Regulated hAAT Expression from a Novel rAAV Vector and Its Application in the Prevention of Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vector Construction, Production, and Administration
2.3. Detection of Transgene Expression
3. Results
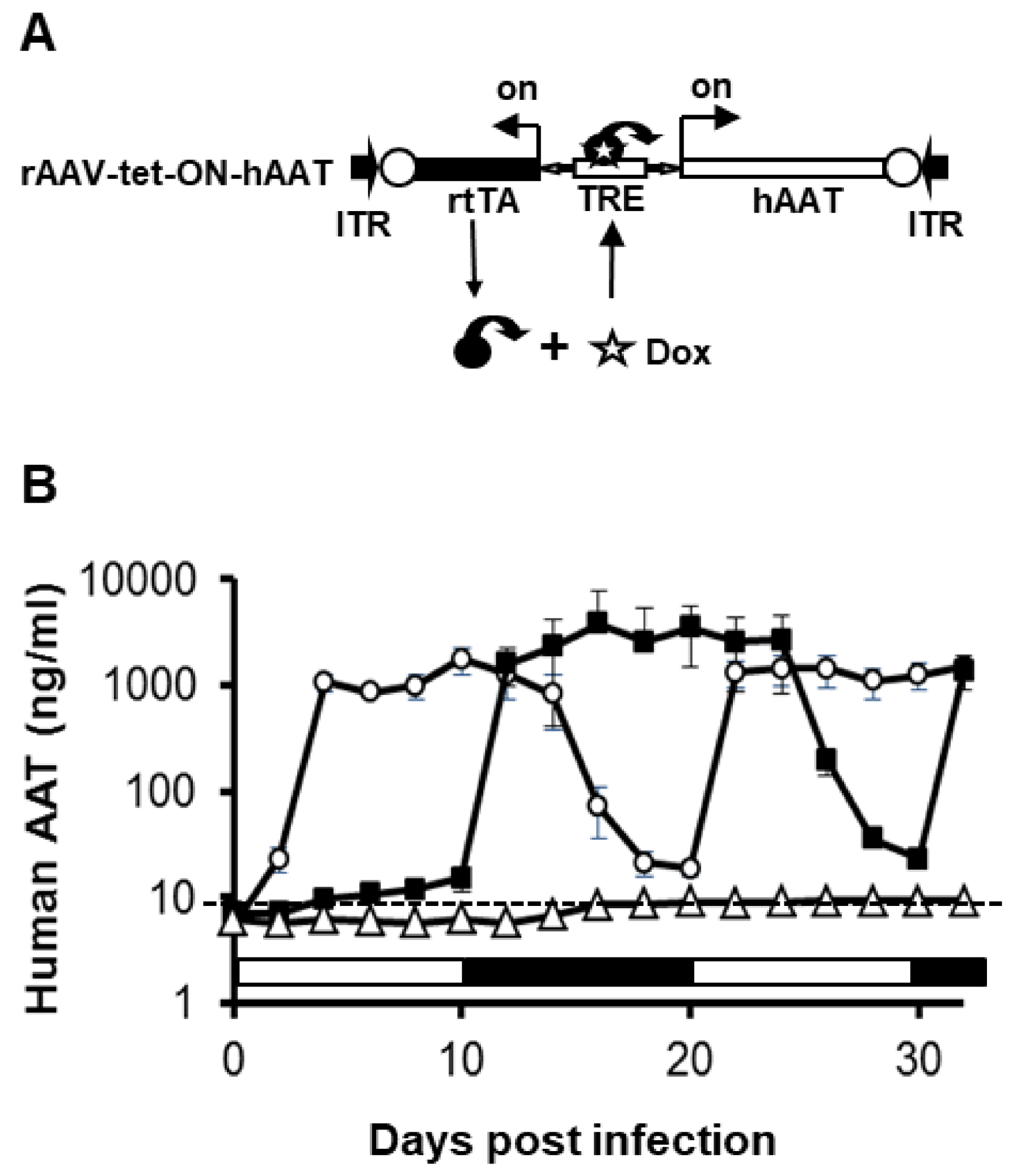
3.1. Construction and In Vitro Evaluation of rAAV1-tet-on-hAAT Vector
3.2. Regulated hAAT Expression from rAAV1-tet-on-hAAT in Muscle
3.3. Prevention of Type 1 Diabetes in NOD Mice by rAAV1-tet-on-hAAT and Dox
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rossini, A.A. Autoimmune diabetes and the circle of tolerance. Diabetes 2004, 53, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Lemarchand, P.; Trucco, M.; Giannoukakis, N. Gene and cell-based therapeutics for type I diabetes mellitus. Gene Ther. 2003, 10, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Carnaud, C.; Boitard, C.; Bach, J.F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J. Exp. Med. 1987, 166, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Appel, M.C.; O’Neil, J.J.; Wicker, L.S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J. Immunol. 1988, 140, 52–58. [Google Scholar] [PubMed]
- Wang, Y.; Hao, L.; Gill, R.G.; Lafferty, K.J. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes 1987, 36, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Like, A.A.; Biron, C.A.; Weringer, E.J.; Byman, K.; Sroczynski, E.; Guberski, D.L. Prevention of diabetes in BioBreeding/Worcester rats with monoclonal antibodies that recognize T lymphocytes or natural killer cells. J. Exp. Med. 1986, 164, 1145–1159. [Google Scholar] [CrossRef]
- Sibley, R.K.; Sutherland, D.E. Pancreas transplantation. An immunohistologic and histopathologic examination of 100 grafts. Am. J. Pathol. 1987, 128, 151–170. [Google Scholar]
- Haskins, K.; Portas, M.; Bradley, B.; Wegmann, D.; Lafferty, K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes 1988, 37, 1444–1448. [Google Scholar] [CrossRef]
- von Boehmer, H. Type 1 diabetes: Focus on prevention. Nat. Med. 2004, 10, 783–784. [Google Scholar] [CrossRef]
- Tarbell, K.V.; Yamazaki, S.; Olson, K.; Toy, P.; Steinman, R.M. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1467–1477. [Google Scholar] [CrossRef]
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, K.; Hamerman, J.A.; Ehrlich, L.R.; Bour-Jordan, H.; Santamaria, P.; Bluestone, J.A.; Lanier, L.L. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 2004, 20, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Dotta, F.; Di Mario, U.; Perfetti, R. Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J. Cell. Physiol. 2004, 200, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lu, Y.; Campbell-Thompson, M.; Spencer, T.; Wasserfall, C.; Atkinson, M.; Song, S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes 2007, 56, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Goudy, K.; Song, S.; Wasserfall, C.; Zhang, Y.C.; Kapturczak, M.; Muir, A.; Powers, M.; Scott-Jorgensen, M.; Campbell-Thompson, M.; Crawford, J.M.; et al. Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice. Proc. Natl. Acad. Sci. USA 2001, 98, 13913–13918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudy, K.S.; Wasserfall, C.; Burknhardt, B.; Brusko, T.; Song, S.; Ellis, T.; Flotte, T.; Atkinson, M. Elucidation of time and dose dependencies using AAV-IL-10 gene therapy for prevention of type 1 diabetes in the NOD mouse. Mol. Ther. 2002, 5, S17. [Google Scholar]
- Song, S. Alpha-1 Antitrypsin Therapy for Autoimmune Disorders. Chronic Obstr. Pulm. Dis. 2018, 5, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Kohnlein, T.; Welte, T. The discovery of alpha1-antitrypsin and its role in health and disease. Respir. Med. 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Fijalkowska, I.; Medler, T.R.; Skirball, J.; Cruz, P.; Zhen, L.; Petrache, H.I.; Flotte, T.R.; Tuder, R.M. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am. J. Pathol. 2006, 169, 1155–1166. [Google Scholar] [CrossRef]
- Toldo, S.; Seropian, I.M.; Mezzaroma, E.; Van Tassell, B.W.; Salloum, F.N.; Lewis, E.C.; Voelkel, N.; Dinarello, C.A.; Abbate, A. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2011, 51, 244–251. [Google Scholar] [CrossRef]
- Bergin, D.A.; Reeves, E.P.; Hurley, K.; Wolfe, R.; Jameel, R.; Fitzgerald, S.; McElvaney, N.G. The circulating proteinase inhibitor alpha-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci. Transl. Med. 2014, 6, 217ra211. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Goudy, K.; Campbell-Thompson, M.; Wasserfall, C.; Scott-Jorgensen, M.; Wang, J.; Tang, Q.; Crawford, J.M.; Ellis, T.M.; Atkinson, M.A.; et al. Recombinant adeno-associated virus-mediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004, 11, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Tang, M.; Wasserfall, C.; Kou, Z.; Campbell-Thompson, M.; Gardemann, T.; Crawford, J.; Atkinson, M.; Song, S. Alpha1-antitrypsin gene therapy modulates cellular immunity and efficiently prevents type 1 diabetes in nonobese diabetic mice. Hum. Gene Ther. 2006, 17, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lu, Y.; Li, H.; Campbell-Thompson, M.; Parker, M.; Wasserfall, C.; Haller, M.; Brantly, M.; Schatz, D.; Atkinson, M.; et al. Intradermal alpha1-antitrypsin therapy avoids fatal anaphylaxis, prevents type 1 diabetes and reverses hyperglycaemia in the NOD mouse model of the disease. Diabetologia 2010, 53, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C.; Mizrahi, M.; Toledano, M.; Defelice, N.; Wright, J.L.; Churg, A.; Shapiro, L.; Dinarello, C.A. Alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 16236–16241. [Google Scholar] [CrossRef]
- Lewis, E.C.; Shapiro, L.; Bowers, O.J.; Dinarello, C.A. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 12153–12158. [Google Scholar] [CrossRef]
- Grimstein, C.; Choi, Y.K.; Satoh, M.; Lu, Y.; Wang, X.; Campbell-Thompson, M.; Song, S. Combination of alpha-1 antitrypsin and doxycycline suppresses collagen-induced arthritis. J. Gene Med. 2010, 12, 35–44. [Google Scholar] [CrossRef]
- Grimstein, C.; Choi, Y.K.; Wasserfall, C.H.; Satoh, M.; Atkinson, M.A.; Brantly, M.L.; Campbell-Thompson, M.; Song, S. Alpha-1 Antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model. J. Transl. Med. 2011, 9, 21. [Google Scholar] [CrossRef]
- Tawara, I.; Sun, Y.; Lewis, E.C.; Toubai, T.; Evers, R.; Nieves, E.; Azam, T.; Dinarello, C.A.; Reddy, P. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc. Natl. Acad. Sci. USA 2012, 109, 564–569. [Google Scholar] [CrossRef]
- Moldthan, H.L.; Hirko, A.C.; Thinschmidt, J.S.; Grant, M.B.; Li, Z.; Peris, J.; Lu, Y.; Elshikha, A.S.; King, M.A.; Hughes, J.A.; et al. Alpha 1-Antitrypsin Therapy Mitigated Ischemic Stroke Damage in Rats. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, e355–e363. [Google Scholar] [CrossRef] [Green Version]
- Akbar, M.A.; Cao, J.J.; Lu, Y.; Nardo, D.; Chen, M.J.; Elshikha, A.S.; Ahamed, R.; Brantly, M.; Holliday, L.S.; Song, S. Alpha-1 Antitrypsin Gene Therapy Ameliorates Bone Loss in Ovariectomy-Induced Osteoporosis Mouse Model. Hum. Gene Ther. 2016, 27, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Elshikha, A.S.; Lu, Y.; Chen, M.J.; Akbar, M.; Zeumer, L.; Ritter, A.; Elghamry, H.; Mahdi, M.A.; Morel, L.; Song, S. Alpha-1 Antitrypsin Inhibits Dendritic Cell Activation and Attenuates Nephritis in a Mouse Model of Lupus. PLoS ONE 2016, 11, e0156583. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; DiCiaccio, B.; Li, Y.; Elshikha, A.S.; Titov, D.; Brenner, B.; Seifer, L.; Pan, H.; Karic, N.; Akbar, M.A.; et al. Anti-inflammaging effects of human alpha-1 antitrypsin. Aging Cell 2018, 17, e12694. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Morgan, M.; Ellis, T.; Poirier, A.; Chesnut, K.; Wang, J.; Brantly, M.; Muzyczka, N.; Byrne, B.J.; Atkinson, M.; et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA 1998, 95, 14384–14388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, P.D.; Podsakoff, G.M.; Chen, X.; McQuiston, S.A.; Colosi, P.C.; Matelis, L.A.; Kurtzman, G.J.; Byrne, B.J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 1996, 93, 14082–14087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Li, J.; Samulski, R.J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 1996, 70, 8098–8108. [Google Scholar] [PubMed]
- Clark, K.R.; Sferra, T.J.; Johnson, P.R. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum. Gene Ther. 1997, 8, 659–669. [Google Scholar] [CrossRef]
- Snyder, R.O.; Spratt, S.K.; Lagarde, C.; Bohl, D.; Kaspar, B.; Sloan, B.; Cohen, L.K.; Danos, O. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum. Gene Ther. 1997, 8, 1891–1900. [Google Scholar] [CrossRef]
- Flotte, T.R.; Afione, S.A.; Conrad, C.; McGrath, S.A.; Solow, R.; Oka, H.; Zeitlin, P.L.; Guggino, W.B.; Carter, B.J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 1993, 90, 10613–10617. [Google Scholar] [CrossRef]
- Snyder, R.O.; Miao, C.H.; Patijn, G.A.; Spratt, S.K.; Danos, O.; Nagy, D.; Gown, A.M.; Winther, B.; Meuse, L.; Cohen, L.K.; et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 1997, 16, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Berta, S.C.; Lu, M.M.; Moscioni, A.D.; Tazelaar, J.; Wilson, J.M. Adeno-associated virus as a vector for liver-directed gene therapy. J. Virol. 1998, 72, 10222–10226. [Google Scholar] [PubMed]
- Song, S.; Embury, J.; Laipis, P.J.; Berns, K.I.; Crawford, J.M.; Flotte, T.R. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001, 8, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Daly, T.; Gao, C.; Flotte, T.R.; Song, S.; Byrne, B.J.; Sands, M.S.; Parker Ponder, K. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum. Gene Ther. 2001, 12, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kaplitt, M.G.; Leone, P.; Samulski, R.J.; Xiao, X.; Pfaff, D.W.; O’Malley, K.L.; During, M.J. Long-term gene expression and phenotypic correction using adeno- associated virus vectors in the mammalian brain. Nat. Genet. 1994, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Flannery, J.G.; Zolotukhin, S.; Vaquero, M.I.; LaVail, M.M.; Muzyczka, N.; Hauswirth, W.W. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc. Natl. Acad. Sci. USA 1997, 94, 6916–6921. [Google Scholar] [CrossRef] [PubMed]
- Elshikha, A.S.; Yuan, Y.; Lu, Y.; Chen, M.J.; Abboud, G.; Akbar, M.A.; Plate, H.; Wolney, H.; Hoffmann, T.; Tagari, E.; et al. Alpha 1 Antitrypsin Gene Therapy Extends the Lifespan of Lupus-Prone Mice. Mol. Ther. Methods Clin. Dev. 2018, 11, 131–142. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S. Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc. Natl. Acad. Sci. USA 2009, 106, 17158–17162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Choi, Y.K.; Campbell-Thompson, M.; Li, C.; Tang, Q.; Crawford, J.M.; Flotte, T.R.; Song, S. Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J. Gene Med. 2006, 8, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Manfredsson, F.P.; Burger, C.; Rising, A.C.; Zuobi-Hasona, K.; Sullivan, L.F.; Lewin, A.S.; Huang, J.; Piercefield, E.; Muzyczka, N.; Mandel, R.J. Tight Long-term dynamic doxycycline responsive nigrostriatal GDNF using a single rAAV vector. Mol. Ther. J. Am. Soc. Gene Ther. 2009, 17, 1857–1867. [Google Scholar] [CrossRef]
- Kalsheker, N.; Morley, S.; Morgan, K. Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin. Biochem. Soc. Trans. 2002, 30, 93–98. [Google Scholar] [CrossRef]
- Lu, Y.; Parker, M.; Pileggi, A.; Zhang, B.; Choi, Y.K.; Molano, R.D.; Wasserfall, C.; Ricordi, C.; Inverardi, L.; Brantly, M.; et al. Human alpha 1-antitrypsin therapy induces fatal anaphylaxis in non-obese diabetic mice. Clin. Exp. Immunol. 2008, 154, 15–21. [Google Scholar] [CrossRef]
- Petrache, I.; Hajjar, J.; Campos, M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biol. Targets Ther. 2009, 3, 193–204. [Google Scholar] [CrossRef]
- Schwartz, R.F.; Neu, J.; Schatz, D.; Atkinson, M.A.; Wasserfall, C. Comment on: Brugman S et al. (2006) Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49:2105–2108. Diabetologia 2007, 50, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Klatter, F.A.; Visser, J.T.; Wildeboer-Veloo, A.C.; Harmsen, H.J.; Rozing, J.; Bos, N.A. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 2006, 49, 2105–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Lu, Y.; Lowe, K.; van der Meijden-Erkelens, L.; Wasserfall, C.; Atkinson, M.A.; Song, S. Regulated hAAT Expression from a Novel rAAV Vector and Its Application in the Prevention of Type 1 Diabetes. J. Clin. Med. 2019, 8, 1321. https://doi.org/10.3390/jcm8091321
Ma H, Lu Y, Lowe K, van der Meijden-Erkelens L, Wasserfall C, Atkinson MA, Song S. Regulated hAAT Expression from a Novel rAAV Vector and Its Application in the Prevention of Type 1 Diabetes. Journal of Clinical Medicine. 2019; 8(9):1321. https://doi.org/10.3390/jcm8091321
Chicago/Turabian StyleMa, Hongxia, Yuanqing Lu, Keith Lowe, Lonneke van der Meijden-Erkelens, Clive Wasserfall, Mark A. Atkinson, and Sihong Song. 2019. "Regulated hAAT Expression from a Novel rAAV Vector and Its Application in the Prevention of Type 1 Diabetes" Journal of Clinical Medicine 8, no. 9: 1321. https://doi.org/10.3390/jcm8091321
APA StyleMa, H., Lu, Y., Lowe, K., van der Meijden-Erkelens, L., Wasserfall, C., Atkinson, M. A., & Song, S. (2019). Regulated hAAT Expression from a Novel rAAV Vector and Its Application in the Prevention of Type 1 Diabetes. Journal of Clinical Medicine, 8(9), 1321. https://doi.org/10.3390/jcm8091321




