The Regulatory Role of Rac1, a Small Molecular Weight GTPase, in the Development of Diabetic Retinopathy
Abstract
1. Introduction
2. Molecular Mechanisms of Diabetic Retinopathy
3. NADPH Oxidases
4. Rac1
5. The Role of Rac1 in Diabetic Retinopathy
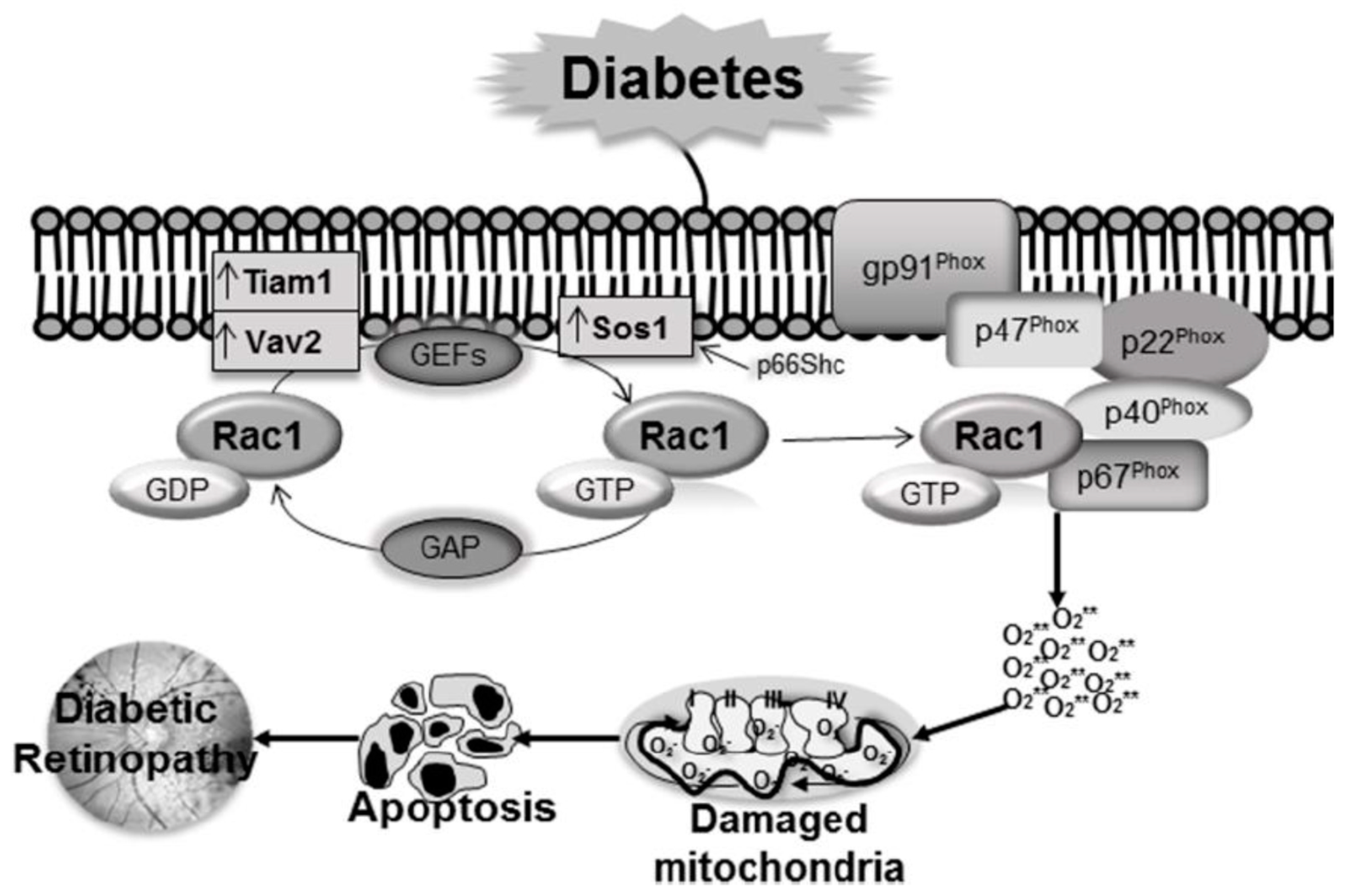
6. Regulation of Rac1 in Diabetic Retinopathy
6.1. Functional Regulation of Rac1
6.1.1. Guanine Nucleotide Exchange Factors (GEFs)
6.1.2. Guanine Nucleotide Dissociation Inhibitors
6.1.3. Post-Translational Modifications
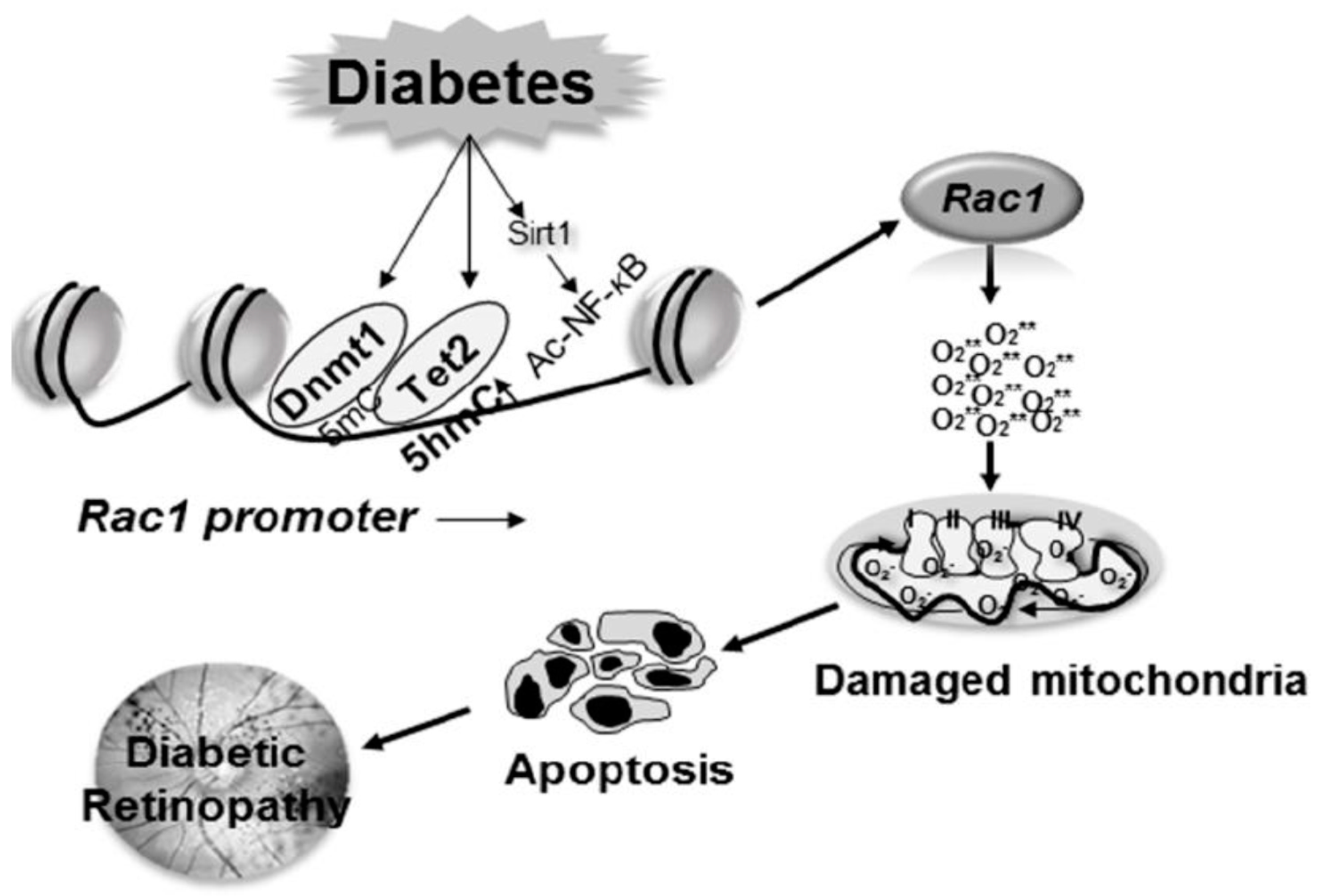
6.2. Transcriptional Regulation and Epigenetic Modifications
7. Therapeutic Targets
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Orasanu, G.; Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009, 53, S35–S42. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar] [PubMed]
- Cunha-Vaz, J.; Faria de Abreu, J.R.; Campos, A.J. Early breakdown of the blood-retinal barrier in diabetes. Br. J. Ophthalmol. 1975, 59, 649–656. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.P.; Gonzalez-Correa, J.A.; Guerrero, A.; De La Cuesta, F.S. Pharmacological approach to diabetic retinopathy. Diabetes Metab. Res. Rev. 2004, 20, 91–113. [Google Scholar] [CrossRef]
- Ting, D.S.; Cheung, G.C.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Kalra, S.; Mukherjee, J.J.; Venkataraman, S.; Bantwal, G.; Shaikh, S.; Saboo, B.; Das, A.K.; Ramachandran, A. Hypoglycemia: The neglected complication. Indian J. Endocrinol. Metab. 2013, 17, 819–834. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Diabetic retinopathy: Mitochondrial dysfunction and retinal capillary cell death. Antioxid. Redox Signal. 2005, 7, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G. Oxidative stress profiling: Part I. Its potential importance in the optimization of human health. Ann. N. Y. Acad. Sci. 2005, 1055, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, A.; Veluthakal, R.; Mohammad, G.; Syed, I.; Santos, J.M.; Mishra, M. Tiam1-rac1 signalling axis-mediated activation of nadph oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 2014, 57, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Abbas, S.N. Diabetes-induced mitochondrial dysfunction in the retina. Invesig. Ophtahlmol. Vis. Sci. 2003, 44, 5327–5334. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef]
- Kowluru, A. Small g proteins in islet beta-cell function. Endocr. Rev. 2010, 31, 52–78. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Phys. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Kowluru, A. Friendly, and not so friendly, roles of rac1 in islet beta-cell function: Lessons learnt from pharmacological and molecular biological approaches. Biochem. Pharmacol. 2011, 81, 965–975. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Elshafey, S.; Sellak, H.; Hussein, K.A.; El-Sherbiny, M.; Abdelsaid, M.; Rizk, N.; Beasley, S.; Tawfik, A.M.; Smith, S.B.; et al. A lipidomic screen of hyperglycemia-treated hrecs links 12/15-lipoxygenase to microvascular dysfunction during diabetic retinopathy via nadph oxidase. J. Lipid Res. 2015, 56, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.M.; Kim, C.S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting nadph oxidase-related oxidative stress. Free Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Shah, K.P.; Pollack, S.; Toppila, I.; Hebert, H.L.; McCarthy, M.I.; Groop, L.; Ahlqvist, E.; Lyssenko, V.; Agardh, E.; et al. A genome-wide association study suggests new evidence for an association of the nadph oxidase 4 (nox4) gene with severe diabetic retinopathy in type 2 diabetes. Acta Ophthalmol. 2018, 96, e811–e819. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Banfi, B.; Jesaitis, A.J.; Dinauer, M.C.; Allen, L.A.; Nauseef, W.M. Critical roles for p22phox in the structural maturation and subcellular targeting of nox3. Biochem. J. 2007, 403, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Matos, P.; Skaug, J.; Marques, B.; Beck, S.; Verissimo, F.; Gespach, C.; Boavida, M.G.; Scherer, S.W.; Jordan, P. Small gtpase rac1: Structure, localization, and expression of the human gene. Biochem. Biophys. Res. Commun. 2000, 277, 741–751. [Google Scholar] [CrossRef]
- Bhat, H.F.; Baba, R.A.; Adams, M.E.; Khanday, F.A. Role of snta1 in rac1 activation, modulation of ros generation, and migratory potential of human breast cancer cells. Br. J. Cancer 2014, 110, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Biro, M.; Munoz, M.A.; Weninger, W. Targeting rho-gtpases in immune cell migration and inflammation. Br. J. Pharmacol. 2014, 171, 5491–5506. [Google Scholar] [CrossRef]
- Cuadrado, A.; Martin-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors nrf2 and nf-kappab are coordinated effectors of the rho family, gtp-binding protein rac1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho gtpases: Biochemistry and biology. Ann. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Simon, A.R.; Vikis, H.G.; Stewart, S.; Fanburg, B.L.; Cochran, B.H.; Guan, K.L. Regulation of stat3 by direct binding to the rac1 gtpase. Science 2000, 290, 144–147. [Google Scholar] [CrossRef]
- Bustelo, X.R.; Ojeda, V.; Barreira, M.; Sauzeau, V.; Castro-Castro, A. Rac-ing to the plasma membrane: The long and complex work commute of rac1 during cell signaling. Small GTPases 2012, 3, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Chang, P.; Zhang, Q.; Reddy, P.G.; Bokoch, G.M.; Greenberg, S. Requirements for both rac1 and cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 1997, 186, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Bokoch, G.M.; Waterman-Storer, C.M. Regulation of leading edge microtubule and actin dynamics downstream of rac1. J. Cell Biol. 2003, 161, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Velaithan, R.; Kang, J.; Hirpara, J.L.; Loh, T.; Goh, B.C.; Le Bras, M.; Brenner, C.; Clement, M.V.; Pervaiz, S. The small gtpase rac1 is a novel binding partner of bcl-2 and stabilizes its antiapoptotic activity. Blood 2011, 117, 6214–6226. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, N.; Xia, P.; Wang, E.; Guo, Q.; Ye, Z. Inhibition of rac1 ameliorates neuronal oxidative stress damage via reducing bcl-2/rac1 complex formation in mitochondria through pi3k/akt/mtor pathway. Exp. Neurobiol. 2018, 300, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chong, S.J.; Ooi, V.Z.; Vali, S.; Kumar, A.; Kapoor, S.; Abbasi, T.; Hirpara, J.L.; Loh, T.; Goh, B.C.; et al. Overexpression of bcl-2 induces stat-3 activation via an increase in mitochondrial superoxide. Oncotarget 2015, 6, 34191–34205. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Schelle, I.; Brakebusch, C.; Rottner, K.; Genth, H. Rac1-dependent recruitment of pak2 to g2 phase centrosomes and their roles in the regulation of mitotic entry. Cell Cycle 2014, 13, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Li, L.; Ballermann, B.; Wang, Z. Phosphorylation of rac1 t108 by extracellular signal-regulated kinase in response to epidermal growth factor: A novel mechanism to regulate rac1 function. Mol. Cell. Biol. 2013, 33, 4538–4551. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, A.; Kowluru, R.A. Phagocyte-like nadph oxidase [nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochem. Pharmacol. 2014, 88, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of reactive oxygen species by lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of nadph oxidase 4. Diabetes 2010, 59, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Veluthakal, R.; Kumar, B.; Mohammad, G.; Kowluru, A.; Kowluru, R.A. Tiam1-rac1 axis promotes activation of p38 map kinase in the development of diabetic retinopathy: Evidence for a requisite role for protein palmitoylation. Cell. Phys. Biochem. 2015, 36, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M.; Kumar, B. Diabetic retinopathy and transcriptional regulation of a small molecular weight g-protein, rac1. Exp. Eye Res. 2016, 147, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.C.; Martins, J.; Voabil, P.; Liberal, J.; Chiavaroli, C.; Bauer, J.; Cunha-Vaz, J.; Ambrosio, A.F. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes 2010, 59, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Flaga, J.; Kowluru, R.A. Molecular mechanism of transcriptional regulation of matrix metalloproteinase-9 in diabetic retinopathy. J. Cell. Physiol. 2016, 231, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab. Investig. 2010, 90, 1365–1372. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, D.; Bao, S.; Hambly, B.D.; Gillies, M.C. Triamcinolone acetonide inhibits p38mapk activation and neuronal apoptosis in early diabetic retinopathy. Curr. Mol. Med. 2013, 13, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Zhang, M.; Hu, Z. Silencing of rac1 expression via rna interference inhibits retinal neovascularization in rats. Mol. Vis. 2012, 18, 1354–1360. [Google Scholar]
- Wang, H.; Fotheringham, L.; Wittchen, E.S.; Hartnett, M.E. Rap1 gtpase inhibits tumor necrosis factor-alpha-induced choroidal endothelial migration via nadph oxidase- and nf-kappab-dependent activation of rac1. Am. J. Pathol. 2015, 185, 3316–3325. [Google Scholar] [CrossRef]
- Kowluru, A. Regulatory roles for small g proteins in the pancreatic beta-cell: Lessons from models of impaired insulin secretion. Am. J. Phys. Endocr. Metab. 2003, 285, E669–E684. [Google Scholar] [CrossRef]
- Kowluru, A. Protein prenylation in glucose-induced insulin secretion from the pancreatic islet beta cell: A perspective. J. Cell Mol. Med. 2008, 12, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Cherfils, J.; Zeghouf, M. Regulation of small gtpases by gefs, gaps, and gdis. Physiol Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, A. Tiam1/vav2-rac1 axis: A tug-of-war between islet function and dysfunction. Biochem. Pharmacol. 2017, 132, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, N.; Waschke, J. Camp with other signaling cues converges on rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res. 2014, 355, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Duraisamy, A.J.; Kowluru, A.; Kowluru, R.A. Functional regulation of an oxidative stress mediator, rac1, in diabetic retinopathy. Mol. Neurobiol 2019, in press. [Google Scholar]
- Ingersoll, M.A.; Chou, Y.W.; Lin, J.S.; Yuan, T.C.; Miller, D.R.; Xie, Y.; Tu, Y.; Oberley-Deegan, R.E.; Batra, S.K.; Lin, M.F. P66shc regulates migration of castration-resistant prostate cancer cells. Cell. Signal. 2018, 46, 1–14. [Google Scholar] [CrossRef]
- Mishra, M.; Duraisamy, A.J.; Bhattacharjee, S.; Kowluru, R.A. Adaptor protein p66shc: A link between cytosolic and mitochondrial dysfunction in the development of diabetic retinopathy. Antioxid. Redox Signal. 2019, 30, 1621–1634. [Google Scholar] [CrossRef]
- DerMardirossian, C.; Bokoch, G.M. Gdis: Central regulatory molecules in rho gtpase activation. Trends Cell Biol. 2005, 15, 356–363. [Google Scholar] [CrossRef]
- Navarro-Lerida, I.; Sanchez-Perales, S.; Calvo, M.; Rentero, C.; Zheng, Y.; Enrich, C.; Del Pozo, M.A. A palmitoylation switch mechanism regulates rac1 function and membrane organization. EMBO J. 2012, 31, 534–551. [Google Scholar] [CrossRef]
- Li, L.; Shi, X.; Guo, X.; Li, H.; Xu, C. Ionic protein-lipid interaction at the plasma membrane: What can the charge do? Trends Biochem. Sci. 2014, 39, 130–140. [Google Scholar] [CrossRef]
- Remorino, A.; De Beco, S.; Cayrac, F.; Di Federico, F.; Cornilleau, G.; Gautreau, A.; Parrini, M.C.; Masson, J.B.; Dahan, M.; Coppey, M. Gradients of rac1 nanoclusters support spatial patterns of rac1 signaling. Cell Rep. 2017, 21, 1922–1935. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Syeda, K.; Hadden, T.; Kowluru, A. Upregulation of phagocyte-like nadph oxidase by cytokines in pancreatic beta-cells: Attenuation of oxidative and nitrosative stress by 2-bromopalmitate. Biochem. Pharmacol. 2013, 85, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Di-Poi, N.; Faure, J.; Grizot, S.; Molnar, G.; Pick, E.; Dagher, M.C. Mechanism of nadph oxidase activation by the rac/rho-gdi complex. Biochemistry 2001, 40, 10014–10022. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.F. Rho gtpases, their post-translational modifications, disease-associated mutations and pharmacological inhibitors. Small GTPases 2018, 9, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lluva, S.; Tatham, M.H.; Jones, R.C.; Jaffray, E.G.; Edmondson, R.D.; Hay, R.T.; Malliri, A. Sumoylation of the gtpase rac1 is required for optimal cell migration. Nat. Cell Biol. 2010, 12, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Fraga, M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Holliday, R. Epigenetics: A historical overview. Epigenetics 2006, 1, 76–80. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Meaney, M.J. Epigenetics and the environmental regulation of the genome and its function. Annu. Rev. Psychol. 2010, 61, 439–466. [Google Scholar] [CrossRef]
- Turner, B.M. Epigenetic responses to environmental change and their evolutionary implications. Philos. Trans. R. Soc. 2009, 364, 3403–3418. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef] [PubMed]
- Javaid, N.; Choi, S. Acetylation- and methylation-related epigenetic proteins in the context of their targets. Genes 2017, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Willbanks, A.; Leary, M.; Greenshields, M.; Tyminski, C.; Heerboth, S.; Lapinska, K.; Haskins, K.; Sarkar, S. The evolution of epigenetics: From prokaryotes to humans and its biological consequences. Genet. Epigent. 2016, 8, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.P.; Taggart, M.H.; Nicholls, R.D.; Higgs, D.R. Non-methylated cpg-rich islands at the human alpha-globin locus: Implications for evolution of the alpha-globin pseudogene. EMBO J. 1987, 6, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Hisano, M.; Ohta, H.; Nishimune, Y.; Nozaki, M. Methylation of cpg dinucleotides in the open reading frame of a testicular germ cell-specific intronless gene, tact1/actl7b, represses its expression in somatic cells. Nucleic Acids Res. 2003, 31, 4797–4804. [Google Scholar] [CrossRef] [PubMed]
- Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y. Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- Duraisamy, A.J.; Mishra, M.; Kowluru, A.; Kowluru, R.A. Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invesig. Ophtahlmol. Vis. Sci. 2018, 59, 4831–4840. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Shan, Y.; Mishra, M. Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab. Investig. 2016, 96, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Kowluru, R.A. The role of DNA methylation in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. Investig. Ophtahlmol. Vis. Sci. 2016, 57, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Cooper, N.G. Glutamate-induced nfkappab activation in the retina. Investig. Ophtahlmol. Vis. Sci. 2009, 50, 917–925. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kowluru, R.A.; Santos, J.M.; Zhong, Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Investig. Ophtahlmol. Vis. Sci. 2014, 55, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Duraisamy, A.J.; Kowluru, R.A. Sirt1-a guardian of the development of diabetic retinopathy. Diabetes 2018, 67, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, A.D.; Kowluru, A.; Kowluru, R.A. Epigenetic regulation of an oxidative stress mediator Rac1, in the development of diabetic retinopathy. Free Rad. Biol. Med. 2018, S137. [Google Scholar] [CrossRef]
- Payapilly, A.; Malliri, A. Compartmentalisation of rac1 signalling. Curr. Opin. Cell Biol. 2018, 54, 50–56. [Google Scholar] [CrossRef]
- Cheng, W.H.; Ho, W.Y.; Chang, C.F.; Lu, P.J.; Cheng, P.W.; Yeh, T.C.; Hong, L.Z.; Sun, G.C.; Hsiao, M.; Tseng, C.J. Simvastatin induces a central hypotensive effect via ras-mediated signalling to cause enos up-regulation. Br. J. Pharmacol. 2013, 170, 847–858. [Google Scholar] [CrossRef]
- Liang, S.L.; Liu, H.; Zhou, A. Lovastatin-induced apoptosis in macrophages through the rac1/cdc42/jnk pathway. J. Immunol. 2006, 177, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Liao, J.K. The pleiotropic effects of statins-from coronary artery disease and stroke to atrial fibrillation and ventricular tachyarrhythmia. Curr. Vasc. Pharmacol. 2019, 17, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.Y.; Chen, T.H.; Garg, S.J.; Sun, C.C.; Kang, J.H.; Wu, W.C.; Hung, M.J.; Lai, C.C.; Cherng, W.J.; Hwang, Y.S. Association of statin therapy with prevention of vision-threatening diabetic retinopathy. JAMA Ophthalmol. 2019, 137, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Eller-Borges, R.; Batista, W.L.; da Costa, P.E.; Tokikawa, R.; Curcio, M.F.; Strumillo, S.T.; Sartori, A.; Moraes, M.S.; de Oliveira, G.A.; Taha, M.O.; et al. Ras, rac1, and phosphatidylinositol-3-kinase (pi3k) signaling in nitric oxide induced endothelial cell migration. Nitric Oxide 2015, 47, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Fidilio, A.; Lazzara, F.; Platania, C.B.M.; Salerno, L.; Foresti, R.; Drago, F.; Bucolo, C. Effects of novel nitric oxide-releasing molecules against oxidative stress on retinal pigmented epithelial cells. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Fardi, M.; Solali, S.; Farshdousti Hagh, M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis. 2018, 5, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Heerboth, S.; Lapinska, K.; Snyder, N.; Leary, M.; Rollinson, S.; Sarkar, S. Use of epigenetic drugs in disease: An overview. Genet. Epigenet. 2014, 6, 9–19. [Google Scholar] [CrossRef]
- Robak, T. New nucleoside analogs for patients with hematological malignancies. Expert Opin. Investig. Drugs 2011, 20, 343–359. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group b. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Treatment of cardiovascular pathology with epigenetically active agents: Focus on natural and synthetic inhibitors of DNA methylation and histone deacetylation. Int. J. Cardiol. 2017, 227, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Foulks, J.M.; Parnell, K.M.; Nix, R.N.; Chau, S.; Swierczek, K.; Saunders, M.; Wright, K.; Hendrickson, T.F.; Ho, K.K.; McCullar, M.V.; et al. Epigenetic drug discovery: Targeting DNA methyltransferases. J. Biolmol. Screen. 2012, 17, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Pechalrieu, D.; Etievant, C.; Arimondo, P.B. DNA methyltransferase inhibitors in cancer: From pharmacology to translational studies. Biochem. Pharmacol. 2017, 129, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Boer, V.C.; de Goffau, M.C.; Arts, I.C.; Hollman, P.C.; Keijer, J. Sirt1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of resveratrol and other polyphenols on sirt1: Relevance to brain function during aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Purrucker, J.C.; Mahlknecht, U. Targeting the epigenome: Effects of epigenetic treatment strategies on genomic stability in healthy human cells. Clin. Epigenet. 2010, 1, 45–54. [Google Scholar] [CrossRef][Green Version]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158, 13–26. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Bucolo, C.; Drago, F.; Salomone, S. Ocular drug delivery: A clue from nanotechnology. Front. Pharmacol. 2012, 3, 188. [Google Scholar] [CrossRef]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahajpal, N.; Kowluru, A.; Kowluru, R.A. The Regulatory Role of Rac1, a Small Molecular Weight GTPase, in the Development of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 965. https://doi.org/10.3390/jcm8070965
Sahajpal N, Kowluru A, Kowluru RA. The Regulatory Role of Rac1, a Small Molecular Weight GTPase, in the Development of Diabetic Retinopathy. Journal of Clinical Medicine. 2019; 8(7):965. https://doi.org/10.3390/jcm8070965
Chicago/Turabian StyleSahajpal, Nikhil, Anjan Kowluru, and Renu A. Kowluru. 2019. "The Regulatory Role of Rac1, a Small Molecular Weight GTPase, in the Development of Diabetic Retinopathy" Journal of Clinical Medicine 8, no. 7: 965. https://doi.org/10.3390/jcm8070965
APA StyleSahajpal, N., Kowluru, A., & Kowluru, R. A. (2019). The Regulatory Role of Rac1, a Small Molecular Weight GTPase, in the Development of Diabetic Retinopathy. Journal of Clinical Medicine, 8(7), 965. https://doi.org/10.3390/jcm8070965




