Development of a Novel Nanotextured Titanium Implant. An Experimental Study in Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Surface Modification
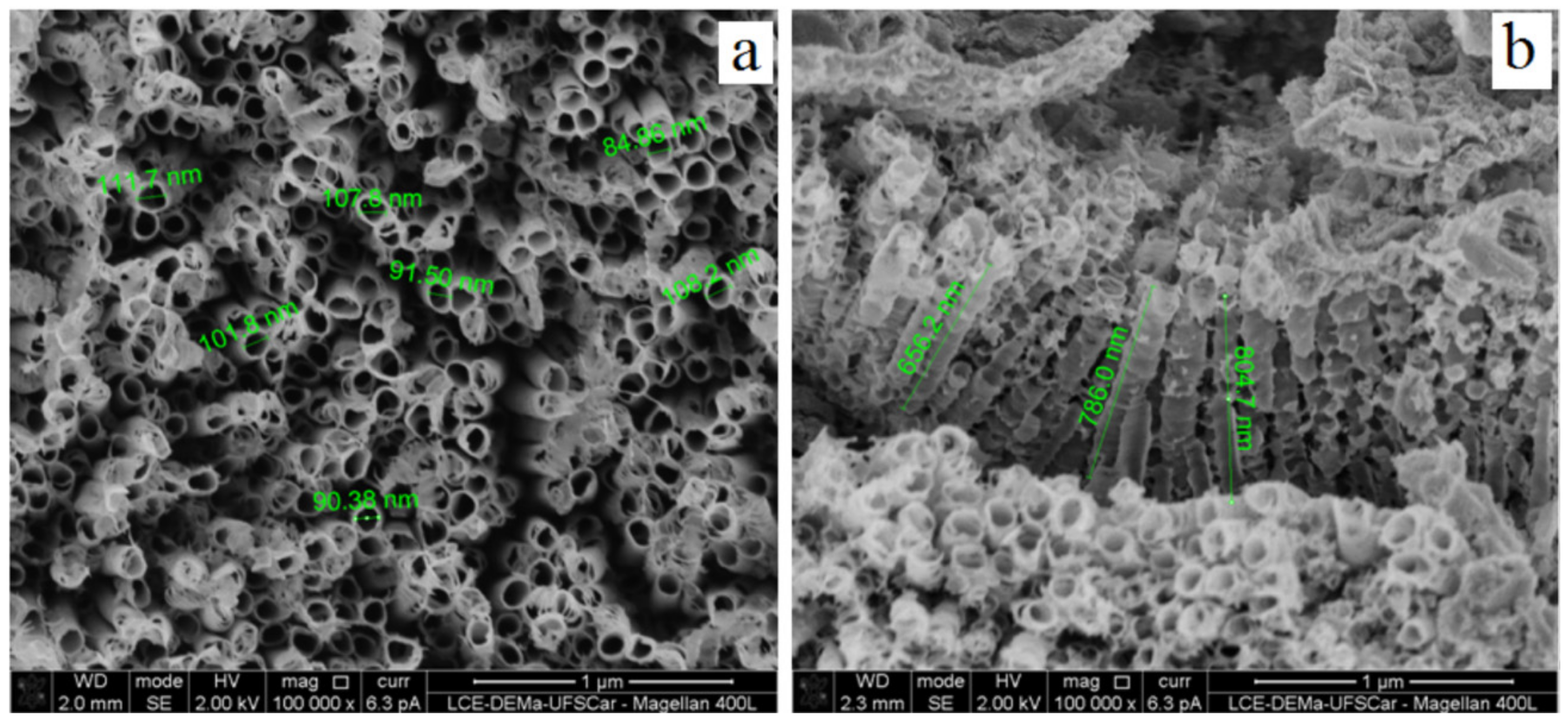
2.2. Surface Characterization
2.3. Animal Study
2.4. Surgical Technique
2.5. Histological Preparation and Histomorphometric Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balazic, M.; Kopac, J.; Jackson, M.J.; Ahmed, W. Review: Titanium and titanium alloy applications in medicine. Int. J. Nano Biomat. 2007, 1, 3–34. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Sawase, T.; Wennerberg, A.; Baba, K.; Tsuboi, Y.; Sennerby, L.; Johansson, C.B.; Albrektsson, T. Application of oxygen ion implantation to titanium surfaces: Effects on surface characteristics, corrosion resistance, and bone response. Clin. Implant Dent. Relat. Res. 2001, 3, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Feng, X.J.; Grimes, C.A. Self-Assembled fabrication of vertically oriented Ta2O5 nanotube arrays and membranes thereof, by one-step tantalum anodization. Chem. Mater. 2008, 20, 6477–6481. [Google Scholar] [CrossRef]
- Schroeder, A.; Pohler, O.; Sutter, F. Tissue reaction to an implant of a titanium hollow cylinder with a titanium surface spray layer. SSO Schweiz Monatsschr Zahnheilkd 1976, 86, 713–727. [Google Scholar] [PubMed]
- Ito, K.; Nanba, K.; Nishida, T.; Sato, H.; Murai, S. Comparison of osseointegration between hydroxyapatite-coated and uncoated threaded titanium dental implants placed into surgically created bone defect in rabbit tibia. J. Oral Sci. 1998, 40, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rocci, A.; Martignoni, M.; Gottlow, J. Immediate loading of Brånemark System TiUnite and machined-surface implants in the posterior mandible: A randomized open-ended clinical trial. Clin. Implant Dent. Relat. Res. 2003, 5, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Liu, B.L.; Zou, J.C.; Xu, K.W. Improvement of osseointegration of titanium dental implants by a modified sandblasting surface treatment: An in vivo interfacial biomechanics study. Implant Dent. 1999, 8, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Lazzara, R.; Rebaudi, A.; Rao, W.; Testori, T.; Porter, S.S. Bone-implant contact on machined and dual acid-etched surfaces after 2 months of healing in the human maxilla. J. Periodontol. 2003, 74, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Wilson, T. A prospective study evaluating a protocol for 6 weeks’ loading of SLA implants in the posterior maxilla: Ane year results. Clin. Oral Implants Res. 2002, 13, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, C.; Reimers, H.; Chakarov, D.; Gold, J.; Wennerberg, A. An in vivo study of bone response to implants topographically modified by laser micromachining. Biomaterials 2003, 24, 701–710. [Google Scholar] [CrossRef]
- Esteban, J.; Cordero-Ampuero, J. Treatment of prosthetic osteoarticular infections. Expert. Opin. Pharmacother. 2011, 12, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Rangel, A.L.; Chaves, J.A.; Escada, A.L.; Konatu, R.T.; Popat, K.C.; Alves Claro, A.P.R. Modification of the Ti15Mo alloy surface through TiO2 nanotube growth—An in vitro study. J. Appl. Biomater. Funct. Mater. 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Takeshita, F.; Yoshinari, M.; Inoue, T.; Ohtsuka, Y.; Shimono, M.; Suetsugu, T.; Tanaka, T. An immunocytochemical study for lysosomal cathepsins B and D related to the intracellular degradation of titanium at the bone-titanium interface. J. Periodontol. 1998, 69, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Surmeneva, M.; Nikityuk, P.; Hans, M.; Surmenev, R. Deposition of Ultrathin Nano-Hydroxyapatite Films on Laser Micro-Textured Titanium Surfaces to Prepare a Multiscale Surface Topography for Improved Surface Wettability/Energy. Materials 2016, 9, 862. [Google Scholar] [CrossRef]
- Kim, I.H.; Son, J.S.; Choi, S.H.; Kim, K.H.; Kwon, T.Y. Nano- and Micro-Scale Oxidative Patterning of Titanium Implant Surfaces for Improved Surface Wettability. J. Nanosci. Nanotechnol. 2016, 16, 1883–1886. [Google Scholar] [CrossRef]
- Lazzara, R.J.; Testori, T.; Trisi, P.; Porter, S.S.; Weinstein, R.L. A human histologic analysis of osseotite and machined surfaces using implants with 2 opposing surfaces. Int. J. Periodontics Restor. Dent. 1999, 19, 117–129. [Google Scholar]
- Yin, C.; Zhang, Y.; Cai, Q.; Li, B.; Yang, H.; Wang, H.; Qi, H.; Zhou, Y.; Meng, W. Effects of the micro-nano surface topography of titanium alloy on the biological responses of osteoblast. J. Biomed. Mater. Res. A 2017, 105, 757–769. [Google Scholar] [CrossRef]
- Mafra, C.E.S.; Sirolli, M.; Cavalcanti, M.C.; Santos, R.B.A.D.; Pannuti, C.M.; Romito, G.A.; César Neto, J.B. Effect of Different Doses of Synthetic Parathyroid Hormone (1–34) on Bone around Implants: A Preclinical Rat Model. Braz. Dent. J. 2019, 30, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu, K.; Kido, H.; Sato, A.; Watazu, A.; Matsuura, M. Ultrastructure of the interface between titanium and surrounding tissue in rat tibiae—A comparison study on titanium-coated and -uncoated plastic implants. Clin. Implant. Dent. Relat. Res. 2007, 9, 100–111. [Google Scholar] [CrossRef]
- Bozoglan, A.; Dundar, S.; Yildirim, T.T.; Bulmus, O.; Ertugrul, A.S.; Bozoglan, M.Y.; Tekin, S.; Toy, V.E. Effects of Different Levels of Restraint Stress on Bone-Implant Contact. J. Craniofac. Surg. 2019, 30, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.; Steinemann, S.; Fiorellini, J.; Fox, C.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. A 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Webster, T.J. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, C.; Wang, D.; Ji, Q. Research status about surface modification of biomedical Ti and its alloys by micro-arc oxidation. Surf. Rev. Lett. 2006, 13, 35–43. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Wen, J.; Huang, S.; Sun, Y.; Chen, Z.; Wang, Y.; Li, H.; Liu, X. Titanium Dioxide NanotubeBased Oxygen Indicator for Modified Atmosphere Packaging: Efficiency and Accuracy. Materials 2018, 29, 11. [Google Scholar] [CrossRef]
- Chiang, H.J.; Hsu, H.J.; Peng, P.W.; Wu, C.Z.; Ou, K.L.; Cheng, H.Y.; Walinski, C.J.; Sugiatno, E. Early bone response to machined, sandblasting acid etching (SLA) and novel surfacefunctionalization (SLAffinity) titanium implants: Characterization, biomechanical analysis and histological evaluation in pigs. J. Biomed. Mater. Res. A 2016, 104, 397–405, Epub 2015 Oct 15. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Weng, D.; Hoffmeyer, M.; Huürzeler, M.B.; Richter, E.J. Osseotite vs. machined surface in poor bone quality. A study in dogs. Clin. Oral Implant. Res. 2003, 14, 703–708. [Google Scholar] [CrossRef]
- Bowers, M.; Yoo, D.; Marin, C.; Gil, L.; Shabaka, N.; Goldstein, M.; Janal, M.; Tovar, N.; Hirata, R.; Bonfante, E.; et al. Surface characterization and in vivo evaluation of laser sintered and machined implants followed by resorbable-blasting media process: A study in sheep. Med. Oral. Patol. Oral. Cir. Bucal. 2016, 21, e206–e213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klokkevold, P.R.; Nishimura, R.D.; Adachi, M.; Caputo, A. Osseointegration enhanced by chemical etching of the titanium surface. A torque removal study in the rabbit. Clin. Oral Implant. Res. 1997, 8, 442–447. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Johnson, P.; Dadgostari, S.; Caputo, A.; Davies, J.E.; Nishimura, R.D. Early endosseous integration enhanced by dual acid etching of titanium: A torque removal study in the rabbit. Clin. Oral Implant. Res. 2001, 12, 350–357. [Google Scholar] [CrossRef]
- Yang, G.L.; He, F.M.; Yang, X.F.; Wang, X.X.; Zhao, S.F. Bone responses to titanium implants surface-roughened by sandblasted and double etchedtreatments in a rabbit model. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 516–524. [Google Scholar] [CrossRef]
- Ding, X.; Zhou, L.; Wang, J.; Zhao, Q.; Lin, X.; Gao, Y.; Li, S.; Wu, J.; Rong, M.; Guo, Z.; et al. The effects of hierarchical micro/nanosurfaces decorated with TiO2 nanotubes on the bioactivity of titanium implants in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6955–6973. [Google Scholar]
- Lee, J.; Park, S.; Kang, S.; Park, C.; Yun, K. Bonding Evaluation Between Nanotube by Anodic Oxidation and Machined Dental Titanium Implant in Beagle Dog. J. Nanosci. Nanotechnol. 2019, 19, 912–914. [Google Scholar] [CrossRef]
- Ballo, A.M.; Broke, J. Evaluation of implant osseointegration in small laboratory animals. In Implant Dentistry Research Guide: Basic, Translation, and Clinical Research; Ballo, A.M., Ed.; Nova Publishers: Hauppauge, NY, USA, 2012; pp. 151–176. [Google Scholar]
- Futami, T.; Fujii, N.; Ohnishi, H.; Tagushi, N.; Kusakari, H.; Ohshima, H.; Maeda, T. Tissue response to titanium implants in the rat maxilla: Ultrastructural and histochemical observations of the bone-titanium interface. J. Periodontol. 2000, 71, 287–298. [Google Scholar] [CrossRef]
- Murai, K.; Takeshita, F.; Ayukawa, Y.; Kiyoshima, T.; Suetsugu, T.; Tanaka, T. Light and electron microscopic studies of bone-titanium interface in the tibiae of young and mature rats. J. Biomed. Mater. Res. 1999, 30, 523–533. [Google Scholar] [CrossRef]
- Rizzo, S.; Zampetti, P.; Rodriguez, Y.; Baena, R.; Svanosio, D.; Lupi, S.M. Retrospective analysis of 521 endosseous implants placed under antibiotic prophylaxis and review of literature. Minerva Stomatol. 2010, 59, 75–88. [Google Scholar] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.A.; Li, H.; Lu, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, Y.; Xu, L.; Sun, S.; Jiang, X.; Zhang, F. Microarray-based bioinformatics analysis of osteoblasts on TiO2 nanotube layers. Colloids Surf. B 2012, 93, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Ivanovski, S. Dental implants modified with drug releasing titania nanotubes: Therapeutic potential and developmental challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelegrine, A.A.; Moy, P.K.; Moshaverinia, A.; do Amaral Escada, A.L.; Calvo-Guirado, J.L.; Rosifini Alves Claro, A.P. Development of a Novel Nanotextured Titanium Implant. An Experimental Study in Rats. J. Clin. Med. 2019, 8, 954. https://doi.org/10.3390/jcm8070954
Pelegrine AA, Moy PK, Moshaverinia A, do Amaral Escada AL, Calvo-Guirado JL, Rosifini Alves Claro AP. Development of a Novel Nanotextured Titanium Implant. An Experimental Study in Rats. Journal of Clinical Medicine. 2019; 8(7):954. https://doi.org/10.3390/jcm8070954
Chicago/Turabian StylePelegrine, André Antonio, Peter Karyen Moy, Alireza Moshaverinia, Ana Lúcia do Amaral Escada, José Luis Calvo-Guirado, and Ana Paula Rosifini Alves Claro. 2019. "Development of a Novel Nanotextured Titanium Implant. An Experimental Study in Rats" Journal of Clinical Medicine 8, no. 7: 954. https://doi.org/10.3390/jcm8070954
APA StylePelegrine, A. A., Moy, P. K., Moshaverinia, A., do Amaral Escada, A. L., Calvo-Guirado, J. L., & Rosifini Alves Claro, A. P. (2019). Development of a Novel Nanotextured Titanium Implant. An Experimental Study in Rats. Journal of Clinical Medicine, 8(7), 954. https://doi.org/10.3390/jcm8070954





