subsets in Patients with Rheumatoid Arthritis. Iran. J. Allergy Asthma Immunol. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Dang, L.T.-T.; Bui, A.N.-T.; Nguyen, C.L.-T.; Truong, N.C.; Thi-Van Bui, A.; Kim, N.P.; Truong, K.D.; Van Pham, P. Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells to Treat Type 1 Diabetic Mellitus in Mice: An Evaluation of Grafted Cell Doses. In Stem Cells: Biology and Engineering; Springer: Berlin/Heidelberg, Germany, 2017; pp. 145–156. [Google Scholar]
- Wang, M.; Song, L.; Strange, C.; Dong, X.; Wang, H. Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes. Mol. Ther. 2018, 26, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ma, T.; Gong, K.; Ao, Q.; Zhang, X.; Gong, Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen. Res. 2014, 9, 798. [Google Scholar]
- Kim, S.; Chang, K.-A.; Kim, J.A.; Park, H.-G.; Ra, J.C.; Kim, H.-S.; Suh, Y.-H. The Preventive and Therapeutic Effects of Intravenous Human Adipose-Derived Stem Cells in Alzheimer’s Disease Mice. PLoS ONE 2012, 7, e45757. [Google Scholar] [CrossRef]
- Schwerk, A.; Altschüler, J.; Roch, M.; Gossen, M.; Winter, C.; Berg, J.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy 2015, 17, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Roch, M.; Altschüler, J.; Winter, C.; Schwerk, A.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stem cells improve motor functions and are neuroprotective in the 6-hydroxydopamine-rat model for Parkinson’s disease when cultured in monolayer cultures but suppress hippocampal neurogenesis and hippocampal memory function when cultured in spheroids. Stem Cell Rev. Rep. 2015, 11, 133–149. [Google Scholar]


| Phase | Trials |
|---|---|
| Early Phase I | 6 |
| Phase I | 97 |
| Phase I, II | 125 |
| Phase III | 7 |
| Phase IV | 2 |
| Not applicable | 34 |
| Device | Open/Semi-Closed/Closed | Automatic/Semi-Automatic/Manual | Capacity | Collagenenase Provided (yes/no) | Time (min) | Original Country |
|---|---|---|---|---|---|---|
| Celution | Semi-closed | Automatic | 300 g | Yes | 90 | Cytori Therapeutics, Inc. San Diego, CA, USA |
| Multi Station | Open | Manual | 150 g | No | 110 | PNC International Co., Ltd. Gyeonggi-do, Korea |
| Lipokit with 416D | Semi-closed | Manual | 100 g | No | 110 | Medi-Khan Seoul, Korea |
| Cha-Station | Semi-closed | Semi-automatic | 200 g | No | 90 | PNC International Co., Ltd. Gyeonggi-do, Korea |
| GID-SVF1 & SVF2 | Closed | Manual | 300 g | SVF1 (No) SVF2 (Yes) | 90 | GID Group, Inc. Louisville, Colorado |
| Stem.pras | Closed | Manual | 200 g | No | 110 | Proteal Spain Mexico |
| Puregraft 250 | Semi-closed | Manual | 250 g | No | 100 | Eurosilicone Zone Industrielle de la Peyrol B.P., France |
| IcellatorR X | Semi-closed | Semi-automatic | 60–100 mL | No | 60 | Tissue Genesis, Inc. Honolulu, Hawaii |
| STEM-X | Closed | Automatic | 20–800 cc | yes | Not provided | Medikan Co., Ltd. Seoul, Korea |
| SynGenX-1000 | Closed | Semiautomated | 250 mL | No | Not provided | SynGen Inc. Sacramento. CA, USA |
| Sepax-2 | Semiclosed | Semiautomated | 300 g | No | 90 | Biosafe Group SA Route Du Petit-Eysins 1 Eysins, Switzerland |
| StromaCell | Closed | Semiautomated | Not provided | No | Not provided | MicroAire Surgical Instruments, LLC Charlottesville, VA, USA |
| Diseases | Pre-Clinical Studies | Clinical Trials | Routine Treatment | Effect of ASCs Therapy | Autologous or Heterologous | Ref. |
|---|---|---|---|---|---|---|
| Knee osteoarthritis | Yes | - | Intra-articular injections | Improve pain, function and cartilage volume of the knee joint | Autologous | [113] |
| - | Phase I | Intra-articular injection | Decrease pain and improve WOMUA index | Autologous | [114] | |
| Degenerative disc disease | - | Yes | Injection | Decrease low back pain | Autologous | NCT02097862 |
| - | Yes | Intradiscal implantation | Improvement of flexion, pain ratings, VAS, PPI | Stromal vascular fractions | NCT02097862 | |
| Hip osteoarthritis | Yes | - | Intra-articular injection | Decrease pain and improve the function subscales | ASCs | [115] |
| - | Yes | Percutaneous injections | Regenerate cartilage-like tissue | ASCs | [116] | |
| Cardiac disease | Yes | - | Intracoronary reperfusion | Improve LVEF & reduce infarct area | Autologous ASCs | [117] |
| Heart failure | - | Phase II | Intramyocardial injection | Heterologous | NCT0267316 | |
| Ischemic heart disease | - | Phase I/II | Intramyocardial injection | Increase myocardial perfusion | Autologous | [118] |
| Ischemic cardiomyopathy | - | Phase I | Intravenous injection | Angiogenic effect | Autologous | NCT00426868 |
| Critical limb ischemia | - | Phase I/II | Intramuscular injection | Angiogenic effect | Autologous | NCT01211028 |
| Chronic myocardial ischemia | - | Phase I/II | Intramyocardial injection | Angiogenic effect | Heterologous | NCT01556022 |
| Ischemic stroke | - | Phase II | Intravenous injection | Angiogenic effect | Heterologous | NCT01678534 |
| Stroke | - | Phase II/III | Intravenous infusion | Angiogenic effect | Heterologous | NCT02849613 |
| Amyotrophic lateral sclerosis | - | Phase I | Intravenous injection | Safety Improvement of ALS function, FVC | ASCs | NCT02492516 |
| Yes | - | Transplantation | Neuroprotective effects by increasing cytokine & growth factors | ASCs | [119] | |
| Yes | - | Intravenous injection | Enhance the viability and motor activity | Autologous | [98,99] | |
| Multiple system atrophy | - | Phase I | Intrathecal injections | Safety | Autologous | NCT02315027 * |
| - | Phase I/II | intrathecally via lumbar puncture | Safety at high dose | Autologous | [120,121] | |
| Spinal cord injury | - | Phase I/II | Intrathecal transplantation | Recover ASIA and sensory score | Autologous | [104] |
| Rheumatoid arthritis | yes | - | Infusion | Increase IL10, T-reg production | Autologous | [122] |
| yes | - | Co-culture | Inhibit inflammation | Autologous | [123,124] | |
| Type I diabetes mellitus | Yes | - | Intravenous transfusion | Improve glucose & insulin tolerance, increase insulin production | Autologous | [125] |
| Type 2 Diabetes | Yes | - | Intravenous injection | Increase insulin sensitivity, reduce inflammation and fat mass | Autologous | [126] |
| Alzheimer’s disease | Yes | - | Transplantation | Enhance neurogenic activity, reduce oxidative stress | Autologous | [127] |
| Yes | - | Intravenously or intracerebrally injection | Increase cytokine IL10 & VEGF | Autologous | [128] | |
| Parkinson’s disease | Yes | - | Transplantation | Neurogenesis increase cytokine secretion and brain-derived neurotrophic factor | Autologous | [129] |
| Yes | - | Transplantation | Improve motor function & neuroprotective effects | Monolayer-cultured ASCs | [130] | |
| Traumatic brain injury | - | Phase I/II | Injection | Safety Benefits | Autologous | NCT02959294 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, D.-T.; Nguyen Thi Phuong, T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Gia Anh, P.; Pham, V.H.; Thi Nga, V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917. https://doi.org/10.3390/jcm8070917
Chu D-T, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH, Thi Nga V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. Journal of Clinical Medicine. 2019; 8(7):917. https://doi.org/10.3390/jcm8070917
Chicago/Turabian StyleChu, Dinh-Toi, Thuy Nguyen Thi Phuong, Nguyen Le Bao Tien, Dang Khoa Tran, Le Bui Minh, Vo Van Thanh, Pham Gia Anh, Van Huy Pham, and Vu Thi Nga. 2019. "Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application" Journal of Clinical Medicine 8, no. 7: 917. https://doi.org/10.3390/jcm8070917
APA StyleChu, D.-T., Nguyen Thi Phuong, T., Tien, N. L. B., Tran, D. K., Minh, L. B., Thanh, V. V., Gia Anh, P., Pham, V. H., & Thi Nga, V. (2019). Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. Journal of Clinical Medicine, 8(7), 917. https://doi.org/10.3390/jcm8070917






