Twin-Twin Transfusion Syndrome with Anemia-Polycythemia: Prevalence, Characteristics, and Outcome
Abstract
:1. Introduction
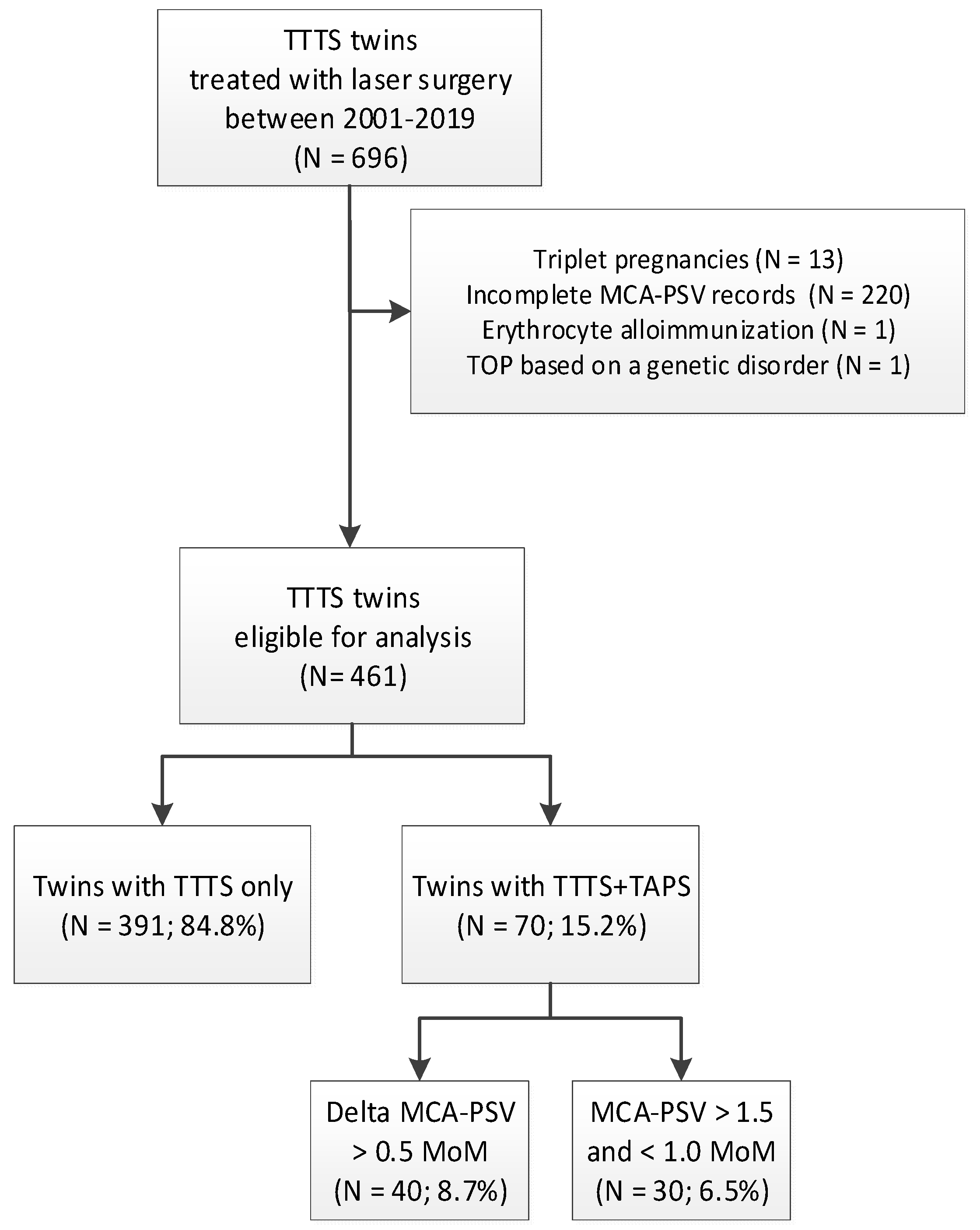
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lewi, L.; Van Schoubroeck, D.; Gratacós, E.; Witters, I.; Timmerman, D.; Deprest, J. Monochorionic diamniotic twins: Complications and management options. Curr. Opin. Obstet. Gynecol. 2003, 15, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Middeldorp, J.M.; Oepkes, D.; Kanhai, H.H.; Walther, F.J.; Vandenbussche, F.P. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta 2007, 28, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Jani, J.; Blickstein, I.; Huber, A.; Gucciardo, L.; Van Mieghem, T.; Doné, E.; Boes, A.S.; Hecher, K.; Gratacós, E.; et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: A prospective cohort study. Am. J. Obstet. Gynecol. 2008, 199, 514. [Google Scholar] [CrossRef] [PubMed]
- Habli, M.; Bombrys, A.; Lewis, D.; Lim, F.Y.; Polzin, W.; Maxwell, R.; Crombleholme, T. Incidence of complications in twin-twin transfusion syndrome after selective fetoscopic laser photocoagulation: A single-center experience. Am. J. Obstet. Gynecol. 2009, 201, 417. [Google Scholar] [CrossRef] [PubMed]
- Robyr, R.; Lewi, L.; Salomon, L.J.; Yamamoto, M.; Bernard, J.P.; Deprest, J.; Ville, Y. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2006, 194, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Gucciardo, L.; Lewi, L.; Vaast, P.; Debska, M.; De Catte, L.; Van Mieghem, T.; Done, E.; Devlieger, R.; Deprest, J. Twin anemia polycythemia sequence from a prenatal perspective. Prenat. Diagn. 2010, 30, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.; Lopriore, E.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.J.; Oepkes, D.; Slaghekke, F. Improved antenatal prediction of twin anemia polycythemia sequence by delta middle cerebral artery peak systolic velocity—a new antenatal classification system. Ultrasound Obstet. Gynecol. 2018, 53, 788–793. [Google Scholar]
- Donepudi, R.; Papanna, R.; Snowise, S.; Johnson, A.; Bebbington, M.; Moise, K.J., Jr. Does anemia-polycythemia complicating twin-twin transfusion syndrome affect outcome after fetoscopic laser surgery? Ultrasound Obstet. Gynecol. 2016, 47, 340–344. [Google Scholar] [CrossRef]
- Van Winden, K.R.; Quintero, R.A.; Kontopoulos, E.V.; Korst, L.M.; Llanes, A.; Chmait, R.H. Pre-Operative Twin Anemia/Polycythemia in the Setting of Twin-Twin Transfusion Syndrome (TTTS). Fetal Diagn. Ther. 2015, 37, 274–280. [Google Scholar] [CrossRef]
- Senat, M.V.; Deprest, J.; Boulvain, M.; Paupe, A.; Winer, N.; Ville, Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N. Engl. J. Med. 2004, 351, 136–144. [Google Scholar] [CrossRef]
- Mari, G.; Deter, R.L.; Carpenter, R.L.; Rahman, F.; Zimmerman, R.; Moise, K.J., Jr.; Dorman, K.F.; Ludomirsky, A.; Gonzalez, R.; Gomez, R.; et al. Noninvasive diagnosis by doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative group for doppler assessment of the blood velocity in anemic fetuses. N. Engl. J. Med. 2000, 342, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Klaritsch, P.; Deprest, J.; Van Mieghem, T.; Gucciardo, L.; Doné, E.; Jani, J.; Lewi, P.; Rasmussen, S.; Lewi, L. Reference ranges for middle cerebral artery peak systolic velocity in monochorionic diamniotic twins: A longitudinal study. Ultrasound. Obstet. Gynecol. 2009, 34, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Intraventricular hemorrhage and brain injury in the premature infant. Diagnosis, prognosis, and prevention. Clin. Perinatol. 1989, 16, 387–411. [Google Scholar] [CrossRef]
- De Vries, L.S.; Eken, P.; Dubowitz, L.M. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 1992, 49, 1–6. [Google Scholar] [CrossRef]
- Levene, M.I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child 1981, 56, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.P.; Cambiaso, O.; Otaño, L.; Lewi, L.; Deprest, J.; Sun, L.M.; Duan, T.; Oepkes, D.; Shapiro, S.D.; Paepe, M.E.; et al. Veno-venous anastomoses in twin-twin transfusion syndrome: A multicenter study. Placenta 2015, 36, 911–914. [Google Scholar] [CrossRef]
- De Villiers, S.F.; Slaghekke, F.; Middeldorp, J.M.; Walther, F.J.; Oepkes, D.; Lopriore, E. Arterio-arterial vascular anastomoses in monochorionic placentas with and without twin-twin transfusion syndrome. Placenta 2012, 33, 652–654. [Google Scholar] [CrossRef]
- De Villiers, S.; Slaghekke, F.; Middeldorp, J.M.; Klumper, F.J.; Walther, F.J.; Oepkes, D.; Lopriore, E. Arterio-arterial vascular anastomoses in monochorionic twin placentas with and without twin anemia-polycythemia sequence. Placenta 2012, 33, 227–229. [Google Scholar] [CrossRef]
- De Villiers, S.; Slaghekke, F.; Middeldorp, J.M.; Klumper, F.J.; Walther, F.J.; Oepkes, D.; Lopriore, E. Placental characteristics in monochorionic twins with spontaneous versus post-laser twin anemia-polycythemia sequence. Placenta 2013, 34, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Souka, A.; Skentou, H.; Geerts, L.; Nicolaides, K.H. Early prediction of severe twin-to-twin transfusion syndrome. Hum. Reprod. 2000, 15, 2008–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| TTTS-only (N = 391 Pregnancies, n = 782 Fetuses) | TTTS+AP (N = 70 Pregnancies n = 140 Fetuses) | p-Value | |
|---|---|---|---|
| Maternal age (years) | 32 (28–35) | 30 (27–34) | 0.438 |
| Gravidity | 2 (1–3) | 2 (1–3) | 0.380 |
| Parity | 1 (0–1) | 1 (0–1) | 0.778 |
| Male | 189/388 (49) | 37/69 (54) | 0.552 |
| Cesarean | 286/776 (37) | 58/140 (41) | 0.423 |
| Delta MCA-PSV (MoM) | 0.2 (0.1–0.3) | 0.7 (0.6–0.9) | <0.0001 |
| Quintero stage | 0.198 | ||
| I | 58/391 (15) | 11/70 (16) | |
| II | 140/391 (36) | 16/70 (23) | |
| III | 180/391 (46) | 40/70 (57) | |
| IV | 13/391 (3) | 3/70 (4) |
| TTTS-only (N = 391 Pregnancies, n = 782 Fetuses) | TTTS+AP (N = 70 Pregnancies n = 140 Fetuses) | p-Value | |
|---|---|---|---|
| Gestational age at laser | 19.3 (17.3–21.9) | 21.0 (18.8–24.0) | <0.0001 |
| Total number of anastomoses on fetoscopy | 6 (5–8) | 5 (4–6) | <0.0001 |
| Number of AV-anastomoses | 3 (3–5) | 3 (2–4) | 0.018 |
| Number of VA-anastomoses | 2 (1–3) | 2 (1–3) | 0.012 |
| Presence of AA-anastomoses | 54/371 (15) | 5/67 (7) | 0.118 |
| Presence of VV-anastomoses | 35/371 (9) | 0/67 (0) | 0.009 |
| Presence of residual anastomoses | 53/277 (19) | 9/50 (18) | 0.742 |
| Recurrent TTTS | 3/291 (1) | 1/70 (1) | 0.458 |
| Recurrent TTTS with AP | 2/391 (1) | 0/70 (0) | 0.549 |
| Post-laser TAPS | 37/387 (10) | 6/70 (9) | 0.855 |
| TTTS-only (N = 391 Pregnancies, n = 782 Fetuses) | TTTS+AP (N = 70 Pregnancies n = 140 Fetuses) | p-Value | |
|---|---|---|---|
| Gestational age at birth (weeks) | 33.0 (29.2–35.6) | 33.1 (29.9–35.6) | 0.556 |
| Birth-weight discordance (%) | 11.5 (4.9–21.3) | 10.8 (4.6–20.8) | 0.953 |
| Perinatal survival | 599/782 (77) | 118/142 (83) | 0.130 |
| Fetal demise | 157/782 (20) | 22/140 (16) | 0.283 |
| Neonatal mortality | 26/625 (4) | 2/118 (2) | 0.090 |
| Severe neonatal morbidity | 132/575 (23) | 13/115 (11) | 0.005 |
| Respiratory distress syndrome | 114/575 (20) | 8/115 (7) | <0.0001 |
| Patent ductus arteriosus | 19/575 (3) | 1/115 (1) | 0.054 |
| Necrotizing enterocolitis | 17/575 (3) | 3/115 (3) | 0.873 |
| Severe cerebral injury | 33/575 (6) | 3/115 (3) | 0.082 |
| Severe NDI | 28/370 (8) | 2/74 (3) | 0.053 |
| Severe cognitive delay | 11/370 (3) | 0/74 (0) | 0.007 |
| Severe motor delay | 14/370 (4) | 2/74 (3) | 0.605 |
| Bilateral blindness | 0/370 (0) | 0/74 (0) | 1.000 |
| Bilateral deafness | 4/370 (1) | 0/74 (0) | 0.177 |
| Cerebral palsy | 12/370 (3) | 2/74 (3) | 0.682 |
| Disease-free survival | 341/548 (62) | 72/98 (73) | 0.046 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tollenaar, L.S.A.; Slaghekke, F.; van Klink, J.M.M.; Groene, S.G.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.J.C.M.; Oepkes, D.; Lopriore, E. Twin-Twin Transfusion Syndrome with Anemia-Polycythemia: Prevalence, Characteristics, and Outcome. J. Clin. Med. 2019, 8, 1129. https://doi.org/10.3390/jcm8081129
Tollenaar LSA, Slaghekke F, van Klink JMM, Groene SG, Middeldorp JM, Haak MC, Klumper FJCM, Oepkes D, Lopriore E. Twin-Twin Transfusion Syndrome with Anemia-Polycythemia: Prevalence, Characteristics, and Outcome. Journal of Clinical Medicine. 2019; 8(8):1129. https://doi.org/10.3390/jcm8081129
Chicago/Turabian StyleTollenaar, Lisanne S. A., Femke Slaghekke, Jeanine M. M. van Klink, Sophie G. Groene, Johanna M. Middeldorp, Monique C. Haak, Frans J. C. M. Klumper, Dick Oepkes, and Enrico Lopriore. 2019. "Twin-Twin Transfusion Syndrome with Anemia-Polycythemia: Prevalence, Characteristics, and Outcome" Journal of Clinical Medicine 8, no. 8: 1129. https://doi.org/10.3390/jcm8081129
APA StyleTollenaar, L. S. A., Slaghekke, F., van Klink, J. M. M., Groene, S. G., Middeldorp, J. M., Haak, M. C., Klumper, F. J. C. M., Oepkes, D., & Lopriore, E. (2019). Twin-Twin Transfusion Syndrome with Anemia-Polycythemia: Prevalence, Characteristics, and Outcome. Journal of Clinical Medicine, 8(8), 1129. https://doi.org/10.3390/jcm8081129






