Markers of Cancer Cell Invasion: Are They Good Enough?
Abstract
:1. Introduction
2. Potential Markers of Cancer Cell Invasion
2.1. ECM Components
2.2. EMT Factors
2.3. Cell–Cell and Cell–ECM Interaction Molecules
2.4. Serine Proteases and Matrix Metalloproteinases
2.5. Actin Cytoskeleton Proteins
2.6. Other Proteins
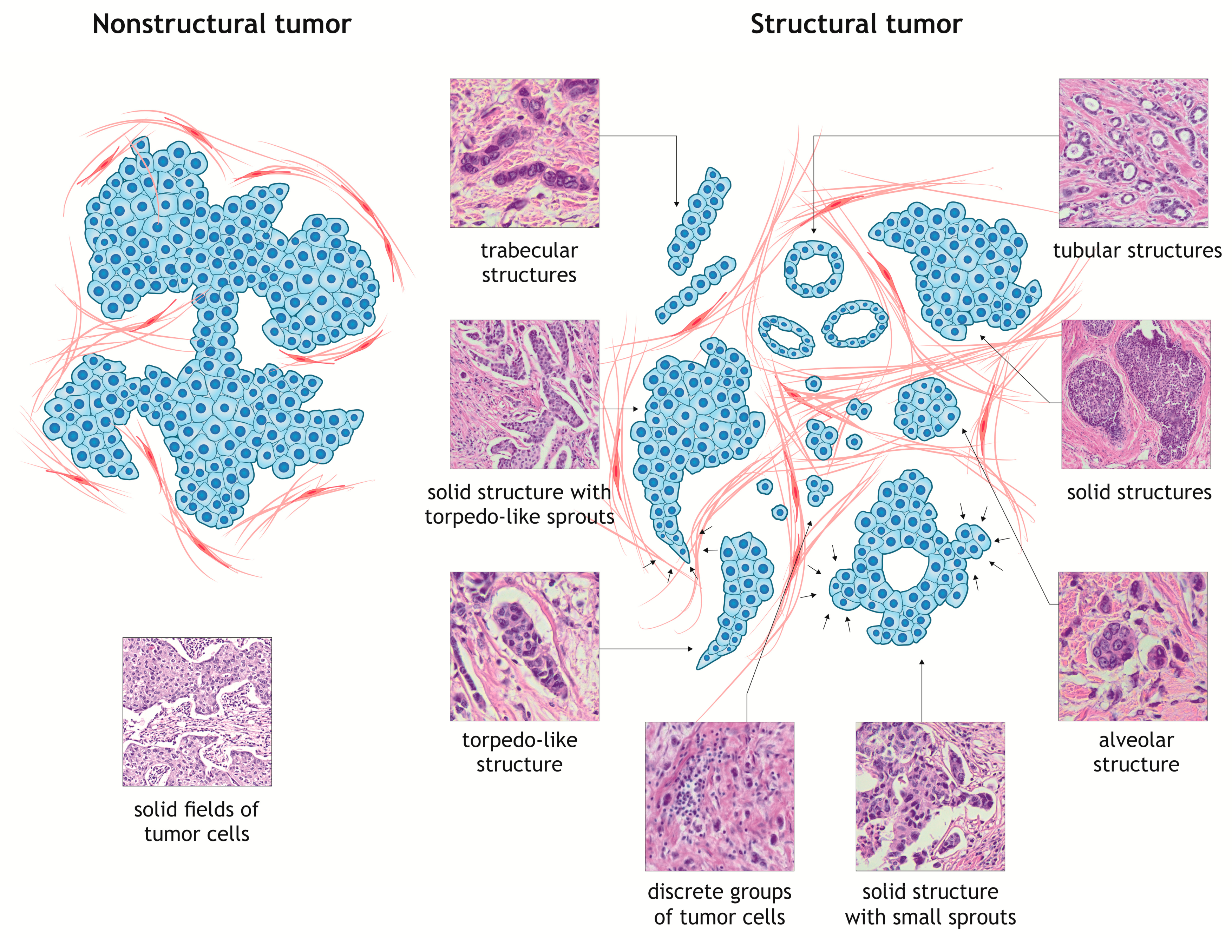
3. Intratumor Morphological Heterogeneity as a Model for Studying Cancer Cell Invasion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, 244–275. [Google Scholar]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [PubMed]
- Bànkfalvi, A.; Piffkò, J. Prognostic and predictive factors in oral cancer: The role of the invasive tumour front. J. Oral Pathol. Med. 2000, 29, 291–298. [Google Scholar] [PubMed]
- Zlobec, I.; Lugli, A. Invasive front of colorectal cancer: Dynamic interface of pro-/anti-tumor factors. World J. Gastroenterol. 2009, 15, 5898–5906. [Google Scholar] [PubMed]
- Rivera, C.; Venegas, B. Histological and molecular aspects of oral squamous cell carcinoma. Oncol. Lett. 2014, 8, 7–11. [Google Scholar] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar]
- Wolf, K.; Friedl, P. Molecular mechanisms of cancer cell invasion and plasticity. Br. J. Dermatol. 2006, 154, 11–15. [Google Scholar]
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer invasion: Patterns and mechanisms. Acta Naturae 2015, 7, 17–28. [Google Scholar]
- Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J. Biomech. Eng. 2017, 139, 021005. [Google Scholar]
- Wang, X.; Enomoto, A.; Asai, N.; Kato, T.; Takahashi, M. Collective invasion of cancer: Perspectives from pathology and development. Pathol. Int. 2016, 66, 183–192. [Google Scholar] [PubMed] [Green Version]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Modes of invasion during tumour dissemination. Mol. Oncol. 2017, 11, 5–27. [Google Scholar] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [PubMed]
- Nguyen-Ngoc, K.-V.; Cheung, K.J.; Brenot, A.; Shamir, E.R.; Gray, R.S.; Hines, W.C.; Yaswen, P.; Werb, Z.; Ewald, A.J. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. 2012, 109, E2595–E2604. [Google Scholar] [PubMed]
- Cheung, K.J.; Gabrielson, E.; Werb, Z.; Ewald, A.J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 2013, 155, 1639–1651. [Google Scholar] [PubMed]
- Cheung, K.J.; Ewald, A.J. Illuminating breast cancer invasion: Diverse roles for cell-cell interactions. Curr. Opin. Cell Biol. 2014, 30, 99–111. [Google Scholar] [PubMed]
- Paňková, K.; Rösel, D.; Novotný, M.; Brábek, J. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell. Mol. Life Sci. 2010, 67, 63–71. [Google Scholar] [PubMed]
- Chikina, A.S.; Alexandrova, A.Y. The cellular mechanisms and regulation of metastasis formation. Mol. Biol. 2014, 48, 165–180. [Google Scholar]
- Paluch, E.K.; Raz, E. The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 2013, 25, 582–590. [Google Scholar] [Green Version]
- Huang, B.; Lu, M.; Jolly, M.K.; Tsarfaty, I.; Onuchic, J.; Ben-Jacob, E. The three-way switch operation of Rac1/RhoA GTPase-based circuit controlling amoeboid-hybrid-mesenchymal transition. Sci. Rep. 2014, 4, 1–11. [Google Scholar]
- Kalluri, R.; Weinberg, R. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [PubMed] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 1–45. [Google Scholar]
- Bonde, A.K.; Tischler, V.; Kumar, S.; Soltermann, A.; Schwendener, R.A. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer 2012, 12, 35. [Google Scholar]
- Nieto, M.A.; Huang, R.Y.Y.J.; Jackson, R.A.A.; Thiery, J.P.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [PubMed] [Green Version]
- Gao, D.; Vahdat, L.T.; Wong, S.; Chang, J.C.; Mittal, V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012, 72, 4883–4889. [Google Scholar] [PubMed]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Onuchic, J.N.; Levine, H. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol. 2015, 5, 1–19. [Google Scholar]
- Grigore, A.D.; Jolly, M.K.; Jia, D. Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 2016, 5, 1–23. [Google Scholar]
- Zhang, S.; Wang, X.; Gupta, A.; Fang, X.; Wang, L.; Zhang, C. Expression of IL-17 with tumor budding as a prognostic marker in oral squamous cell carcinoma. Am. J. Transl. Res. 2019, 11, 1876–1883. [Google Scholar]
- Lorenzo Soriano, L.; Ordaz Jurado, G.; Pontones Moreno, J.L.; Villarroya Castillo, S.; Hernández Girón, S.; Sáez Moreno, I.; Ramos Soler, D. Tumor Budding: Prognostic Value in Muscle-Invasive Bladder Cancer. Urology 2019. [Google Scholar]
- Sirin, A.H.; Sokmen, S.; Unlu, S.M.; Ellidokuz, H.; Sarioglu, S. The prognostic value of tumor budding in patients who had surgery for rectal cancer with and without neoadjuvant therapy. Tech. Coloproctol. 2019, 23, 333–342. [Google Scholar] [PubMed]
- Ekmekci, S.; Kucuk, U.; Kokkoz, S.; Cakir, E.; Gumussoy, M. Tumor budding in laryngeal carcinoma. Indian J. Pathol. Microbiol. 2019, 62, 7–10. [Google Scholar] [PubMed]
- Agarwal, R.; Khurana, N.; Singh, T.; Agarwal, P.N. Tumor budding in infiltrating breast carcinoma: Correlation with known clinicopathological parameters and hormone receptor status. Indian J. Pathol. Microbiol. 2019, 62, 222–225. [Google Scholar] [PubMed]
- Ogino, M.; Nakanishi, Y.; Mitsuhashi, T.; Hatanaka, Y.; Amano, T.; Marukawa, K.; Nitta, T.; Ueno, T.; Ono, M.; Kuwabara, S.; et al. Impact of Tumour Budding Grade in 310 Patients Who Underwent Surgical Resection for Extrahepatic Cholangiocarcinoma. Histopathology 2019, 74, 861–872. [Google Scholar] [PubMed]
- Huang, B.; Jolly, M.K.; Lu, M.; Tsarfaty, I.; Ben-Jacob, E.; Onuchic, J.N. Modeling the Transitions between Collective and Solitary Migration Phenotypes in Cancer Metastasis. Sci. Rep. 2015, 5, 1–13. [Google Scholar]
- Lehmann, S.; Boekhorst, V.; Odenthal, J.; Bianchi, R.; van Helvert, S.; Ikenberg, K.; Ilina, O.; Stoma, S.; Xandry, J.; Jiang, L.; et al. Hypoxia Induces a HIF-1-Dependent Transition from Collective-to-Amoeboid Dissemination in Epithelial Cancer Cells. Curr. Biol. 2017, 27, 392–400. [Google Scholar] [PubMed] [Green Version]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448. [Google Scholar] [Green Version]
- Iguchi, T.; Aishima, S.; Taketomi, A.; Nishihara, Y.; Fujita, N.; Sanefuji, K.; Maehara, Y.; Tsuneyoshi, M. Extracapsular penetration is a new prognostic factor in human hepatocellular carcinoma. Am. J. Surg. Pathol. 2008, 32, 1675–1682. [Google Scholar] [PubMed]
- Mehes, G.; Witt, A.; Kubista, E.; Ambros, P.F. Circulating Breast Cancer Cells Are Frequently Apoptotic. Am. J. Pathol. 2001, 159, 17–20. [Google Scholar] [PubMed] [Green Version]
- Larson, C.J.; Moreno, J.G.; Pienta, K.J.; Gross, S.; Repollet, M.; O’Hara, S.M.; Russell, T.; Terstappen, L.W. Apoptosis of circulating tumor cells in prostate cancer patients. Cytom. Part A 2004, 62, 46–53. [Google Scholar]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. 2002, 2, 1–10. [Google Scholar]
- Rowe, R.G.; Weiss, S.J. Navigating ECM Barriers at the Invasive Front: The Cancer Cell—Stroma Interface. Annu. Rev. Cell Dev. Biol. 2009, 25, 567–595. [Google Scholar] [PubMed]
- Sentani, K.; Matsuda, M. Clinicopathological significance of MMP-7, laminin γ2 and EGFR expression at the invasive front of gastric carcinoma. Gastric Cancer 2014, 17, 412–422. [Google Scholar] [PubMed]
- Halfter, W.; Oertle, P.; Monnier, C.A.; Camenzind, L.; Reyes-Lua, M.; Hu, H.; Candiello, J.; Labilloy, A.; Balasubramani, M.; Henrich, P.B.; et al. New concepts in basement membrane biology. FEBS J. 2015, 282, 4466–4479. [Google Scholar] [PubMed]
- Hintermann, E.; Quaranta, V. Epithelial cell motility on laminin-5: Regulation by matrix assembly, proteolysis, integrins and erbB receptors. Matrix Biol. 2004, 23, 75–85. [Google Scholar] [PubMed]
- Masuda, R.; Kijima, H.; Imamura, N.; Aruga, N.; Nakazato, K.; Oiwa, K.; Nakano, T.; Watanabe, H.; Ikoma, Y.; Tanaka, M.; et al. Laminin-5 gamma 2 chain expression is associated with tumor cell invasiveness and prognosis of lung squamous cell carcinoma. Biomed. Res. 2012, 33, 309–317. [Google Scholar] [PubMed]
- Sharma, M.; Sah, P.; Sharma, S.S.; Radhakrishnan, R. Molecular changes in invasive front of oral cancer. J. Oral Maxillofac. Pathol. 2013, 17, 240–247. [Google Scholar] [PubMed]
- Ramovs, V.; te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57, 213–243. [Google Scholar]
- Ishikawa, T.; Wondimu, Z.; Oikawa, Y.; Gentilcore, G.; Kiessling, R.; Egyhazi Brage, S.; Hansson, J.; Patarroyo, M. Laminins 411 and 421 differentially promote tumor cell migration via α6β1 integrin and MCAM (CD146). Matrix Biol. 2014, 38, 69–83. [Google Scholar]
- Wang, H.; Fu, W.; Im, J.H.; Zhou, Z.; Santoro, S.A.; Iyer, V.; DiPersio, C.M.; Yu, Q.C.; Quaranta, V.; Al-Mehdi, A.; et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell Biol. 2004, 164, 935–941. [Google Scholar]
- Maltseva, D.V.; Rodin, S.A. Laminins in Metastatic Cancer. Mol. Biol. 2018, 52, 350–371. [Google Scholar]
- Miyazaki, K. Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006, 97, 91–98. [Google Scholar] [PubMed]
- Yamamoto, H.; Kitadai, Y.; Yamamoto, H.; Oue, N.; Ohdan, H.; Yasui, W.; Kikuchi, A. Laminin γ2 Mediates Wnt5a-Induced Invasion of Gastric Cancer Cells. Gastroenterology 2009, 137, 242–252. [Google Scholar] [PubMed]
- Okada, K.-I.; Kijima, H.; Imaizumi, T.; Hirabayashi, K.; Matsuyama, M.; Yazawa, N.; Oida, Y.; Tobita, K.; Tanaka, M.; Dowaki, S.; et al. Stromal laminin-5γ2 chain expression is associated with the wall-invasion pattern of gallbladder adenocarcinoma. Biomed. Res. 2009, 30, 53–62. [Google Scholar] [PubMed]
- Niki, T.; Kohno, T.; Iba, S.; Moriya, Y.; Takahashi, Y.; Saito, M.; Maeshima, A. Frequent Co-Localization of Cox-2 and Laminin-5 γ2 Chain at the Invasive Front of Early-Stage Lung Adenocarcinomas. Am. J. Pathol. 2002, 160, 1129–1141. [Google Scholar] [PubMed]
- Karamitopoulou, E.; Zlobec, I.; Panayiotides, I.; Patsouris, E.S.; Peros, G.; Rallis, G.; Lapas, C.; Karakitsos, P.; Terracciano, L.M.; Lugli, A. Systematic analysis of proteins from different signaling pathways in the tumor center and the invasive front of colorectal cancer. Hum. Pathol. 2011, 42, 1888–1896. [Google Scholar] [PubMed]
- Lowy, C.M.; Oskarsson, T.; Lowy, C.M.; Oskarsson, T. Tenascin C in metastasis: A view from the invasive front. Cell Adh. Migr. 2015, 9, 112–124. [Google Scholar] [Green Version]
- García-Solano, J.; Conesa-Zamora, P.; Trujillo-Santos, J.; Torres-Moreno, D.; Mäkinen, M.J.; Pérez-Guillermo, M. Immunohistochemical expression profile of β-catenin, E-cadherin, P-cadherin, laminin-5γ2 chain, and SMAD4 in colorectal serrated adenocarcinoma. Hum. Pathol. 2012, 43, 1094–1102. [Google Scholar]
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum. Pathol. 2013, 44, 2028–2037. [Google Scholar]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Adwanska, A.; Beghelli-De La Forest Divonne, S.; et al. Fibronectin-guided migration of carcinoma collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar]
- De Oliveira Ramos, G.; Bernardi, L.; Lauxen, I.; Filho, M.S.A.; Horwitz, A.R.; Lamers, M.L. Fibronectin modulates cell adhesion and signaling to promote single cell migration of highly invasive oral squamous cell carcinoma. PLoS ONE 2016, 11, 1–18. [Google Scholar]
- Aishima, S.; Taguchi, K.; Terashi, T.; Matsuura, S.; Shimada, M.; Tsuneyoshi, M. Tenascin Expression at the Invasive Front Is Associated with Poor Prognosis in Intrahepatic Cholangiocarcinoma. Mod. Pathol. 2003, 16, 1019–1027. [Google Scholar] [PubMed] [Green Version]
- Gurzu, S. Epithelial-mesenchymal, mesenchymal-epithelial, and endothelial-mesenchymal transitions in malignant tumors: An update. World J. Clin. Cases 2015, 3, 393–404. [Google Scholar] [PubMed]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [PubMed]
- Zhong, J.; Ogura, K.; Wang, Z.; Inuzuka, H. Degradation of the Transcription Factor Twist, an Oncoprotein that Promotes Cancer Metastasis. Discov Med. 2013, 15, 7–15. [Google Scholar] [PubMed]
- Krakhmal, N.V.; Zavyalova, M.V.; Perelmuter, V.M.; Vtorushin, S.V.; Slonimskaya, E.M.; Denisov, E.V. Heterogeneous expression of markers associated with invasive breast cancer (in Russ). Sib. J. Oncol. 2016, 15, 56–61. [Google Scholar]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar]
- Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L.A.; Knuechel, R.; Kirchner, T. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. 2002, 98, 10356–10361. [Google Scholar]
- Pereira, C.H.; Morais, M.O.; Martins, A.F.L.; Soares, M.Q.S.; de C.G. Alencar, R.; Batista, A.C.; Leles, C.R.; Mendonça, E.F. Expression of adhesion proteins (E-cadherin and β-catenin) and cell proliferation (Ki-67) at the invasive tumor front in conventional oral squamous cell and basaloid squamous cell carcinomas. Arch. Oral Biol. 2016, 61, 8–15. [Google Scholar]
- Suzuki, H.; Masuda, N.; Shimura, T.; Araki, K.; Kobayashi, T.; Tsutsumi, S.; Asao, T.; Kuwano, H. Nuclear β-Catenin Expression at the Invasive Front and in the Vessels Predicts Liver Metastasis in Colorectal Carcinoma. Anticancer Res. 2008, 28, 1821–1830. [Google Scholar]
- Wang, L.; Cheng, H.; Liu, Y.; Wang, L.; Yu, W.; Zhang, G.; Chen, B.; Yu, Z.; Hu, S. Prognostic value of nuclear β-catenin overexpression at invasive front in colorectal cancer for synchronous liver metastasis. Ann. Surg. Oncol. 2011, 18, 1553–1559. [Google Scholar] [PubMed]
- Shamir, E.R.; Ewald, A.J. Adhesion in mammary development: Novel roles for E-cadherin in individual and collective cell migration. Curr. Top. Dev. Biol. 2015, 112, 353–382. [Google Scholar] [PubMed]
- Yang, G.Y.; Guo, S.; Dong, C.Y.; Wang, X.Q.; Hu, B.Y.; Liu, Y.F.; Chen, Y.W.; Niu, J.; Dong, J.H. Integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression. World J. Gastroenterol. 2015, 21, 7457–7467. [Google Scholar] [PubMed]
- Stewart, R.L.; West, D.; Wang, C.; Weiss, H.L.; Gal, T.; Durbin, E.B.; O’Connor, W.; Chen, M.; O’Connor, K.L. Elevated integrin α6β4 expression is associated with venous invasion and decreased overall survival in non-small cell lung cancer. Hum. Pathol. 2016, 54, 174–183. [Google Scholar] [PubMed]
- Albelda, S.M.; Mette, S.A.; Elder, D.E.; Stewart, R.M.; Damjanovich, L.; Herlyn, M.; Buck, C.A. Integrin Distribution in Malignant Melanoma: Association of the β3 Subunit with Tumor Progression. Cancer Res. 1990, 50, 6757–6764. [Google Scholar] [PubMed]
- Koopman Van Aarsen, L.A.; Leone, D.R.; Ho, S.; Dolinski, B.M.; McCoon, P.E.; LePage, D.J.; Kelly, R.; Heaney, G.; Rayhorn, P.; Reid, C.; et al. Antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res. 2008, 68, 561–570. [Google Scholar]
- Chou, F.C.; Chen, H.Y.; Kuo, C.C.; Sytwu, H.K. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar]
- Richard, O. Hynes. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar]
- Wu, M.-H.; Hong, H.-C.; Cheng, H.-W.; Pan, S.-H.; Liang, Y.-R.; Hong, T.-M.; Chiang, W.-F.; Wong, T.-Y.; Shieh, D.-B.; Shiau, A.-L.; et al. Galectin-1-Mediated Tumor Invasion and Metastasis, Up-Regulated Matrix Metalloproteinase Expression, and Reorganized Actin Cytoskeletons. Mol. Cancer Res. 2009, 7, 311–318. [Google Scholar]
- Toussaint, L.G.; Nilson, A.E.; Goble, J.M.; Ballman, K.V.; James, C.D.; Lefranc, F.; Kiss, R.; Uhm, J.H. Galectin-1, a gene preferentially expressed at the tumor margin, promotes glioblastoma cell invasion. Mol. Cancer 2012, 11, 32. [Google Scholar] [PubMed]
- Gavert, N.; Conacci-sorrell, M.; Gast, D.; Schneider, A.; Altevogt, P.; Brabletz, T.; Ben-Ze’ev, A. L1, a novel target of β-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J. Cell Biol. 2005, 168, 633–642. [Google Scholar] [PubMed]
- Tsutsumi, S.; Morohashi, S.; Kudo, Y.; Akasaka, H.; Ogasawara, H.; Ono, M.; Takasugi, K.; Ishido, K.; Hakamada, K.; Kijima, H. L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2011, 103, 669–673. [Google Scholar] [PubMed]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in human cancer. Int. J. Cancer 2016, 138, 1565–1576. [Google Scholar]
- Shi, Z.; Stack, M.S. Urinary-type plasminogen activator (uPA) and its receptor (uPAR) in squamous cell carcinoma of the oral cavity. Biochem. J. 2007, 407, 153–159. [Google Scholar] [PubMed] [Green Version]
- Rømer, J.; Pyke, C.; Lund, L.R.; Ralfkiær, E.; Danø, K. Cancer cell expression of urokinase-type plasminogen activator receptor mRNA in squamous cell carcinomas of the skin. J. Invest. Dermatol. 2001, 116, 353–358. [Google Scholar] [PubMed]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [PubMed] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [PubMed] [Green Version]
- Ondruschka, C.; Buhtz, P.; Motsch, C.; Freigang, B.; Schneider-Stock, R.; Roessner, A.; Boltze, C. Prognostic value of MMP-2, -9 and TIMP-1,-2 immunoreactive protein at the invasive front in advanced head and neck squamous cell carcinomas. Pathol. Res. Pract. 2002, 198, 509–515. [Google Scholar] [PubMed]
- Ntayi, C.; Labrousse, A.L.; Debret, R.; Birembaut, P.; Bellon, G.; Antonicelli, F.; Hornebeck, W.; Bernard, P. Elastin-Derived Peptides Upregulate Matrix Metalloproteinase-2-ediated Melanoma Cell Invasion Through Elastin-Binding Protein. J. Invest. Dermatol. 2004, 122, 256–265. [Google Scholar]
- Liu, X.P.; Kawauchi, S.; Oga, A.; Tsushimi, K.; Tsushimi, M. Prognostic significance of matrix metalloproteinase-7 (MMP-7) expression at the invasive front in gastric carcinoma. Jpn. J. Cancer Res. 2002, 7, 291–295. [Google Scholar]
- Kitoh, T.; Yanai, H.; Saitoh, Y.; Nakamura, Y.; Matsubara, Y.; Kitoh, H.; Yoshida, T.; Okita, K. Increased expression of matrix metalloproteinase-7 in invasive early gastric cancer. J. Gastroenterol. 2004, 39, 434–440. [Google Scholar] [PubMed]
- Adachi, Y.; Yamamoto, H.; Itoh, F.; Hinoda, Y.; Okada, Y.; Imai, K. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut 1999, 45, 252–258. [Google Scholar] [PubMed]
- Planagumà, J.; Liljeström, M.; Alameda, F.; Bützow, R.; Virtanen, I.; Reventós, J.; Hukkanen, M. Matrix metalloproteinase-2 and matrix metalloproteinase-9 codistribute with transcription factors RUNX1 / AML1 and ETV5 / ERM at the invasive front of endometrial and ovarian carcinoma. Hum. Pathol. 2011, 42, 57–67. [Google Scholar] [PubMed]
- Sterz, C.M.; Kulle, C.; Dakic, B.; Makarova, G.; Böttcher, M.C.; Bette, M.; Werner, J.A.; Mandic, R. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the tumor and is expressing MMP-9. Oral Oncol. 2010, 46, 116–122. [Google Scholar] [PubMed]
- Li, Q.; Gao, H.; Xu, H.; Wang, X.; Pan, Y.; Hao, F.; Qiu, X.; Stoecker, M.; Wang, E.; Wang, E. Expression of ezrin correlates with malignant phenotype of lung cancer, and in vitro knockdown of ezrin reverses the aggressive biological behavior of lung cancer cells. Tumour Biol. 2012, 33, 1493–1504. [Google Scholar] [PubMed]
- Li, J.; Wei, K.; Yu, H.; Jin, D.; Wang, G.; Yu, B. Prognostic Value of Ezrin in Various Cancers: A Systematic Review and Updated Meta-analysis. Sci. Rep. 2015, 5, 1–13. [Google Scholar]
- Iwaya, K.; Norio, K.; Mukai, K. Coexpression of Arp2 and WAVE2 predicts poor outcome in invasive breast carcinoma. Mod. Pathol. 2007, 20, 339–343. [Google Scholar] [PubMed]
- Ambrosio, P.E.; Rosa, E.F.; Aparecida, M.; Domingues, C.; André, R.; Villacis, R.; Coudry, R.D.A.; Tagliarini, J.V.; Soares, F.A. Cortactin is associated with perineural invasion in the deep invasive front area of laryngeal carcinomas. Hum. Pathol. 2011, 42, 1221–1229. [Google Scholar] [PubMed]
- Yamada, S.I.; Yanamoto, S.; Kawasaki, G.; Mizuno, A.; Nemoto, T.K. Overexpression of cortactin increases invasion potential in oral squamous cell carcinoma. Pathol. Oncol. Res. 2010, 16, 523–531. [Google Scholar] [PubMed]
- Toyoda, A.; Yokota, A.Y.A.; Saito, T.; Kawana, H. Overexpression of human ortholog of mammalian enabled (hMena) is associated with the expression of mutant p53 protein in human breast cancers. Int. J. Oncol. 2011, 38, 89–96. [Google Scholar]
- Vignjevic, D.; Schoumacher, M.; Gavert, N.; Janssen, K.; Jih, G.; Lae, M.; Louvard, D.; Ben-ze, A.; Robine, S. Fascin, a Novel Target of β-Catenin-TCF Signaling, Is Expressed at the Invasive Front of Human Colon Cancer. Cancer Res. 2007, 67, 6844–6854. [Google Scholar] [PubMed]
- Won, K.Y.; Kim, G.Y.; Lim, S.J.; Park, Y.K.; Kim, Y.W. Prognostic significance of fascin expression in extrahepatic bile duct carcinomas. Pathol. Res. Pract. 2009, 205, 742–748. [Google Scholar] [PubMed]
- Stewart, C.J.R.; Crook, M.; Loi, S. Fascin expression in endocervical neoplasia: Correlation with tumour morphology and growth pattern. J. Clin. Pathol. 2012, 65, 213–217. [Google Scholar] [PubMed]
- Stewart, C.J.R.; Crook, M.L. Fascin expression in undifferentiated and dedifferentiated endometrial carcinoma. Hum. Pathol. 2015, 46, 1514–1520. [Google Scholar] [PubMed]
- Kurokawa, H.; Zhang, M.; Matsumoto, S.; Yamashita, Y.; Tanaka, T.; Tomoyose, T.; Takano, H.; Funaki, K.; Fukuyama, H.; Takahashi, T.; et al. The relationship of the histologic grade at the deep invasive front and the expression of Ki-67 antigen and p53 protein in oral squamous cell carcinoma. J. Oral Pathol. Med. 2005, 34, 602–607. [Google Scholar] [PubMed]
- Horre, N.; Van Diest, P.J.; Sie-Go, D.M.; Heintz, A.P. The invasive front in endometrial carcinoma: higher proliferation and associated derailment of cell cycle regulators. Hum. Pathol. 2007, 38, 1232–1238. [Google Scholar]
- Gong, P.; Wang, Y.; Liu, G.; Zhang, J.; Wang, Z. New Insight into Ki67 Expression at the Invasive Front in Breast Cancer. PLoS ONE 2013, 8, 1–5. [Google Scholar]
- Rubio, C.A. Further studies on the arrest of cell proliferation in tumor cells at the invading front of colonic adenocarcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1877–1881. [Google Scholar]
- Matsuda, Y.; Ishiwata, T.; Yamahatsu, K.; Kawahara, K. Overexpressed fibroblast growth factor receptor 2 in the invasive front of colorectal cancer: A potential therapeutic target in colorectal cancer. Cancer Lett. 2011, 309, 209–219. [Google Scholar]
- Kawase, R.; Ishiwata, T.; Matsuda, Y.; Onda, M.; Kudo, M.; Takeshita, T.; Naito, Z. Expression of fibroblast growth factor receptor 2 IIIc in human uterine cervical intraepithelial neoplasia and cervical cancer. Int. J. Oncol. 2010, 38, 257–266. [Google Scholar]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [PubMed]
- Pal, S.; Ganguly, K.K.; Chatterjee, A. Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3. Cell Commun. Adhes. 2013, 20, 105–114. [Google Scholar] [PubMed]
- Wang, J.P.; Hielscher, A. Fibronectin: How its aberrant expression in tumors may improve therapeutic targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.; Lebleu, V.S.; Kalluri, R.; Biology, C. EMT Program is Dispensable for Metastasis but Induces Chemoresistance in Pancreatic Cancer. Nature 2016, 527, 525–530. [Google Scholar]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [PubMed]
- Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top Dev. Biol. 2015, 112, 273–300. [Google Scholar] [PubMed]
- Shamir, E.R.; Pappalardo, E.; Jorgens, D.M.; Coutinho, K.; Tsai, W.T.; Aziz, K.; Auer, M.; Tran, P.T.; Bader, J.S.; Ewald, A.J. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol. 2014, 204, 839–856. [Google Scholar]
- Isaeva, A.V.; Zima, A.P.; Shabalova, I.P.; Ryazantseva, N.V.; Vasil’eva, O.A.; Kasoayn, K.T.; Saprina, T.V.; Latypova, V.N.; Berezkina, I.S.; Novitskii, V.V. β-catenin: Structure, function and role in malignant transformation of epithelial cells (in Russ). Vestn. Ross. Akad. Meditsinskikh Nauk 2015, 70, 475–483. [Google Scholar]
- Jolly, M.K.; Boareto, M.; Debeb, B.G.; Aceto, N.; Farach-Carson, M.C.; Woodward, W.A.; Levine, H. Inflammatory breast cancer: A model for investigating cluster-based dissemination. NPJ Breast Cancer 2017, 3, 1–7. [Google Scholar]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. 2009, 10, 455–456. [Google Scholar]
- Rathinam, R.; Alahari, S.K. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010, 29, 223–237. [Google Scholar] [PubMed]
- Huttenlocher, A.; Horwitz, A.R. Integrins in Cell Migration. Cold Spring Harb. Perspect. Biol. 2011, 1–17. [Google Scholar]
- Ganguly, K.K.; Pal, S.; Moulik, S.; Chatterjee, A. Integrins and metastasis. Cell Adhes. Migr. 2013, 7, 251–261. [Google Scholar] [Green Version]
- Morini, M.; Marcella, M.; Nicoletta, F.; Federica, G.; Simonetta, B.; Roberta, M.; Douglas, M.N.; Pier Giorgio, N.; Adriana, A. The α3β1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (mmp-9) activity. Int. J. Cancer 2000, 87, 336–342. [Google Scholar] [PubMed]
- He, Y.; Liu, X.D.; Chen, Z.Y.; Zhu, J.; Xiong, Y.; Li, K.; Dong, J.H.; Li, X. Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clin. Cancer Res. 2007, 13, 3115–3124. [Google Scholar] [PubMed]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [PubMed]
- Chan, B.M.; Matsuura, N.; Takada, Y.; Zetter, B.R.; Hemler, M.E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science 1991, 251, 1600–1602. [Google Scholar]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar]
- Wu, L.; Bernard-Trifilo, J.A.; Lim, Y.; Lim, S.T.; Mitra, S.K.; Uryu, S.; Chen, M.; Pallen, C.J.; Cheung, N.K.V.; Mikolon, D.; et al. Distinct FAK-Src activation events promote α5β1 and α4β1 integrin-stimulated neuroblastoma cell motility. Oncogene 2008, 27, 1439–1448. [Google Scholar]
- Schöttelndreier, H.; Potter, B.V.; Mayr, G.W.; Guse, A.H. Mechanisms involved in α6β1-integrin-mediated Ca(2+) signalling. Cell. Signal. 2001, 13, 895–899. [Google Scholar] [PubMed]
- Inoue, O.; Suzuki-Inoe, K.; McCarty, O.J.T.; Moroi, M.; Ruggeri, Z.M.; Kunicki, T.J. Laminin stimulates spreading of platelets through integrin α6β1–dependent activation of GPVI. Northwest Sci. 2006, 107, 1405–1411. [Google Scholar]
- Rapoport, E.M.; Kurmyshkina, O.V.; Bovin, N.V. Mammalian galectins: Structure, carbohydrate specificity, and functions. Biochem. 2008, 73, 393–405. [Google Scholar]
- Kowitz, A.; Kadmon, G.; Verschueren, H.; Remels, L.; De Baetselier, P.; Hubbe, M.; Schachner, M.; Schirrmacher, V.; Altevogt, P. Expression of L1 cell adhesion molecule is associated with lymphoma growth and metastasis. Clin. Exp. Metastasis 1993, 11, 419–429. [Google Scholar] [PubMed]
- Sidenius, N.; Blasi, F. The urokinase plasminogen activator system in cancer: Recent advances and implication for prognosis and therapy. Cancer Metastasis Rev. 2003, 22, 205–222. [Google Scholar] [PubMed]
- Banys-Paluchowski, M.; Witzel, I.; Aktas, B.; Fasching, P.A.; Hartkopf, A.; Janni, W.; Kasimir-Bauer, S.; Pantel, K.; Schön, G.; Rack, B.; et al. The prognostic relevance of urokinase-type plasminogen activator (uPA) in the blood of patients with metastatic breast cancer. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Pavón, M.A.; Arroyo-Solera, I.; Céspedes, M.V.; Casanova, I.; León, X.; Mangues, R. uPA/uPAR and SERPINE1 in head and neck cancer: Role in tumor resistance, metastasis, prognosis and therapy. Oncotarget 2016, 7, 57351–57366. [Google Scholar]
- Bozzuto, G.; Ruggieri, P.; Molinari, A. Molecular aspects of tumor cell migration and invasion. Ann Ist Super Sanita 2010, 46, 66–80. [Google Scholar]
- Mohtasham, N.; Babakoohi, S.; Shiva, A.; Shadman, A.; Kamyab-Hesari, K.; Shakeri, M.-T.; Sharifi-Sistan, N. Immunohistochemical study of p53, Ki-67, MMP-2 and MMP-9 expression at invasive front of squamous cell and verrucous carcinoma in oral cavity. Pathol. Res. Pract. 2013, 209, 110–114. [Google Scholar]
- Mu, D.; Cambier, S.; Fjellbirkeland, L.; Baron, J.L.; Munger, J.S.; Kawakatsu, H.; Sheppard, D.; Courtney Broaddus, V.; Nishimura, S.L. The integrin ανβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 2002, 157, 493–507. [Google Scholar]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [PubMed]
- Tatti, O.; Vehviläinen, P.; Lehti, K.; Keski-Oja, J. MT1-MMP releases latent TGF-β1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp. Cell Res. 2008, 314, 2501–2514. [Google Scholar] [PubMed]
- Mitsiades, N.; Yu, W.H.; Poulaki, V.; Tsokos, M.; Stamenkovic, I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001, 61, 577–581. [Google Scholar] [PubMed]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [PubMed]
- Ahn, G.O.; Brown, J.M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008, 13, 193–205. [Google Scholar] [PubMed]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. 2007, 104, 20262–20267. [Google Scholar] [PubMed] [Green Version]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 2007, 642–652. [Google Scholar]
- Clucas, J.; Valderrama, F. ERM proteins in cancer progression. J. Cell Sci. 2015, 128, 1253. [Google Scholar] [PubMed]
- Martín-Villar, E.; Scholl, F.G.; Gamallo, C.; Yurrita, M.M.; Muñoz-Guerra, M.; Cruces, J.; Quintanilla, M. Characterization of human PA2.26 antigen (T1α-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int. J. Cancer 2005, 113, 899–910. [Google Scholar] [PubMed]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [PubMed]
- Clark, E.S.; Brown, B.; Whigham, A.S.; Kochaishvili, A.; Yarbrough, W.G.; Weaver, A.M. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene 2009, 28, 431–444. [Google Scholar] [PubMed]
- Roussos, E.T.; Balsamo, M.; Alford, S.K.; Wyckoff, J.B.; Gligorijevic, B.; Wang, Y.; Pozzuto, M.; Stobezki, R.; Goswami, S.; Segall, J.E.; et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J. Cell Sci. 2011, 124, 2120–2131. [Google Scholar] [PubMed] [Green Version]
- Weidmann, M.D.; Surve, C.R.; Eddy, R.J.; Chen, X.; Gertler, F.B.; Sharma, V.P.; Condeelis, J.S. MenaINV dysregulates cortactin phosphorylation to promote invadopodium maturation. Sci. Rep. 2016, 6, 1–18. [Google Scholar]
- Machesky, L.M.; Li, A. Fascin. Commun. Integr. Biol. 2010, 3, 263–270. [Google Scholar] [PubMed]
- Papaspyrou, K.; Brochhausen, C.; Schmidtmann, I.; Fruth, K.; Gouveris, H.; Kirckpatrick, J.; Mann, W.; Brieger, J. Fascin upregulation in primary head and neck squamous cell carcinoma is associated with lymphatic metastasis. Oncol. Lett. 2014, 7, 2041–2046. [Google Scholar] [PubMed]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [PubMed]
- Bauer, A.L.; Jackson, T.L.; Jiang, Y.; Rohlf, T. Receptor cross-talk in angiogenesis: Mapping environmental cues to cell phenotype using a stochastic, Boolean signaling network model. J. Theor. Biol. 2010, 264, 838–846. [Google Scholar] [PubMed]
- Zavyalova, M.V.; Perelmuter, V.M.; Slonimskaya, E.M.; Vtorushin, S.V.; Garbukov, E.Y.; Gluschenko, S.A. Conjugation of lymphogenous metastatic spread and histologic pattern of infiltrative component of ductal breast cancer (in Russ). Sib. J. Oncol. 2006, 1, 32–35. [Google Scholar]
- Perelmuter, V.M.; Zavyalova, M.V.; Slonimskaya, E.M.; Vtorushin, S.V.; Garbukov, E.Y. Hematogenous metastasis depending on histologic tumor pattern in breast cancer (In Russ). Sib. J. Oncol. 2006, 3, 29–33. [Google Scholar]
- Denisov, E.V.; Skryabin, N.A.; Gerashchenko, T.S.; Tashireva, L.A.; Wilhelm, J.; Buldakov, M.A.; Sleptcov, A.A.; Lebedev, I.N.; Vtorushin, S.V.; Zavyalova, M.V.; et al. Clinically relevant morphological structures in breast cancer represent transcriptionally distinct tumor cell populations with varied degrees of epithelial-mesenchymal transition and CD44+CD24− stemness. Oncotarget 2017, 8, 61163–61180. [Google Scholar]
- Zavyalova, M.V.; Perelmuter, V.M.; Vtorushin, S.V.; Denisov, E.V.; Litvyakov, N.V.; Slonimskaya, E.M.; Cherdyntseva, N.V. The Presence of Alveolar Structures in Invasive Ductal NOS Breast Carcinoma is Associated with Lymph Node Metastasis. Diagn. Cytopathol. 2013, 41, 279–282. [Google Scholar] [PubMed]
- Gerashchenko, T.S.; Zavyalova, M.V.; Denisov, E.V.; Krakhmal, N.V.; Pautova, D.N.; Litviakov, N.V.; Vtorushin, S.V.; Cherdyntseva, N.V.; Perelmuter, V.M. Intratumoral morphological heterogeneity of breast cancer as an indicator of the metastatic potential and tumor chemosensitivity. Acta Naturae 2017, 9, 56–67. [Google Scholar] [PubMed]
- Denisov, E.V.; Litviakov, N.V.; Zavyalova, M.V.; Perelmuter, V.M.; Vtorushin, S.V.; Tsyganov, M.M.; Gerashchenko, T.S.; Garbukov, E.Y.; Slonimskaya, E.M.; Cherdyntseva, N.V. Intratumoral morphological heterogeneity of breast cancer: neoadjuvant chemotherapy efficiency and multidrug resistance gene expression. Sci. Rep. 2014, 4, 1–7. [Google Scholar]
| Markers | Functions | Expression at the Invasive Front | Limitations | |
|---|---|---|---|---|
| ECM components | Laminin-5, γ2 chain | ECM components, triggering MMP production through interaction with integrins | Breast, pancreatic, colon, lung, and other cancers [46,51,52,55,56] | Expression not only in the invasive front, but in other regions of the tumor [43,46,57,58,59] |
| Fibronectin | Oral and head and neck cancers [60,61] | |||
| Tenascin C | Modulation of cell adhesion | Melanoma, breast, lung, liver, and gallbladder cancers [57,62] | ||
| EMT molecules | Snail, Twist, vimentin | EMT induction and regulation | Various cancers [63] | Snail and Twist: Unstable molecules [64,65], total expression in breast tumors [66]. Vimentin may not be expressed in invasive carcinomas [67] |
| Cell–cell and cell–ECM interaction molecules | Cadherin-catenin complex | Adherens junctions | Colorectal, oral, and basaloid carcinomas (loss of E-cadherin and nuclear localization of β-catenin) [68,69,70,71] | In some tumors, loss of E-cadherin is not indispensable for invasive growth [72] |
| Integrins | Cell–ECM adhesion, involvement in MMP production | Melanoma (αvβ3), colon (αvβ6), head and neck (αvβ6), and lung (α6β4) cancers [73,74,75,76] | Involvement in other biological processes [77,78] | |
| Galectin 1 | Modulation of cell–cell and cell–ECM interactions | Oral and lung cancers, glioblastoma [47,79,80] | ||
| L1CAM | Cell adhesion | Colorectal and pancreatic cancers [81,82] | Dualistic role in cancer progression [83] | |
| Serine proteases and MMPs | uPA | Proteolysis of plasminogen to plasmin | Oral and skin carcinomas [84,85] | Involvement in other biological processes [86,87] |
| MMPs | ECM proteolysis | Melanoma (MMP-2), colorectal (MMP-7), gastric (MMP-7), endometrial (MMP-2, 9), ovarian (MMP-2, 9), and head and neck (MMP-2, 9) cancers [56,88,89,90,91,92,93,94] | ||
| Actin cytoskeleton proteins | Ezrin | Actin polymerization, cytoskeletal dynamics | Lung cancer [95,96] | Involvement in other biological processes. Contradictory data on the role in cancer progression [96] |
| WAVE2 | Breast cancer [97] | - | ||
| Cortactin | Oral and laryngeal cancers [98,99] | - | ||
| MENAinv | Breast cancer [100] | - | ||
| Fascin-1 | Liver, colon, cervical, and endometrial cancers [101,102,103,104] | - | ||
| Other proteins | Ki-67 | Cell proliferation | Breast, oral, and endometrial cancers [6,105,106,107] | Contradictory data on the level of Ki-67 expression at the invasive front [56,69,108] |
| FGFR2 | Cell division, growth and differentiation | Colorectal and cervical cancers [109,110] | Involvement in other biological processes [111] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerashchenko, T.S.; Novikov, N.M.; Krakhmal, N.V.; Zolotaryova, S.Y.; Zavyalova, M.V.; Cherdyntseva, N.V.; Denisov, E.V.; Perelmuter, V.M. Markers of Cancer Cell Invasion: Are They Good Enough? J. Clin. Med. 2019, 8, 1092. https://doi.org/10.3390/jcm8081092
Gerashchenko TS, Novikov NM, Krakhmal NV, Zolotaryova SY, Zavyalova MV, Cherdyntseva NV, Denisov EV, Perelmuter VM. Markers of Cancer Cell Invasion: Are They Good Enough? Journal of Clinical Medicine. 2019; 8(8):1092. https://doi.org/10.3390/jcm8081092
Chicago/Turabian StyleGerashchenko, Tatiana S., Nikita M. Novikov, Nadezhda V. Krakhmal, Sofia Y. Zolotaryova, Marina V. Zavyalova, Nadezhda V. Cherdyntseva, Evgeny V. Denisov, and Vladimir M. Perelmuter. 2019. "Markers of Cancer Cell Invasion: Are They Good Enough?" Journal of Clinical Medicine 8, no. 8: 1092. https://doi.org/10.3390/jcm8081092
APA StyleGerashchenko, T. S., Novikov, N. M., Krakhmal, N. V., Zolotaryova, S. Y., Zavyalova, M. V., Cherdyntseva, N. V., Denisov, E. V., & Perelmuter, V. M. (2019). Markers of Cancer Cell Invasion: Are They Good Enough? Journal of Clinical Medicine, 8(8), 1092. https://doi.org/10.3390/jcm8081092





