Identification of Retinal Biomarkers in Alzheimer’s Disease Using Optical Coherence Tomography: Recent Insights, Challenges, and Opportunities
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. General Findings
3.2. OCT Findings and Normative Data
3.3. OCT Findings Concerning Neuroimaging Features and Cognitive Tests
3.4. OCT Findings and AD Biomarkers
3.5. OCT Findings in Relation to Advanced Imaging and Electrophysiological Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puliafito, C.A. OCT Angiography: The next era of OCT technology emerges. Ophthalmic Surg. Lasers Imaging Retina 2014, 45, 360. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Cabrera DeBuc, D.; Somfai, G.M.; Koller, A. Retinal microvascular network alterations: Potential biomarkers of cerebrovascular and neural diseases. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H201–H212. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, M. Dementia: A global health priority—Highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 2012, 4, 40. [Google Scholar] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS—ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 2011, 77, 333. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 1997, 18, 351–357. [Google Scholar] [CrossRef]
- La Rue, A.; Jarvik, L.F. Cognitive function and prediction of dementia in old age. Int. J. Aging Hum. Dev. 1987, 25, 79–89. [Google Scholar] [CrossRef]
- Linn, R.T.; Wolf, P.A.; Bachman, D.L.; Knoefel, J.E.; Cobb, J.L.; Belanger, A.J.; Kaplan, E.F.; D’Agostino, R.B. The “preclinical phase” of probable Alzheimer’s disease. A 13-year prospective study of the Framingham Cohort. Arch. Neurol. 1995, 52, 485–490. [Google Scholar] [CrossRef]
- Elias, M.F.; Beiser, A.; Wolf, P.A.; Au, R.; White, R.F.; D’Agostino, R.B. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch. Neurol. 2000, 57, 808–813. [Google Scholar] [CrossRef]
- Kawas, C.H.; Corrada, M.M.; Brookmeyer, R.; Morrison, A.; Resnick, S.M.; Zonderman, A.B.; Arenberg, D. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology 2003, 60, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, D.A.; Kemper, S.J.; Mortimer, J.A.; Greiner, L.H.; Wekstein, D.R.; Markesbery, W.R. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the nun study. JAMA 1996, 275, 528–532. [Google Scholar] [CrossRef]
- Katz, B.; Rimmer, S. Ophthalmologic manifestations of Alzheimer’s disease. Surv. Ophthalmol. 1989, 34, 31–43. [Google Scholar] [CrossRef]
- Devanand, D.P.; Bansal, R.; Liu, J.; Hao, X.; Pradhaban, G.; Peterson, B.S. MRI hippocampal and entorhinal cortex mapping in predicting conversion to Alzheimer’s disease. Neuroimage 2012, 60, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Au, R.; Cabral, H.J.; Kowall, N.W.; Seshadri, S.; Kubilus, C.A.; Drake, J.; Wolf, P.A. Visual association pathology in preclinical Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 621–630. [Google Scholar] [CrossRef]
- Ong, Y.-L.; Ong, Y.-T.; Ikram, M.K.; Chen, C.L.H.; Wong, T.Y. Potential applications of spectral-domain optical coherence tomography (SD-OCT) in the study of Alzheimer’s disease. Proc. Singap. Healthc. 2014, 23, 74–83. [Google Scholar] [CrossRef]
- Moschos, M.M.; Markopoulos, I.; Chatziralli, I.; Rouvas, A.; Papageorgiou, S.G.; Ladas, I.; Vassilopoulos, D. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Trebbastoni, A.; Parisi, F.; Manganiello, S.; Cruciani, F.; D’Antonio, F.; De Vico, U.; Imbriano, L.; Campanelli, A.; De Lena, C. Choroidal thinning as a new finding in Alzheimer’s disease: Evidence from enhanced depth imaging spectral domain optical coherence tomography. J. Alzheimers Dis. 2014, 40, 907–917. [Google Scholar] [CrossRef]
- Cunha, J.P.; Proença, R.; Dias-Santos, A.; Melancia, D.; Almeida, R.; Águas, H.; Santos, B.O.; Alves, M.; Ferreira, J.; Papoila, A.L.; et al. Choroidal thinning: Alzheimer’s disease and aging. Alzheimers Dement. 2017, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.P.; Lopes, L.C.; Costa-Cunha, L.V.F.; Costa, C.F.; Pires, L.A.; Almeida, A.L.M.; Monteiro, M.L.R. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PLoS ONE 2016, 11, e0153830. [Google Scholar] [CrossRef]
- Szigeti, A.; Tátrai, E.; Varga, B.E.; Szamosi, A.; DeBuc, D.C.; Nagy, Z.Z.; Németh, J.; Somfai, G.M. The Effect of Axial Length on the Thickness of Intraretinal Layers of the Macula. PLoS ONE 2015, 10, e0142383. [Google Scholar] [CrossRef]
- Loh, E.H.-T.; Ong, Y.-T.; Venketasubramanian, N.; Hilal, S.; Thet, N.; Wong, T.Y.; Chen, C.P.L.; Cheung, C.Y.-L. Repeatability and reproducibility of retinal neuronal and axonal measures on spectral-domain optical coherence tomography in patients with cognitive impairment. Front. Neurol. 2017, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J. Psych. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Herranz, A.; Balk, L.J.; Oberwahrenbrock, T.; Saidha, S.; Martinez-Lapiscina, E.H.; Lagreze, W.A.; Schuman, J.S.; Villoslada, P.; Calabresi, P.; Balcer, L.; et al. IMSVISUAL consortium. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016, 86, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Lad, E.M.; Mukherjee, D.; Stinnett, S.S.; Cousins, S.W.; Potter, G.G.; Burke, J.R.; Farsiu, S.; Whitson, H.E. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PLoS ONE 2018, 13, e0192646. [Google Scholar] [CrossRef] [PubMed]
- Trebbastoni, A.; Marcelli, M.; Mallone, F.; D’Antonio, F.; Imbriano, L.; Campanelli, A.; de Lena, C.; Gharbiya, M. Attenuation of choroidal thickness in patients with Alzheimer disease: Evidence from an Italian prospective study. Alzheimer Dis. Assoc. Disord. 2017, 31, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, H.A.; Aslan Bayhan, S.; Celikbilek, A.; Tanık, N.; Gürdal, C. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2015, 43, 145–151. [Google Scholar] [CrossRef]
- Santos, C.Y.; Johnson, L.N.; Sinoff, S.E.; Festa, E.K.; Heindel, W.C.; Snyder, P.J. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement. 2018, 10, 196–209. [Google Scholar] [CrossRef]
- Uchida, A.; Pillai, J.A.; Bermel, R.; Bonner-Jackson, A.; Rae-Grant, A.; Fernandez, H.; Bena, J.; Jones, S.E.; Leverenz, J.B.; Srivastava, S.K.; et al. Outer retinal assessment using spectral-domain optical coherence tomography in patients with Alzheimer’s and Parkinson’s disease. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2768–2777. [Google Scholar] [CrossRef]
- Den Haan, J.; Janssen, S.F.; van de Kreeke, J.A.; Scheltens, P.; Verbraak, F.D.; Bouwman, F.H. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimers Dement. 2018, 10, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Castilla-Marti, M.; Rodríguez-Gómez, O.; Valero, S.; Piferrer, A.; Martínez, G.; Martínez, J.; Serra, J.; Moreno-Grau, S.; Hernández-Olasagarre, B.; et al. Usefulness of peripapillary nerve fiber layer thickness assessed by optical coherence tomography as a biomarker for Alzheimer’s disease. Sci. Rep. 2018, 8, 16345. [Google Scholar] [CrossRef] [PubMed]
- Poroy, C.; Yücel, A.Â. Optical coherence tomography: Is really a new biomarker for Alzheimer’s disease? Ann. Indian Acad. Neurol. 2018, 21, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Huang, S.C.; Magnani, G.; Ambrosi, A.; Comi, G.; Leocani, L. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J. Alzheimers Dis. 2017, 56, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Yang, J.H.; Han, J.S.; Kim, D.G. Analysis of the retinal nerve fiber layer thickness in Alzheimer disease and mild cognitive impairment. Korean J. Ophthalmol. 2017, 31, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, U.; Bonnemaijer, P.W.M.; Ikram, M.A.; Colijn, J.M.; Cremers, L.G.M.; Buitendijk, G.H.S.; Vingerling, J.R.; Niessen, W.J.; Vernooij, M.W.; Klaver, C.C.W.; et al. Retinal neurodegeneration and brain MRI markers: The Rotterdam study. Neurobiol. Aging 2017, 60, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Johnson, L.N.; Lim, Y.Y.; Santos, C.Y.; Alber, J.; Maruff, P.; Fernández, B. Nonvascular retinal imaging markers of preclinical Alzheimer’s disease. Alzheimers Dement. 2016, 4, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Giménez Castejón, D.; Dudekova, M.; Gómez Gallego, M.; Lajara Blesa, J. Macular thickness in subjective memory complaints and mild cognitive impairment: A non-invasive biomarker. Neuroophthalmology 2016, 40, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Park, S.J.; Kim, N.R. Macular ganglion cell-inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS ONE 2016, 11, e0162202. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.; Simonett, J.; Volpe, N.J.; Farsiu, S.; Ward, M.; Rademaker, A.; Weintraub, S.; Fawzi, A.A. Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: Case-control study and meta-analysis. Alzheimer’s Dement. 2016, 4, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ong, Y.-T.; Hilal, S.; Loke, Y.M.; Wong, T.Y.; Chen, C.L.-H.; Cheung, C.Y.; Zhou, J. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2016, 54, 585–595. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Ross-Cisneros, F.N.; Koronyo, Y.; Hannibal, J.; Gallassi, R.; Cantalupo, G.; Sambati, L.; Pan, B.X.; Tozer, K.R.; Barboni, P.; et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 2016, 79, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Bambo, M.P.; Marques, M.L.; Satue, M.; Otin, S.; Larrosa, J.M.; Polo, V.; Pablo, L.E. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 2016, 94, e454–e459. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.A.; Bermel, R.; Bonner-Jackson, A.; Rae-Grant, A.; Fernandez, H.; Bena, J.; Jones, S.E.; Ehlers, J.P.; Leverenz, J.B. Retinal nerve fiber layer thinning in Alzheimer’s disease: A case-control study in comparison to normal aging, Parkinson’s disease, and Non-Alzheimer’s dementia. Am. J. Alzheimers Dis. Other Dement. 2016, 31, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Li, Z.; Zhang, X.; Wu, Y.; Yang, H.; Min, B.; Zhang, X.; Ma, D.; Lu, Y. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Li, X.; Bai, Q.; Liu, P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch. Gerontol. Geriatr. 2015, 60, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.-L.; Ong, Y.T.; Hilal, S.; Ikram, M.K.; Low, S.; Ong, Y.L.; Venketasubramanian, N.; Yap, P.; Seow, D.; Chen, C.L.H.; et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 45–56. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Hoyas, I.; Leal, M.; De Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; et al. Analysis of retinal peripapillary segmentation in early Alzheimer’s disease patients. Biomed. Res. Int. 2015, 2015, 636548. [Google Scholar] [CrossRef]
- Oktem, E.O.; Derle, E.; Kibaroglu, S.; Oktem, C.; Akkoyun, I.; Can, U. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol. Sci. 2015, 36, 1141–1146. [Google Scholar] [CrossRef]
- Eraslan, M.; Çerman, E.; Çekiç, O.; Balci, S.; Dericioğlu, V.; Sahin, Ö.; Süer, D.; Chabou, B.; Tuncer Elmaci, E.N. Neurodegeneration in ocular and central nervous systems: optical coherence tomography study in normal-tension glaucoma and Alzheimer disease. Turk. J. Med. Sci. 2015, 45, 1106–1114. [Google Scholar] [CrossRef]
- Ascaso, F.J.; Cruz, N.; Modrego, P.J.; Lopez-Anton, R.; Santabárbara, J.; Pascual, L.F.; Lobo, A.; Cristóbal, J.A. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: An optical coherence tomography study. J. Neurol. 2014, 261, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.S.; Rojas, B.; Ramirez, A.I.; de Hoz, R.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; Ramirez, J.M. Macular thickness as a potential biomarker of mild Alzheimer’s disease. Ophthalmology 2014, 121, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Polo, V.; Garcia-Martin, E.; Bambo, M.P.; Pinilla, J.; Larrosa, J.M.; Satue, M.; Otin, S.; Pablo, L.E. Reliability and validity of cirrus and spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. Eye 2014, 28, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, L.; Cheng, Y.; Feng, W.; Shi, Z.; Zhu, Y.; Wu, W.; Li, C. Retinal nerve fiber layer thickness is associated with episodic memory deficit in mild cognitive impairment patients. Curr. Alzheimer Res. 2014, 11, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wu, Y.; Wang, M.; Cao, J.; Feng, W.; Cheng, Y.; Li, C.; Shen, Y. Greater attenuation of retinal nerve fiber layer thickness in Alzheimer’s disease patients. J. Alzheimers Dis. 2014, 40, 277–283. [Google Scholar] [CrossRef]
- Kromer, R.; Serbecic, N.; Hausner, L.; Froelich, L.; Aboul-Enein, F.; Beutelspacher, S.C. Detection of retinal nerve fiber layer defects in Alzheimer’s disease using SD-OCT. Front. Psych. 2014, 5, 22. [Google Scholar] [CrossRef]
- Larrosa, J.M.; Garcia-Martin, E.; Bambo, M.P.; Pinilla, J.; Polo, V.; Otin, S.; Satue, M.; Herrero, R.; Pablo, L.E. Potential new diagnostic tool for Alzheimer’s disease using a linear discriminant function for fourier domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3043–3051. [Google Scholar] [CrossRef]
- Bambo, M.P.; Garcia-Martin, E.; Pinilla, J.; Herrero, R.; Satue, M.; Otin, S.; Fuertes, I.; Marques, M.L.; Pablo, L.E. Detection of retinal nerve fiber layer degeneration in patients with Alzheimer’s disease using optical coherence tomography: Searching new biomarkers. Acta Ophthalmol. 2014, 92, e581–e582. [Google Scholar] [CrossRef]
- Moreno-Ramos, T.; Benito-León, J.; Villarejo, A.; Bermejo-Pareja, F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with lewy bodies, and Alzheimer’s disease. J. Alzheimers Dis. 2013, 34, 659–664. [Google Scholar] [CrossRef]
- Marziani, E.; Pomati, S.; Ramolfo, P.; Cigada, M.; Giani, A.; Mariani, C.; Staurenghi, G. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2013, 54, 5953–5958. [Google Scholar] [CrossRef]
- Kirbas, S.; Turkyilmaz, K.; Anlar, O.; Tufekci, A.; Durmus, M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J. Neuroophthalmol. 2013, 33, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Kesler, A.; Vakhapova, V.; Korczyn, A.D.; Naftaliev, E.; Neudorfer, M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin. Neurol. Neurosurg. 2011, 113, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Z.; Zhang, X.; Ming, B.; Jia, J.; Wang, R.; Ma, D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: Evidence in optical coherence tomography. Neurosci. Lett. 2010, 480, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; Boissonnot, M.; Roger, F.; Dighiero, P.; Gil, R.; Hugon, J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2007, 420, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Iseri, P.K.; Altinaş, O.; Tokay, T.; Yüksel, N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuroophthalmol. 2006, 26, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Restuccia, R.; Fattapposta, F.; Mina, C.; Bucci, M.G.; Pierelli, F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin. Neurophysiol. 2001, 112, 1860–1867. [Google Scholar] [CrossRef]
- Van de Pol, L.A.; Korf, E.S.C.; Van der Flier, W.M.; Robert Brashear, H.; Fox, N.C.; Barkhof, F.; Scheltens, P. Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Arch. Neurol. 2007, 64, 1023. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia—Meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.W.; Goodkin, D.E. Screening for cognitive impairment in multiple sclerosis. An evaluation of the mini-mental state examination. Arch. Neurol. 1990, 47, 297–301. [Google Scholar] [CrossRef]
- Swirsky-Sacchetti, T.; Field, H.L.; Mitchell, D.R.; Seward, J.; Lublin, F.D.; Knobler, R.L.; Gonzalez, C.F. The sensitivity of the mini-mental state exam in the white matter dementia of multiple sclerosis. J. Clin. Psychol. 1992, 48, 779–786. [Google Scholar] [CrossRef]
- Moser, D.J.; Cohen, R.A.; Clark, M.M.; Aloia, M.S.; Tate, B.A.; Stefanik, S.; Forman, D.E.; Tilkemeier, P.L. Neuropsychological functioning among cardiac rehabilitation patients. J. Cardiopulm. Rehabil. 1999, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Spencer, R.J.; Wendell, C.R.; Giggey, P.P.; Katzel, L.I.; Lefkowitz, D.M.; Siegel, E.L.; Waldstein, S.R. Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates. Exp. Aging Res. 2013, 39, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Lonie, J.A.; Tierney, K.M.; Ebmeier, K.P. Screening for mild cognitive impairment: A systematic review. Int. J. Geriatr. Psych. 2009, 24, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Smith, P.J.; Mabe, S.; Hinderliter, A.; Lin, P.; Liao, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Kraus, W.E.; Murali Doraiswamy, P.; et al. Lifestyle and neurocognition in older adults with cognitive impairments. Neurology 2019, 92, e212–e223. [Google Scholar] [CrossRef] [PubMed]
- Trzepacz, P.T.; Hochstetler, H.; Wang, S.; Walker, B.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging Initiative. Relationship between the montreal cognitive assessment and mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Toschi, N.; Babiloni, C.; Baldacci, F.; Black, K.L.; Bokde, A.L.W.; Bun, R.S.; Cacciola, F.; Cavedo, E.; Chiesa, P.A.; et al. Revolution of Alzheimer precision neurology passageway of systems biology and neurophysiology. J. Alzheimers Dis. 2018, 64, S47–S105. [Google Scholar] [CrossRef]
- Mukherjee, C.; Al-Fahad, Q.; Elsherbiny, S. The role of optical coherence tomography in therapeutics and conditions, which primarily have systemic manifestations: A narrative review. Ther. Adv. Ophthalmol. 2019, 11. [Google Scholar] [CrossRef]
- Feke, G.T.; Hyman, B.T.; Stern, R.A.; Pasquale, L.R. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C.; Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Woehrer, A.; Ricken, G.; Augustin, M.; Mitter, C.; Pircher, M.; Kovacs, G.G.; Hitzenberger, C.K. Visualization of neuritic plaques in Alzheimer’s disease by polarization-sensitive optical coherence microscopy. Sci. Rep. 2017, 7, 43477. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Salumbides, B.C.; Black, K.L.; Koronyo-Hamaoui, M. Alzheimer’s disease in the retina: Imaging retinal Aβ plaques for early diagnosis and therapy assessment. Neurodegener. Dis. 2012, 10, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011, 54 (Suppl. 1), S204–S217. [Google Scholar] [CrossRef] [PubMed]
- Cognoptix, Inc. Available online: http://www.cognoptix.com/news/inthenews.htm (accessed on 15 August 2018).
- Neurovisionimaging. Available online: http://www.neurovisionimaging.com (accessed on 15 August 2018).
- Lesage, S.R.; Mosley, T.H.; Wong, T.Y.; Szklo, M.; Knopman, D.; Catellier, D.J.; Cole, S.R.; Klein, R.; Coresh, J.; Coker, L.H.; et al. Retinal microvascular abnormalities and cognitive decline: The ARIC 14-year follow-up study. Neurology 2009, 73, 862–868. [Google Scholar] [CrossRef] [Green Version]
- Patton, N.; Pattie, A.; MacGillivray, T.; Aslam, T.; Dhillon, B.; Gow, A.; Starr, J.M.; Whalley, L.J.; Deary, I.J. The association between retinal vascular network geometry and cognitive ability in an elderly population. Invest. Opthalmol. Vis. Sci. 2007, 48, 1995. [Google Scholar] [CrossRef]
- Waldstein, S.R.; Rice, S.C.; Thayer, J.F.; Najjar, S.S.; Scuteri, A.; Zonderman, A.B. Pulse pressure and pulse wave velocity are related to cognitive decline in the baltimore longitudinal study of aging. Hypertension 2008, 51, 99–104. [Google Scholar] [CrossRef]
- Elias, M.F.; Robbins, M.A.; Budge, M.M.; Abhayaratna, W.P.; Dore, G.A.; Elias, P.K. Arterial pulse wave velocity and cognition with advancing age. Hypertension 2009, 53, 668–673. [Google Scholar] [CrossRef]
- Goutagny, R.; Krantic, S. Hippocampal oscillatory activity in Alzheimer’s disease: Toward the identification of early biomarkers? Aging Dis. 2013, 4, 134–140. [Google Scholar]
- Liu, C.; Cao, L.; Yang, S.; Xu, L.; Liu, P.; Wang, F.; Xu, D. Subretinal injection of amyloid-β peptide accelerates RPE cell senescence and retinal degeneration. Int. J. Mol. Med. 2015, 35, 169–176. [Google Scholar] [CrossRef]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Antes, R.; Ezra-Elia, R.; Weinberger, D.; Solomon, A.; Ofri, R.; Michaelson, D.M. ApoE4 induces synaptic and ERG impairments in the retina of young targeted replacement apoE4 mice. PLoS ONE 2013, 8, e64949. [Google Scholar] [CrossRef] [PubMed]
- Cabrera DeBuc, D.; Kostic, M.; Oropes, S.; Somfai, G.M.; Mendoza-Santiesteban, C. Diagnostic biomarkers of Alzheimer’s disease utilizing changes in the retina. In Proceedings of the The 55th Annual Symposium of the International Society for Clinical Electrophysiology of Vision, Miami, FL, USA, 21–26 October 2017; p. 25. [Google Scholar]
- Berisha, F.; Feke, G.T.; Trempe, C.L.; McMeel, J.W.; Schepens, C.L. Retinal abnormalities in early Alzheimer’s disease. Invest. Ophthalmol. Vis. Sci. 2007, 48, 2285–2289. [Google Scholar] [CrossRef]

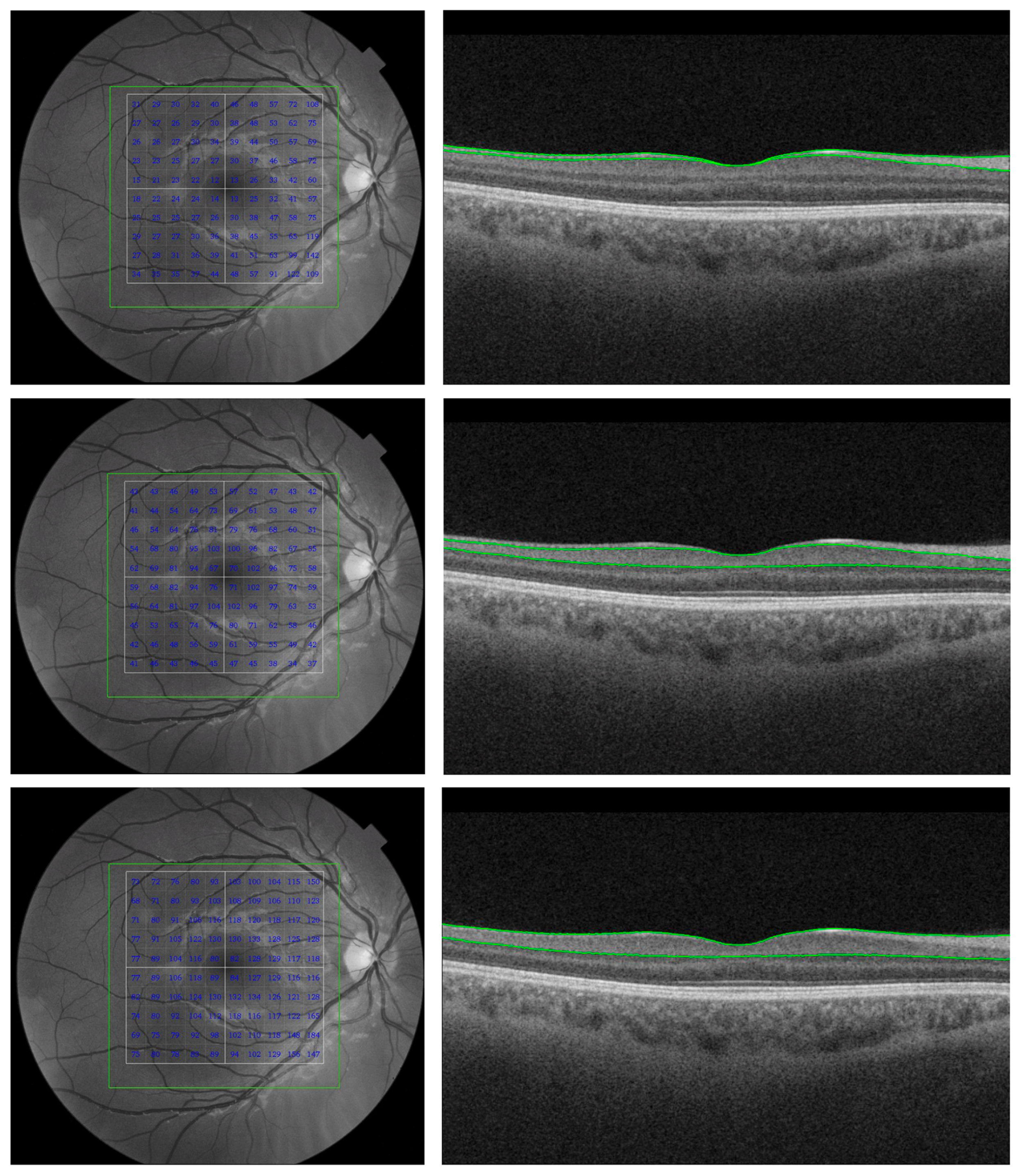
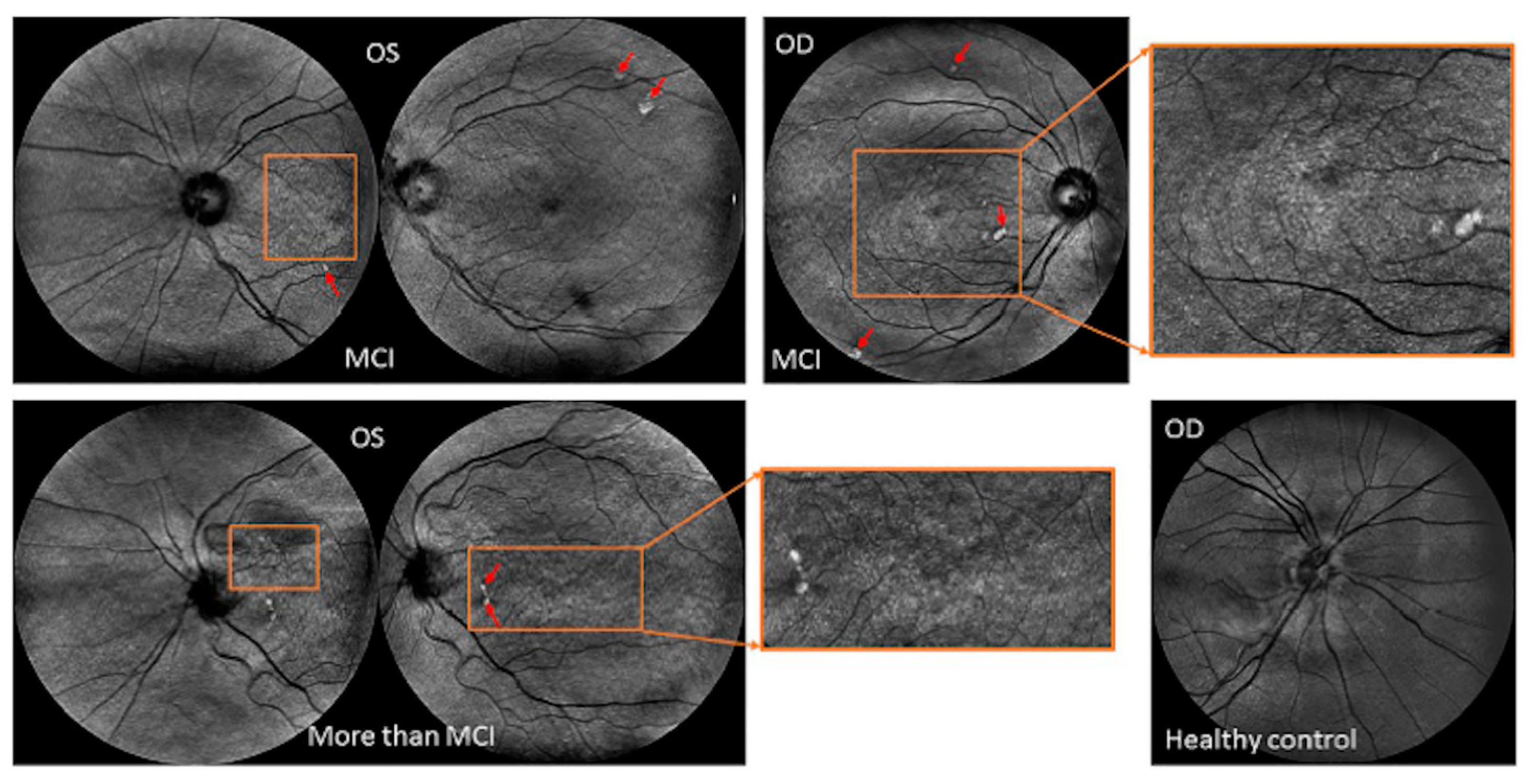


| Source | Sample Size | Criteria Used for Selecting Participants | Findings | MRI or CT (Control Group) & ERG (Both Groups) | OCT Platform | Gender (F/M) & Race | |
|---|---|---|---|---|---|---|---|
| Pathological Group | Control Group | ||||||
| Santos et al. 2018 [29] | (n = 56) older adults with multiple risk factors for AD. | All patients underwent detailed medical screening, patients with AD and MCI according to the NIAAA diagnostic criteria were excluded. | Preclinical stage of AD showed a decrease in macular RNFL volume over a 27-month follow-up interval period, as well as a decrease in ONL and IPL volumes/thickness in the inferior quadrant. Only the RNFL volume was linearly related to neocortical PET amyloid standardized uptake value ratio after controlling for any main effects of age. The magnitude of RNFL volume reduction was correlated with performance on a task of participants’ abilities to efficiently integrate visual and auditory speech information (McGurk effect). | YES (PET with head CT) | Heidelberg Spectralis | 26F/48M Race: unknown | |
| Uchida et al. 2018 [30] | (n = 24) AD Dementia, (n = 22) Amnestic MCI, (n = 20) non-AD dementia, (n = 22) PD, (n = 36) controls | AD diagnosis according to NIAAA criteria. | The outer retinal thickness measures did not show any statistical significance between the groups. However, ellipsoid zone to retinal pigment epithelium volume correlated with cognitive testing scores in all study participants. | YES | Zeiss Cirrus | 14F/8M Amnestic MCI 9F/11M non-AD Dementia 10F/12M PD 22F/14M Controls Race: unknown | |
| Lad et al. 2018 [26] | (n = 15) MCI, (n = 15) mild-moderate AD, (n = 18) cognitively normal adults. | AD diagnosis according to the NINCDS-ADRDA criteria. MCI diagnosis according to the NIAAA criteria. | Regional thicknesses of NFL or GCIPL on macular or nerve OCTs did not differ between groups. Multivariate regression analysis identified macular areas with a significant thickening or thinning in NFL and GC-IPL in MCI and AD patients. | NOT ALWAYS | Heidelberg Spectralis | AD: 7F/8M, 14 Caucasian MCI: 8F/7M, 15 Caucasian Controls: 8F/10M, 14 Caucasian | |
| Den Haan et al. 2018 [31] | (n = 15) early onset amyloid-positive AD, (n = 5) controls. | AD according to NIAAA criteria, evidence of amyloid pathology in CSF and/or amyloid PET. | Total macular thickness correlated with parietal cortical atrophy in both groups. | YES (MRI) | Heidelberg Spectralis | 7F/8M AD 7F/8M Controls Race: unknown | |
| Sánchez et al. 2018 [32] | (n = 24) probable AD (n = 92) probable amnestic MCI (n = 414) controls | Diagnosis according to the MMSE, 7-minute tests and the Hospital Anxiety and Depression Scale | The multivariate adjusted analysis revealed no significant differences in mean overall, or local RNFL thickness between AD, MCI and control groups. These results do not support the usefulness of peripapillary RNFL analysis as a marker of MCI/AD. | NOT ALWAYS | Topcon Maestro | 74.0% women AD 56.2% women MCI 67.7% women Controls Race: unknown | |
| Poroy et al. 2018 [33] | (n = 21) AD (n = 25) controls | AD diagnosis according to DSM-V | Foveal thickness and volume were significantly higher in AD patients than in controls. Compared to controls, peripapillary RNFL and other macular region measurements of AD patient were not statistically different. | N/A | Zeiss Stratus | 15F/6M AD 18F/7M Controls Race: unknown | |
| Cunha et al. 2017 [19] | (n = 50) patients with mild AD, (n = 152) patients without AD (age-matched patients), (n = 50) elderly patients without AD (over 78 years of age) | Each patient sent by the neurology department underwent visual acuity, IOP, mean arterial pressure assessment. | AD patients showed a significant choroidal thinning even when compared with elderly subjects. The reduction of CT may aid in the diagnoses of AD, probably reflecting the importance of vascular factors in their pathogenesis. | NO | Heidelberg Spectralis | 34F/16M AD 97F/55M Controls 33F/17M Controls Race: unknown | |
| Ferrari et al. 2017 [34] | (n = 39) AD, (n = 17) FTD, (n = 27) MCI, (n = 49) controls | Albert criteria for MCI, NINCDS-ADRDA criteria for AD, Rascovsky criteria for FTD. | GCL-IPL was significantly correlated with raw MMSE in AD. | N/A | Heidelberg Spectralis | 24F/13M AD 9F/8M FTD 15F/12M MCI 26F/23M Controls Race: unknown | |
| Kwon et al. 2017 [35] | (n = 15) AD (n = 15) MCI (n = 15) controls | AD diagnosis according to the MMSE, CDR and Activities of Daily Living Scales. MCI according to the Petersen criteria. | Mean RNFL thickness was lower in the AD group than in the MCI group. RNFL thickness in the superior quadrant was lower in AD patients compared to controls. | YES (MRI) | Zeiss Cirrus | Gender and Race unknown | |
| Trebbastioni et al. 2017 [27] | (n = 39) AD (n = 39) age matched controls subjects | AD diagnosis according NINCDS -ADRDA criteria and MMSE/CDR. | Controls underwent physical and neurological assessment, standard laboratory tests, serum vitamin B12, folate and thyroid hormone assays. | Choroidal thickness in patients with AD showed a rate of thinning greater than what could be expected during the natural course of aging. | NO | Heidelberg Spectralis | 21F/18M AD 22F/17M Controls Race: unknown |
| Mutlu et al. 2017 [36] | Included 2124 persons from the Rotterdam Study who had gradable retinal OCT images and brain MRI scans. | Cognitive test battery including the MMSE and the Geriatric Mental State Schedule organic level Dementia-screening was done using the MMSE and the Geriatric Mental State Schedule (GMS) organic level. | Thinner RNFL, GCL and IPL were associated with smaller grey matter and white matter volume. Thinner RNFL and GCL were associated with worse white matter microstructure. No association between retinal sublayer thickness and white matter lesion volumes, cerebral microbleeds or lacunar infarcts. | YES (MRI) | Topcon 3D OCT 1000/2000 | 1190F/934M Race: unknown | |
| Snyder et al. 2016 [37] | Cognitively normal adults, all of whom have a parent with AD and subjective memory complaints (SMC, n = 63) | All participants underwent a detailed medical screening interview. Exclusion criteria were a diagnosis of MCI or AD, history of neurological or psychiatric disorder, any significant systemic illness or unstable medical condition used MMSE and DASS. | Trend toward a selective volume increase in the IPL (which is rich in cholinergic activity) of the retina in Aβ+ relative to Aβ− participants, and IPL volume was correlated with the surface area of retinal inclusion bodies. | NO | Heidelberg Spectralis | 39F/24M Race: unknown | |
| Gimenéz Castejon et al. 2016 [38] | The total number of valid patients was (n = 57), (n = 24) of them diagnosed with SMC and (n = 33) with MCI and (n = 25) control group. | The diagnosis of MCI was based on the standards of the DSM-IV. For the SMC patients, the same cognitive screening as that for the MCI patients was performed. | Statistically significant differences have been found in the macular thickness of the control group and for both MCI and SMC patients. | NO | Zeiss Cirrus | 12F/12M SMC, 10F/15M MCI 15F/18M Controls Race: unknown | |
| Choi et al. 2016 [39] | (n = 42) patients with AD, (n = 26) with MCI, (n = 66) normal elderly controls. | The subjects with AD met the criteria for dementia according to the DSM-IV, as well as the criteria for probable AD established by the NIAAA. The diagnosis of MCI was in accordance with Petersen et al.’s criteria, listed as follows: (1) subjective memory complaints corroborated by an informant; (2) objective memory decline, as defined by a delayed recall score on the Seoul Verbal Learning test (SVLT) less than 1.5 standard deviations (SD) below the age- and education-adjusted normative means; (3) normal general cognitive function, as defined by CDR scale of 0.5, and MMSE scores more than 1.5 SD below the age- and education-adjusted normative means; (4) normal functional activities; and (5) lack of a dementia diagnosis. | The Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) score presented negative relationships with the average GCIPL thickness and the GCIPL thickness in the superotemporal, superonasal, and inferonasal sectors. The composite memory score exhibited significant positive associations with the average GCIPL thickness and the GCIPL thickness in the superotemporal, inferonasal, and inferotemporal sectors. The temporal RNFL thickness, the average and minimum GCIPL thicknesses, and the GCIPL thickness in the inferonasal, inferior, and inferotemporal sectors at baseline were significantly reduced in MCI patients who were converted to AD compared to stable MCI patients. The change of CDR-SB from baseline to 2 years exhibited significant negative associations with the average and minimum GCIPL thicknesses as well as GCIPL thickness in the superotemporal, superior, superonasal, and inferonasal sectors at baseline. Data suggest that macular GCIPL thickness represents a promising biomarker for monitoring the progression of MCI and AD. | YES | Zeiss Cirrus | 38F/4M AD 16F/10M MCI 38F/28M Controls Race: unknown | |
| Knoll et al. 2016 [40] | Participants with a clinical diagnosis of aMCI and Cognitively normal control participants (n = 17/17) | The diagnosis of MCI based on research diagnostic criteria including the following: scores falling 2 or more standard deviations below the mean on neuropsychological tests MMSE within a battery used across the National Institute on Aging ADC programs (i.e., the Uniform Data Set (UDS)) and the absence of impairments of activities of daily living as corroborated by a study partner. | Control subjects had no abnormal test scores on the UDS battery and all had normal activities of daily living as reported by their study partners. | No statistically significant difference in optical coherence tomography (OCT) between aMCI subjects and controls, but uncovered that OCT thickness was significantly (inversely) related to cognitive scores. The meta-analysis showed statistically significant thinning in MCI subjects compared with controls. | NO | Heidelberg Spectralis | 13F/4M aMCI 13F/4M Controls Race: African American 5 aMCI/5 Controls; Caucasian 12 aMCI/12 Controls |
| Liu et al. 2016 [41] | Participants group difference in WM microstructure in (n = 65) no cognitive impairment (NCI), (n = 68) CIND, and (n = 47) AD and the WM-GC-IPL association in a subset of 124 subjects who passed the retina imaging quality control (n = 180) | All subjects underwent MMSE, MoCA, CDR, Geriatric Depression Scale, the informant questionnaire on cognitive decline, and a formal neuropsychological battery. Subjects were also assessed for neuroimaging evidence of significant cerebrovascular disease. | Within those participants with OCT scans of sufficient quality for analysis, GC-IPL was significantly thinner in CIND than in NCI. | YES | Zeiss Cirrus | NCI: 29F/36M CIND: 33F/35M AD: 30F/17M Chinese:Malay:Indian:Mix:Others NCI 60:2:3:0:0 CIND 55:2:11:0:0 AD 38:6:1:1: | |
| Cunha et al. 2016 [20] | A total of 45 eyes from (n = 24) patients with AD and 48 eyes from (n = 24) healthy controls. | Each patient underwent a full neurological examination MMSE and MRI of the brain to rule out alternative diagnoses. | Most OCT peripapillary RNFL and macular full-thickness and segmented inner retinal layers parameters were reduced in AD compared to controls. Average, superior and inferior quadrant RNFL thickness parameters and all but one of the nine full-thickness macular measurements were significantly reduced in AD compared to controls. The segmented layers, GCL+ and GCL++ were significantly reduced in AD eyes. Significant correlation between most OCT parameters and MMSE scores, particularly in macular thickness. | YES | Topcon 3D OCT-2000 | 16F/8M AD group 15F/9M Controls Race: unknown | |
| La Morgia et al. 2016 [42] | (n = 21) AD, (n = 74) age-matched control subjects. | Included patients with a diagnosis of AD according to NINCDS-ADRDA criteria at mild–moderate stage (MMSE>11). Absence of cognitive dysfunction was ascertained in controls. | Age-related optic neuropathy in AD by OCT, with a significant reduction of RNFL thickness, more evident in the superior quadrant. | NO | Zeiss Stratus | 10F/11M AD 43F/31M Controls Race: unknown. | |
| Garcia Martin et al. 2016 [43] | Patients with AD (n = 150) and age-matched healthy controls (n = 75) | Inclusion criteria were AD diagnosis according to the NINCDS-ADRDA, MMSE and DSM-IV criteria. | The controls were family members or caregivers of the patients or health workers. All subjects underwent neurologic and neuro-ophthalmologic evaluations. | The segmentation application revealed ganglion cell and retinal layer atrophy in patients with AD compared with controls, especially in the inner layers of patients with long disease duration. Ganglion cell layer reduction was associated with increased axonal damage and may predict greater disease severity. | NO | Heidelberg Spectralis | 84F/66M AD group 42F/33M Controls Race: unknown |
| Pillai et al. 2016 [44] | AD dementia (n = 21), amnestic MCI (n = 21), non-AD dementia (n = 20), PD (n = 20), (n = 34) age-/sex-matched controls. | As part of neurocognitive testing, study participants completed the MoCA, Logical Memory subtest of the Wechsler Memory Scale—Fourth Edition, Hopkins Verbal Learning Test–Revised phonemic. And semantic verbal fluency, and Trail Making Test (parts A and B). | Among SD-OCT measures, the RNFL, GCL, and MV were not significantly different across all groups. Using all SD-OCT measures in a mixed-effect model did not identify any significant. Differences between the groups. The RNFL thickness measures analysis by group and quadrant also did not show any statistically significant difference between the groups. | YES | Zeiss Cirrus | 13F/8M AD 12F/9M Amnestic MCI 11F/9M NonAD Dementia 11F/9M PD 20F/14M Controls Race: unknown | |
| Liu et al. 2015 [45] | A total of 93 cognitive impaired subjects comprising 26 MCI, 24 mild AD patients, 24 moderate AD patients, 19 severe AD patients, and 39 age-matched controls (n = 39) | All AD patients were diagnosed according to the NINCDS-ADRDA and DSM-IV criteria. | The criteria for controls were: (1) no memory complaints; (2) MMSE scores above 28. | RNFL degeneration is paralleled with dementia progression. Owing to its non-invasive and cost-effective nature, monitoring RNFL thickness may have a value in assessing disease progression and the efficacy of any treatments. The thickness of RNFL in the superior quadrant and total mean values are gradually and significantly decreased from MCI to severe AD when compared to that in the controls. There is also a significant reduction of the RNFL in the inferior quadrant in severe AD patients. | NO | Zeiss Stratus | 52F/41M AD group 22F/17M Controls Race: unknown |
| Gao et al. 2015 [46] | A total of 72 subjects, comprising 25 AD patients, 26 MCI patients (n = 51), and 21 healthy individuals (controls) (n = 21) | All subjects underwent complete neurological MMSE scale and physical examination, ophthalmic examination, laboratory examination of body fluids, neuroimaging evaluation, and psychometric testing to rule out alternative diagnoses. Neuroimaging examination either through MRI or CT was adopted to exclude participants suffering from other neurological or non-neurological diseases that may influence the study results. | Retinal Degeneration in AD and MCI patients results in decreased thickness of the RNFL, and reduced macular volume in AD and MCI patients. However, there seems to be no correlation between these changes and the severity of dementia. | YES | Zeiss Cirrus | 24F/26M AD/MCI 7F/14M Controls Race: unknown | |
| Cheung et al. 2015 [47] | Cognitively normal controls (n = 123), patients with AD (n = 100), MCI (n = 41) | All patients underwent clinical and neuropsychiatric assessment. CT or MRI was reviewed as part of the diagnostic process. AD patients fulfilled the DSM-IV criteria for dementia syndrome (Alzheimer’s type) and NINCDS-ADRDA criteria for probable or possible AD. | AD patients compared with cognitively normal controls had significantly reduced GC-IPL thicknesses in all six (superior, superonasal, inferonasal, inferior, inferotemporal, and superotemporal) sectors and reduced RNFL thickness in superior quadrant. Patients with MCI also had significantly reduced GC-IPL thicknesses compared with controls. Supports the link between retinal ganglion cell neuronal and optic nerve axonal loss with AD, and suggest that assessment of macular GC-IPL can be a test to detect neuronal injury in early AD and MCI. | YES | Zeiss Cirrus | 85F/56M AD/MCI 56F/67M Controls Race: Chinese/Malay/Indian: 108/18/15 in AD/MCI group, and 123/0/0 control group | |
| Salobrar-Garcia et al. 2015 [48] | (n = 23) patients with mild AD (n = 28) age-matched control subjects. | These patients, according to the NINCDS-ADRDA, MMSE and DSM-IV criteria had mild cognitive impairment according to the Clinical Dementia Rating scale. | Eyes of patients with mild-AD patients showed no statistical difference in peripapillary RNFL thickness; however, sectors 2,3,4,8,9, and 11 of the papillae showed thinning, while in sectors 1,5,6,7, and 10 there was thickening. Total macular volume and RNFL thickness of the fovea in all four inner quadrants and in the outer temporal quadrants proved to be significantly decreased. | NO | Topcon 3D OCT-1000 | 14F/9M AD 19F/9M Controls Race: Caucasian | |
| Octem et al. 2015 [49] | (n = 35) patients with AD, (n = 35) patients with MCI, (n = 35) healthy volunteers | Cognitive assessment was done with the standardized MMSE and MoCA test | No significant differences of RNFL were found between the MCI and the AD groups. Significant correlation was found between MMSE scores and the RNFL values. Significant thinning in RNFL along with age was detected. | NO | Zeiss Cirrus | 23F/12M AD 20F/15M MCI 23F/12M Controls Race: unknown | |
| Bayhan et al. 2015 [28] | (n = 31) AD (n = 30) age =matched controls | The diagnosis of probable AD was determined by referring neurologists according to the NINCDS-ADRDA and MMSE. | The control subjects also underwent a detailed neurological examination to rule out the presence of cognitive impairment. | Reduced choroidal and macular ganglion cell complex thicknesses in AD | YES | Zeiss Stratus | 14F/17M AD 14F/16M Controls Race: unknown |
| Eraslan et al. 2015 [50] | (n = 18) normal tension glaucoma (NTG), (n = 20) AD, (n = 20) control subjects | Diagnosis was based on NINCDS/ADRDA. | Each patient underwent neurological examination. | There was a significant reduction in peripapillary RNFL thickness and macular GCC thickness and a significant increase in the global loss volume (GLV) rate in both the NTG and AD patients when compared to the control subjects. The statistical evaluation showed no difference in any RNFL or GCC parameters between the AD and NTG groups. There was a negative correlation between disease duration and average RNFL and GCC thicknesses and a positive correlation between duration and GLV in the AD group. | NO | RTVue 100 | 10F/8M NTG group 13F/7M AD group 14F/6M Controls Race: unknown |
| Ascaso et al. 2014 [51] | (n = 18) patients with AD, (n = 21) aMCI, (n = 41) healthy controls | Diagnosis was based on DSM IV, MMSE scale, of MCI, used Winblad criteria. | Each patient underwent full neurologic examination to rule out the presence of dementia or cognitive impairment. | RNFL was thinner in MCI patients compared with controls, and it was also thinner in AD patients compared with MCI patients and controls. With regard to the macular measurements in mm3, MCI patients had the greatest macular volume in comparison with AD patients and controls. In turn the controls had greater macular volume than AD patients. The decreased RNFL thickness in MCI and AD patients suggests loss of retinal neurons and their axons. | NO | Zeiss Stratus | 21F/18M AD/MCI 21F/20M Controls Race: Hispanic |
| Garcia-Martin et al. 2014 [52] | Twenty patients with mild AD (n = 20), (n = 28) matched control subjects | The AD patients met the criteria for AD according to the NINCDS-ADRDA, MMSE and DSM-IV criteria, having MCI according to the CDR scale. | All the subjects had a complete ophthalmologic examination, including VA, refraction, anterior and posterior segment biomicroscopy, IOP measurement, dilated fundus examination, and OCT. | Mild AD patients, compared with a control group, had a statistically significant decrease in RNFL thickness, of some macular regions and in the total macular volume. | NO | Topcon 3D OCT-1000 | 12F/8M AD group 19F/9M Controls Race: Caucasian |
| Polo et al. 2014 [53] | (n = 70) with AD, (n = 70) sex- and age-matched healthy subjects | Inclusion criteria were confirmed AD diagnosis; Diagnosis of AD was determined by neurologists according to the NINCDS-ADRDA, DSM-IV and MMSE criteria. | Healthy controls had no evidence of disease of any nature, including neurologic disorders by interview. | SD-OCT is a valid and reliable technique for detecting subclinical RNFL and retinal atrophy in AD, especially using the Nsite Axonal application. RNFL thickness decreased with disease duration. | NO | Zeiss Cirrus/Heidelberg Spectralis | 40F/30M AD group 40F/30 M Controls Race: Hispanic |
| Gharbiya et al. 2014 [18] | 42 eyes of (n = 21) patients (mean age, 73.1 ± 6.9 years) with a diagnosis of mild to moderate AD, 42 eyes of (n = 21) age-matched control subjects (mean age, 70.3 ± 7.3 years) | All the subjects underwent neuropsychological (MMSE, ADAS-Cog, and CDR) and ophthalmological evaluation. The SD-OCT images of the choroid were obtained by EDI modality. Choroidal thickness was assessed by manual measurement. The following parameters, measured automatically by the OCT software, were also analyzed for each eye: 1-mm central subfield retinal thickness, peripapillary RNFL thickness. | Compared with healthy subjects, patients with AD showed a significant reduction in choroidal thickness. | NO | Zeiss Stratus | Gender and Race unknown | |
| Shen et al. 2014 [54] | (n = 75) older adults were included in the study, (n = 52) participants had normal cognition (NC), (n = 23) participants were diagnosed with MCI. | Cognitive function was evaluated by the Repeatable Battery for the Assessment of Neuropsychological Status on the same day of the optical examination. | Found that nasal quadrant RNFL thickness was positively associated with episodic memory scores in the participants with normal cognition | NO | Zeiss Stratus | 34F/41M Race: unknown | |
| Shi et al. 2014 [55] | Participants categorized as stable participants whose cognitive status remained unchanged (n = 60) and converted participants whose cognitive status deteriorated, which was diagnosed by DSM-VI (for AD) and Petersen’s definition (for MCI) (n = 18) | The participants were first screened for dementia by using the Chinese version of the MMSE, Chinese version of Activities of the Daily Living Scale, and the Chinese version of the RBANS | The reduction in the inferior quadrant of RNFL thickness might indicate a higher risk for the old adults to develop cognitive deterioration. | NO | Zeiss Cirrus | 34F/26M Stable group 10F/8M Converted group Race: unknown | |
| Kromer et al. 2014 [56] | (n = 22) AD, (n = 22) age-gender-matched healthy subjects | Patients with mild to moderate AD and a cognitively healthy age-matched control subjects were recruited from the Memory Clinic of the Department of Geriatric Psychiatry of the Central Institute of Mental Health, Mannheim, Germany MMSE scale | Patients with AD showed a significant decrease in RNFL thickness in the nasal superior sector compared to the control group (101.0 ± 18.18 μm versus 122.8 ± 28.08 μm; p < 0.0001). In all other sectors, independently of disease duration, no significant difference in RNFL thickness compared to controls was detected. | YES | Heidelberg Spectralis | 14F/8M AD 15F/7M Controls Race: unknown | |
| Larrosa et al. 2014 [57] | (n = 151) AD, (n = 61) age-matched healthy subjects | The AD diagnosis was determined by neurologists according to the NINCDS-ADRDS, DSM IV and MMSE criteria. | Reduced peripapillary RNFL thickness using linear discrimination function in AD | NO | Zeiss Cirrus and Heidelberg Spectralis | 95F/56M AD 38F/23M Controls Race: unknown | |
| Bambo et al. 2014 [58] | (n = 56) AD (n = 56) healthy controls | Confirmed AD diagnosis; and MMSE scale | Reduced peripapillary RNFL thickness in superior and inferior quadrants in AD. | NO | Zeiss Cirrus | Gender and Race unknown | |
| Moreno-Ramos et al 2013 [59] | AD (n = 10), Dementia with Lewy bodies (n = 10), Dementia associated with Parkinson’s disease (n = 10), Cognitively normal age-matched controls (n = 10) | Patients with AD met the NINDS-ADRDA criteria of probable AD, MMSE scale while patients with dementia with Lewy bodies fulfilled McKeith’s criteria. With respect to the diagnosis of the patients with dementia associated with Parkinson’s disease, followed the recommendations of the Movement Disorders Society Task Force. | All controls underwent a detailed neurologic and neuropsycholo-gical examination | The thickness of the RNLF correlated significantly (p < 0.001) with both the MMSE and the Mattis Dementia Rating Scale scores in all types of dementia; that is to say, the greater the cognitive deterioration, the greater the reduction of thickness of the RNLF. The findings from this study show that retinal involvement measured by optical coherence tomography may also be present in non-AD dementias. | NO | Topcon 3D OCT-1000 | 4F/6M Controls 4F/6M Alzheimer’s 5F/5M Dementia with Lewy bodies 4F/6M Dementia associated with Parkinson’s disease Race: unknown |
| Marziani et al. 2013 [60] | (n = 21) AD patients (n = 21) healthy subjects | Inclusion criteria were AD diagnosis according to the NINCDS-ADRDA | Each patient underwent full neurologic examination | RNFL and GCL in AD patients was reduction | NO | RTV-ue100 and Heidelberg Spectralis | 17F/4M AD group 16F/5M Controls Race: unknown |
| Kirbas et al. 2013 [61] | (n = 40) patients with early untreated AD (mean age, 69.3 ± 4.9 years) (n = 40) healthy controls (mean age, 68.9 ± 5.1 years) | Each patient underwent full neurologic examination, MMSE scale and brain MRI to exclude alternative diagnoses. | Thickness of RNFL in patient with AD was lower than that of controls. This suggests that SD- OCT has the potential to be used in the early diagnosis of AD as well as in the study of therapeutic agents. | YES | Heidelberg Spectralis | 18F/22M AD group 20F/20M Controls Race: unknown | |
| Moschos et al. 2012 [17] | (n = 30) patients with AD (n = 30) age and sex matched healthy controls | Diagnosis was based on NINCDS-ADRDA | Patients with AD, even without visual failure there was a decrease in macular and RNFL thickness, as well as a decrease of the electrical activity of the macula | NO ERG/VEP | Zeiss Stratus | 15F/15M AD 15F/15M Controls | |
| Kesler et al. 2011 [62] | AD diagnosis according to DSM-IV criteria-(n = 54) subjects. Cognitively healthy age-matched volunteers were also examined as control subjects (n = 24) | Ophthalmological evaluation included VA, IOP, slit lamp biomicroscopy and visual field examination. OCT measurements were performed by another ophthalmologist. All researchers were familiar with study protocol, but both ophthalmologists were blinded to cognitive status and diagnosis. | The total RNFL thicknesses were significantly different between the three groups: the RNFL was significantly thinner in the MCI Group compared to controls, as well as when the AD group was compared to MCI. | NO | Zeiss Stratus | Gender and Race unknown | |
| Lu et al. 2010 [63] | (n = 22) AD group, (n = 22) healthy controls | AD patients with clinical mild or moderate dementia were diagnosed by the AD group neurologists in the department of Neurology, Xuanwu Hospital, according to the NINCDS-ADRDA | The RNFL thickness of AD patients were much thinner especially in supra-retina and infra-retina, while no difference was found in the other retinal area. | NO | Zeiss Stratus | 12F/10M AD group 12F/10M Controls | |
| Paquet et al. 2007 [64] | (n = 15) healthy, aged-matched subjects, (n = 23) MCI patients, (n = 14) mild AD patients, (n = 12) moderate to severe AD patients enrolled. | AD patients fulfilled the NINCDS-ADRDA criteria, and MMSE scale. Control subjects and MCI patients had no neurological or ophthalmologic diseases. | The results show that RNFL thickness is statistically reduced in patients with MCI, mild AD or moderate to severe AD compared to controls. In addition, no statistical difference was found between the results in MCI patients and mild AD patients. The RNFL seems to be involved early during the course of amnestic MCI and OCT tests could be carried out in patients with cognitive troubles. | NO | Zeiss Stratus | 13F/2M Controls 15F/8M MCI 9F/5M Mild AD 6F/6M Severe AD Race: unknown | |
| Iseri et al 2006 [65] | 28 eyes of (n = 14) patients with AD, 30 eyes of (n = 15) age-matched control subjects. | AD set by the NINCDS-ADRDA, DSM-IV and MMSE scale. AD patients had mild and moderate cognitive impairment according to the CDR scale. | The peripapillary and macular RNFL thickness in all quadrants and positions of AD patients were thinner than in control subjects. The mean total macular volume of AD patients was significantly reduced as compared with control subjects. Total macular volume and MMSE scores were significantly correlated. No significant difference was found in the latency of the VEP P100 of AD patients and control subjects. | NO VEP | Zeiss Stratus | 8F/7M AD 8F/7M Controls Race: unknown | |
| Parisi et al. 2001 [66] | (n = 17) subjects with AD, (n = 14) age-matched controls subjects. | The AD patients met the criteria for AD according to the NINCDS-ADRDA, DSM-IV and MMSE, MRI was performed. | AD patients have reduction of NFL thickness. This morphological abnormality is related to a retinal dysfunction as revealed by abnormal PERG responses. | YES PERG | Zeiss Stratus | Gender and Race unknown | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera DeBuc, D.; Gaca-Wysocka, M.; Grzybowski, A.; Kanclerz, P. Identification of Retinal Biomarkers in Alzheimer’s Disease Using Optical Coherence Tomography: Recent Insights, Challenges, and Opportunities. J. Clin. Med. 2019, 8, 996. https://doi.org/10.3390/jcm8070996
Cabrera DeBuc D, Gaca-Wysocka M, Grzybowski A, Kanclerz P. Identification of Retinal Biomarkers in Alzheimer’s Disease Using Optical Coherence Tomography: Recent Insights, Challenges, and Opportunities. Journal of Clinical Medicine. 2019; 8(7):996. https://doi.org/10.3390/jcm8070996
Chicago/Turabian StyleCabrera DeBuc, Delia, Magdalena Gaca-Wysocka, Andrzej Grzybowski, and Piotr Kanclerz. 2019. "Identification of Retinal Biomarkers in Alzheimer’s Disease Using Optical Coherence Tomography: Recent Insights, Challenges, and Opportunities" Journal of Clinical Medicine 8, no. 7: 996. https://doi.org/10.3390/jcm8070996
APA StyleCabrera DeBuc, D., Gaca-Wysocka, M., Grzybowski, A., & Kanclerz, P. (2019). Identification of Retinal Biomarkers in Alzheimer’s Disease Using Optical Coherence Tomography: Recent Insights, Challenges, and Opportunities. Journal of Clinical Medicine, 8(7), 996. https://doi.org/10.3390/jcm8070996







