The Evolution of the Role of External Ventricular Drainage in Traumatic Brain Injury
Abstract
:1. Introduction
2. Methods
3. Literature Review
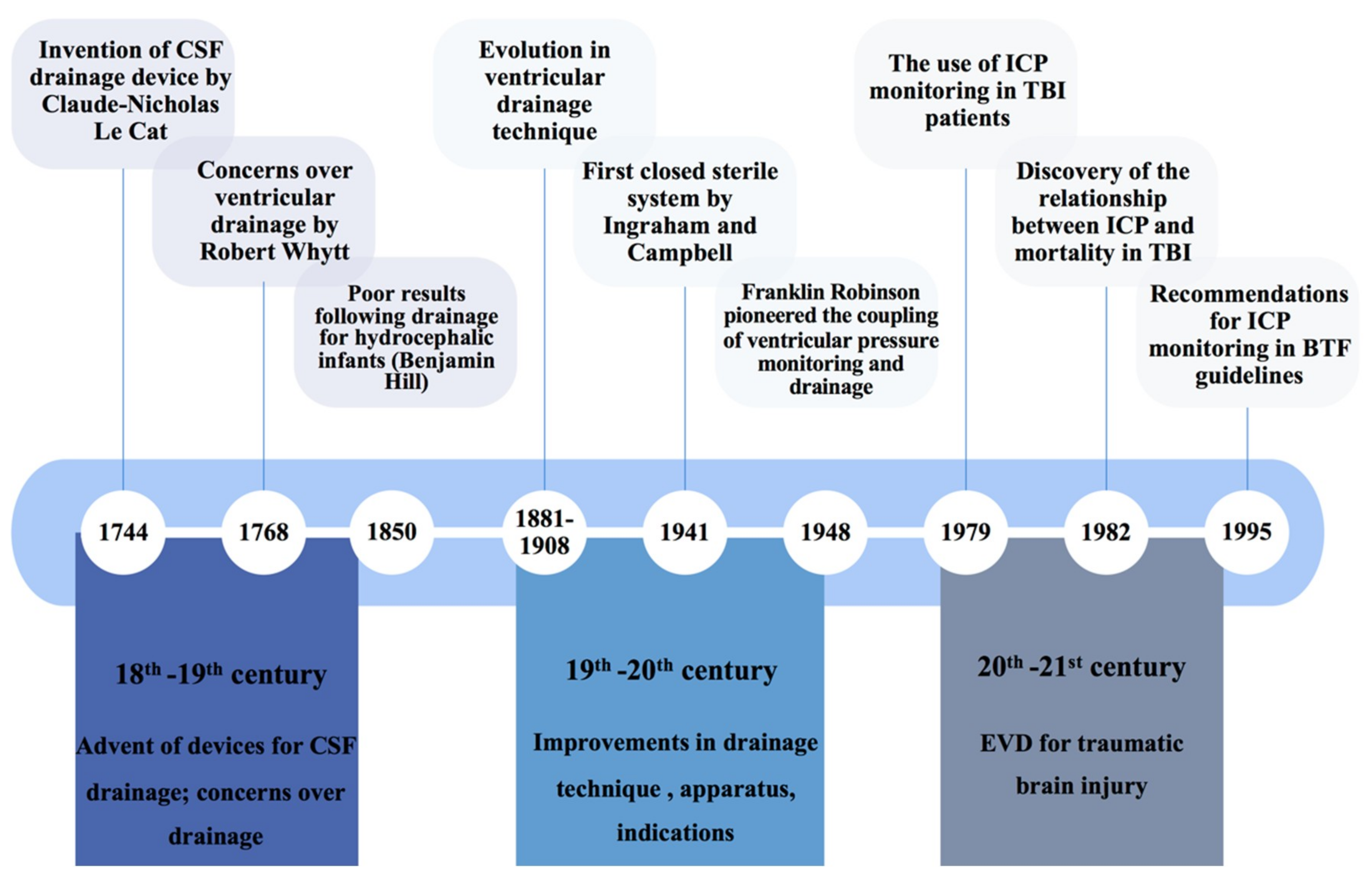
3.1. Historical Overview
3.2. Indications for External Ventricular Drains in TBI: Intracranial Pressure Monitoring
3.2.1. Current International Practice
3.2.2. Gold Standard for ICP Monitoring
3.2.3. ICP Thresholds in TBI
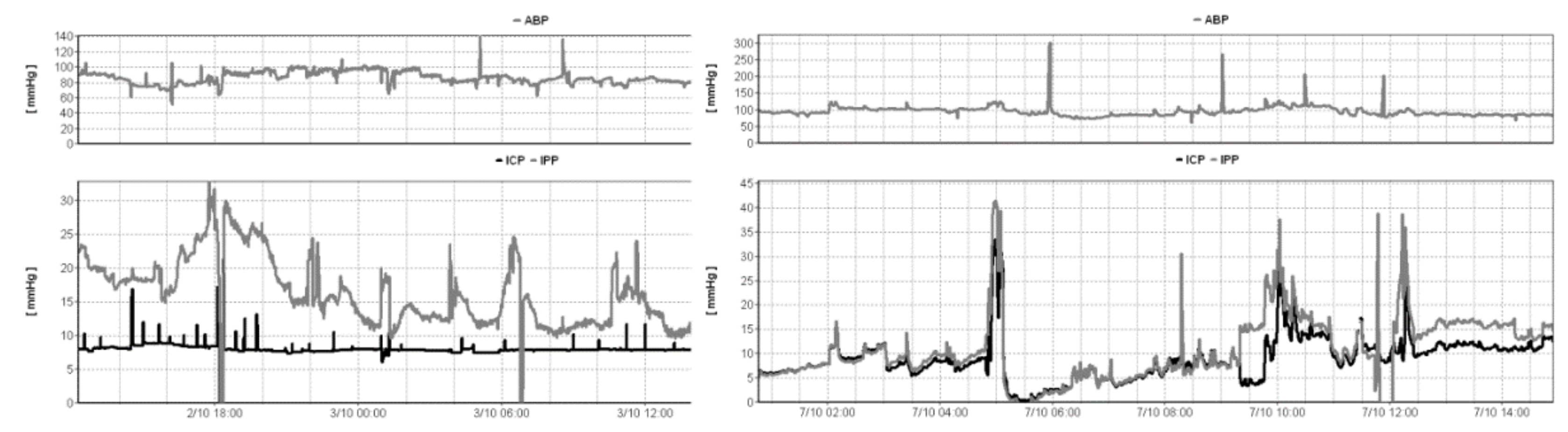
3.2.4. Problems with ICP Monitoring from EVDs
3.2.5. Future Research Directions
3.3. Indications for External Ventricular Drains in TBI: Cerebrospinal Fluid Drainage
3.3.1. Theoretical Indication for EVDs in TBI
3.3.2. Current International Practice
3.3.3. Effect on Intracranial Pressure
3.3.4. Effect on Other Physiological Parameters
3.3.5. Effect on Functional Outcome
3.3.6. Future Research Directions
3.4. Surgical Technique
3.4.1. Landmarks
3.4.2. Tunnelling Recommendations
3.4.3. Bolt EVDs
3.4.4. Accuracy of EVD Placement
3.4.5. Devices to Improve Accuracy
3.5. Complications
3.5.1. Ventriculostomy-Associated Infections (VAIs)
3.5.2. Ventriculostomy-Associated Haemorrhage
3.6. Paediatric Studies
3.6.1. ICP Monitoring
3.6.2. CSF Diversion
3.7. EVDs in Resource-Limited Settings
3.8. Economic Considerations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolias, A.G.; Rubiano, A.M.; Figaji, A.; Servadei, F.; Hutchinson, P.J. Traumatic brain injury: Global collaboration for a global challenge. Lancet Neurol. 2019, 18, 136–137. [Google Scholar] [CrossRef]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- The Trauma Audit & Research Network (TARN). England & Wales: Major Trauma in Older People—2017 Report; The Trauma Audit & Research Network (TARN): England/Wales, UK, 2017. [Google Scholar]
- Bruce, D.A.; Alavi, A.; Bilaniuk, L.; Dolinskas, C.; Obrist, W.; Uzzell, B. Diffuse cerebral swelling following head injuries in children: The syndrome of “malignant brain edema”. J. Neurosurg. 1981, 54, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Fatouros, P.P.; Barzó, P.; Portella, G.; Yoshihara, M.; Tsuji, O.; Yamamoto, T.; Laine, F.; Signoretti, S.; Ward, J.D.; et al. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J. Neurosurg. 2000, 93, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; Vizcaychipi, M.; Gupta, A.K. Traumatic brain injury: Intensive care management. Br. J. Anaesth. 2007, 99, 32–42. [Google Scholar] [CrossRef]
- Alali, A.S.; Fowler, R.A.; Mainprize, T.G.; Scales, D.C.; Kiss, A.; de Mestral, C.; Ray, J.G.; Nathens, A.B. Intracranial pressure monitoring in severe traumatic brain injury: Results from the American College of Surgeons Trauma Quality Improvement Program. J. Neurotrauma 2013, 30, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, R.; Valadka, A.B.; Robertson, C.S. Intracranial hypertension and cerebral ischemia after severe traumatic brain injury. Neurosurg. Focus 2003, 14, 1–4. [Google Scholar] [CrossRef]
- Mayer, S.A.; Coplin, W.M.; Raps, E.C. Cerebral edema, intracranial pressure, and herniation syndromes. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 1999, 8, 183–191. [Google Scholar] [CrossRef]
- Winkler, E.A.; Minter, D.; Yue, J.K.; Manley, G.T. Cerebral Edema in Traumatic Brain Injury: Pathophysiology and Prospective Therapeutic Targets. Neurosurg. Clin. North Am. 2016, 27, 473–488. [Google Scholar] [CrossRef]
- Czosnyka, M.; Pickard, J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Timofeev, I.; Dahyot-Fizelier, C.; Keong, N.; Nortje, J.; Al-Rawi, P.G.; Czosnyka, M.; Menon, D.K.; Kirkpatrick, P.J.; Gupta, A.K.; Hutchinson, P.J. Ventriculostomy for control of raised ICP in acute traumatic brain injury. Acta Neurochir. Suppl. 2008, 102, 99–104. [Google Scholar] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bianchine, J.R.; McConnell, H. Cerebrospinal Fluid in Neurology and Psychiatry; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hajdu, S.I. Discovery of the Cerebrospinal Fluid. Ann. Clin. Lab. Sci. 2003, 33, 334–336. [Google Scholar] [PubMed]
- Kompanje, E.J.; Delwel, E.J. The first description of a device for repeated external ventricular drainage in the treatment of congenital hydrocephalus, invented in 1744 by Claude-Nicolas Le Cat. Pediatric Neurosurg. 2003, 39, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Visish, M.S.; Brent, R.O.N.; Diana, J.; Donald, M.W.; Michael, Y.O. The history of external ventricular drainage. J. Neurosurg. JNS 2014, 120, 228–236. [Google Scholar] [CrossRef]
- Ingraham, D.F.; Campbell, B.J. AN APPARTUS FOR CLOSED DRAINAGE OF THE VENTRICULAR SYSTEM. Ann. Surg. 1941, 114, 1096–1098. [Google Scholar] [CrossRef]
- Crawford, S.A.; Munslow, A.R. A METHOD OF PROLONGED VENTRICULAR DRAINAGE. Ann. Surg. 1943, 117, 798–798. [Google Scholar] [CrossRef]
- Thompson, S.; Baudracco, I.; Craven, C.; Toma, A.; Thorne, L.; Watkins, L. A single centre experience of CSF drainage via a computerised, pressure lead system. Br. J. Neurosurg. 2016, 30, 130–186. [Google Scholar]
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand. Suppl. 1960, 36, 1–193. [Google Scholar] [CrossRef]
- Indications for Intracranial Pressure Monitoring. J. Neurotrauma 1996, 13, 667–679. [CrossRef] [Green Version]
- Lundberg, N.; Troupp, H.; Lorin, H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J. Neurosurg. 1965, 22, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.P.; Miller, J.D.; Ward, J.D.; Greenberg, R.P.; Young, H.F.; Sakalas, R. The outcome from severe head injury with early diagnosis and intensive management. J. Neurosurg. 1977, 47, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.F.; Smith, R.W.; Shapiro, H.M. The outcome with aggressive treatment in severe head injuries. Part I: The significance of intracranial pressure monitoring. J. Neurosurg. 1979, 50, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Saul, T.G.; Ducker, T.B. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J. Neurosurg. 1982, 56, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Recommendations for Intracranial Pressure Monitoring Technology. J. Neurotrauma 1996, 13, 685–692. [CrossRef]
- Shore, P.M.; Thomas, N.J.; Clark, R.S.; Adelson, P.D.; Wisniewski, S.R.; Janesko, K.L.; Bayir, H.; Jackson, E.K.; Kochanek, P.M. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: Effect on biochemical markers. J. Neurotrauma 2004, 21, 1113–1122. [Google Scholar] [CrossRef]
- Cnossen, M.C.; Huijben, J.A.; van der Jagt, M.; Volovici, V.; van Essen, T.; Polinder, S.; Nelson, D.; Ercole, A.; Stocchetti, N.; Citerio, G.; et al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: A survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit. Care 2017, 21, 233–233. [Google Scholar] [CrossRef]
- Zeng, T.; Gao, L. Management of patients with severe traumatic brain injury guided by intraventricular intracranial pressure monitoring: A report of 136 cases. Chin. J. Traumatol. 2010, 13, 146–151. [Google Scholar] [CrossRef]
- Salazar, L.R.M.; Rubiano, A.M.; Miranda, W.G.C.; Aquino-Matus, J. A Colombian-based survey on ventriculostomy and intracranial pressure monitor placement practices. J. Emerg. Pract. Trauma 2017, 3, 1–3. [Google Scholar] [CrossRef]
- O’Neill, B.R.; Velez, D.A.; Braxton, E.E.; Whiting, D.; Oh, M.Y. A survey of ventriculostomy and intracranial pressure monitor placement practices. Surg. Neurol. 2008, 70, 268–273; discussion 273. [Google Scholar] [CrossRef]
- Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 2007, 24 (Suppl. 1), S1–S106. [CrossRef]
- Le Roux, P. Intracranial Pressure Monitoring and Management. In Translational Research in Traumatic Brain Injury; Chapter 15; Laskowitz, D., Grant, G., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2016. [Google Scholar]
- Guillaume, J.; Janny, P. [Continuous intracranial manometry; importance of the method and first results]. Revue Neurologique 1951, 84, 131–142. [Google Scholar] [PubMed]
- Zacchetti, L.; Magnoni, S.; Di Corte, F.; Zanier, E.R.; Stocchetti, N. Accuracy of intracranial pressure monitoring: Systematic review and meta-analysis. Crit. Care 2015, 19, 420. [Google Scholar] [CrossRef] [PubMed]
- Balestreri, M.; Czosnyka, M.; Hutchinson, P.; Steiner, L.A.; Hiler, M.; Smielewski, P.; Pickard, J.D. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit. Care 2006, 4, 8–13. [Google Scholar] [CrossRef]
- Farahvar, A.; Gerber, L.M.; Chiu, Y.L.; Hartl, R.; Froelich, M.; Carney, N.; Ghajar, J. Response to intracranial hypertension treatment as a predictor of death in patients with severe traumatic brain injury. J. Neurosurg. 2011, 114, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Donald, P.B.; John, D.W.; Humbert, G.S.; William, E.A.; Michael, J.R. Significance of intracranial hypertension in severe head injury. J. Neurosurg. 1977, 47, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.; Donnelly, J.; Czosnyka, M.; Kolias, A.G.; Helmy, A.; Menon, D.K.; Smielewski, P.; Hutchinson, P.J. Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: An observational study. PLoS Med. 2017, 14, e1002353. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.D.; Czosnyka, M. Management of raised intracranial pressure. J. Neurol. Neurosurg. Psychiatry 1993, 56, 845–858. [Google Scholar] [CrossRef]
- Stocchetti, N.; Colombo, A.; Ortolano, F.; Videtta, W.; Marchesi, R.; Longhi, L.; Zanier, E.R. Time course of intracranial hypertension after traumatic brain injury. J. Neurotrauma 2007, 24, 1339–1346. [Google Scholar] [CrossRef]
- Bruce, D.A.; Raphaely, R.C.; Goldberg, A.I.; Zimmerman, R.A.; Bilaniuk, L.T.; Schut, L.; Kuhl, D.E. Pathophysiology, treatment and outcome following severe head injury in children. Child’s Brain 1979, 5, 174–191. [Google Scholar] [CrossRef]
- Treggiari, M.M.; Schutz, N.; Yanez, N.D.; Romand, J.A. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: A systematic review. Neurocrit. Care 2007, 6, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Pickard, J.D.; Steiner, L.A. Principles of intracranial pressure monitoring and treatment. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 140, pp. 67–89. [Google Scholar] [CrossRef]
- Sahuquillo, J.; Poca, M.A.; Arribas, M.; Garnacho, A.; Rubio, E. Interhemispheric supratentorial intracranial pressure gradients in head-injured patients: Are they clinically important? J. Neurosurg. 1999, 90, 16–26. [Google Scholar] [CrossRef]
- Zhong, J.; Dujovny, M.; Park, H.K.; Perez, E.; Perlin, A.R.; Diaz, F.G. Advances in ICP monitoring techniques. Neurol. Res. 2003, 25, 339–350. [Google Scholar] [CrossRef]
- Berlin, T.; Murray-Krezan, C.; Yonas, H. Comparison of parenchymal and ventricular intracranial pressure readings utilizing a novel multi-parameter intracranial access system. SpringerPlus 2015, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Volovici, V.; Huijben, J.A.; Ercole, A.; Stocchetti, N.; Dirven, C.M.F.; van der Jagt, M.; Steyerberg, E.W.; Lingsma, H.F.; Menon, D.K.; Maas, A.I.R.; et al. Ventricular Drainage Catheters versus Intracranial Parenchymal Catheters for Intracranial Pressure Monitoring-Based Management of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2019, 36, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Ronning, P.; Helseth, E.; Skaga, N.O.; Stavem, K.; Langmoen, I.A. The effect of ICP monitoring in severe traumatic brain injury: A propensity score-weighted and adjusted regression approach. J. Neurosurg. 2018, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Talving, P.; Karamanos, E.; Teixeira, P.G.; Skiada, D.; Lam, L.; Belzberg, H.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe head injury: Compliance with Brain Trauma Foundation guidelines and effect on outcomes: A prospective study. J. Neurosurg. 2013, 119, 1248–1254. [Google Scholar] [CrossRef]
- Cremer, O.L.; van Dijk, G.W.; van Wensen, E.; Brekelmans, G.J.; Moons, K.G.; Leenen, L.P.; Kalkman, C.J. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit. Care Med. 2005, 33, 2207–2213. [Google Scholar] [CrossRef]
- Shafi, S.; Diaz-Arrastia, R.; Madden, C.; Gentilello, L. Intracranial pressure monitoring in brain-injured patients is associated with worsening of survival. J. Trauma 2008, 64, 335–340. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Temkin, N.; Carney, N.; Dikmen, S.; Rondina, C.; Videtta, W.; Petroni, G.; Lujan, S.; Pridgeon, J.; Barber, J.; et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 2012, 367, 2471–2481. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Czosnyka, M.; Kirkpatrick, P.J.; Pickard, J.D.; Menon, D.K. Intracranial pressure monitoring in severe traumatic brain injury. Br. Med. J. 2013, 346, f1000. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P. Intracranial pressure after the BEST TRIP trial: A call for more monitoring. Curr. Opin. Crit. Care 2014, 20, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.J.; Czosnyka, M.; Budohoski, K.P.; Steiner, L.A.; Lavinio, A.; Kolias, A.G.; Hutchinson, P.J.; Brady, K.M.; Menon, D.K.; Pickard, J.D.; et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med. 2012, 40, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.A.; Yarzebski, J.; Wilkinson, E.C.; Anderson, F.A., Jr. Erroneous measurement of intracranial pressure caused by simultaneous ventricular drainage: A hydrodynamic model study. Neurosurgery 1989, 24, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hockel, K.; Schuhmann, M.U. ICP Monitoring by Open Extraventricular Drainage: Common Practice but Not Suitable for Advanced Neuromonitoring and Prone to False Negativity. In Intracranial Pressure & Neuromonitoring XVI; Springer: Cham, Switzerland, 2018; pp. 281–286. [Google Scholar]
- Möller Medical. LiquoGuard®. Available online: https://www.moeller-medical.com/end-user/liquoguardr.html (accessed on 26 July 2019).
- Integra Lifesciences. Integra Design Verifcation Report for Camino Flex Ventricular Catheter; Integra Lifesciences: Plainsboro Township, NJ, USA, 2013. [Google Scholar]
- Raumedic®. NEUROVENT. In Advanced Neuromonitoring Solutions; Raumedic, AG: Helmbrechts, Germany, 2019. [Google Scholar]
- Liu, X.; Zimmermann, L.L.; Ho, N.; Vespa, P.; Liao, X.; Hu, X. Evaluation of a New Catheter for Simultaneous Intracranial Pressure Monitoring and Cerebral Spinal Fluid Drainage: A Pilot Study. Neurocrit. Care 2019, 30, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Ganau, M.; Syrmos, N.; Paris, M.; Ganau, L.; Ligarotti, G.K.I.; Moghaddamjou, A.; Chibbaro, S.; Soddu, A.; Ambu, R.; Prisco, L. Current and Future Applications of Biomedical Engineering for Proteomic Profiling: Predictive Biomarkers in Neuro-Traumatology. Medicines 2018, 5, 19. [Google Scholar] [CrossRef]
- Bogoslovsky, T.; Gill, J.; Jeromin, A.; Davis, C.; Diaz-Arrastia, R. Fluid Biomarkers of Traumatic Brain Injury and Intended Context of Use. Diagnostics 2016, 6, 37. [Google Scholar] [CrossRef]
- Gubari, M.I.M.; Norouzy, A.; Hosseini, M.; Mohialdeen, F.A.; Hosseinzadeh-Attar, M.J. The Relationship between Serum Concentrations of Pro- and Anti-Inflammatory Cytokines and Nutritional Status in Patients with Traumatic Head Injury in the Intensive Care Unit. Medicina 2019, 55, 486. [Google Scholar] [CrossRef]
- Ganau, L.; Prisco, L.; Ligarotti, G.K.I.; Ambu, R.; Ganau, M. Understanding the Pathological Basis of Neurological Diseases Through Diagnostic Platforms Based on Innovations in Biomedical Engineering: New Concepts and Theranostics Perspectives. Medicines 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J. Cereb. Blood Flow Metab. 2016, 36, 1338–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tameem, A.; Krovvidi, H. Cerebral physiology. Contin. Educ. Anaesth. Crit. Care Pain 2013, 13, 113–118. [Google Scholar] [CrossRef]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.A.; Balestreri, M.; Johnston, A.J.; Coles, J.P.; Smielewski, P.; Pickard, J.D.; Menon, D.K.; Czosnyka, M. Predicting the response of intracranial pressure to moderate hyperventilation. Acta Neurochir. 2005, 147, 477–483; discussion 483. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. American College of Surgeons Trauma Quality Improvement Program: Best Practices in The Management of Traumatic Brain Injury; American College of Surgeons: Chicago, IL, USA, 2015. [Google Scholar]
- Stevens, R.D.; Shoykhet, M.; Cadena, R. Emergency Neurological Life Support: Intracranial Hypertension and Herniation. Neurocrit. Care 2015, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lescot, T.; Boroli, F.; Reina, V.; Chauvet, D.; Boch, A.L.; Puybasset, L. Effect of continuous cerebrospinal fluid drainage on therapeutic intensity in severe traumatic brain injury. Neuro-Chirurgie 2012, 58, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.B.; Feustel, P.J.; Graca, L.; Hasselbarth, J.; Kuehler, D.H. Effect of hyperventilation, mannitol, and ventriculostomy drainage on cerebral blood flow after head injury. J. Trauma 1995, 39, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.E.; Weber, B.B.; Sereika, S.M.; Wilberger, J.; Marion, D.W. Dose response to cerebrospinal fluid drainage on cerebral perfusion in traumatic brain-injured adults. Neurosurg. Focus 2001, 11. [Google Scholar] [CrossRef]
- Nwachuku, E.L.; Puccio, A.M.; Fetzick, A.; Scruggs, B.; Chang, Y.-F.; Shutter, L.A.; Okonkwo, D.O. Intermittent Versus Continuous Cerebrospinal Fluid Drainage Management in Adult Severe Traumatic Brain Injury: Assessment of Intracranial Pressure Burden. Neurocrit. Care 2014, 20, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Akbik, O.S.; Krasberg, M.; Nemoto, E.M.; Yonas, H. Effect of Cerebrospinal Fluid Drainage on Brain Tissue Oxygenation in Traumatic Brain Injury. J. Neurotrauma 2017, 34, 3153–3157. [Google Scholar] [CrossRef]
- Bhargava, D.; Alalade, A.; Ellamushi, H.; Yeh, J.; Hunter, R. Mitigating effects of external ventricular drain usage in the management of severe head injury. Acta Neurochir. 2013, 155, 2129–2132. [Google Scholar] [CrossRef]
- Kinoshita, K.; Sakurai, A.; Utagawa, A.; Ebihara, T.; Furukawa, M.; Moriya, T.; Okuno, K.; Yoshitake, A.; Noda, E.; Tanjoh, K. Importance of cerebral perfusion pressure management using cerebrospinal drainage in severe traumatic brain injury. Acta Neurochir. Suppl. 2006, 96, 37–39. [Google Scholar] [PubMed]
- Kim, G.S.; Amato, A.; James, M.L.; Britz, G.W.; Zomorodi, A.; Graffagnino, C.; Zomorodi, M.; Olson, D.M. Continuous and Intermittent CSF Diversion after Subarachnoid Hemorrhage: A Pilot Study. Neurocrit. Care 2011, 14, 68–72. [Google Scholar] [CrossRef]
- Shyam, S.R.; David, Y.C.; Zoe, W.; Faheem, S.; Ayaz, M.K.; Hang, L.; Mary, M.G.; Thabele, M.L.-M.; Kimberly, W.T.; Aman, B.P.; et al. Intermittent CSF drainage and rapid EVD weaning approach after subarachnoid hemorrhage: Association with fewer VP shunts and shorter length of stay. J. Neurosurg. JNS 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Chung, D.Y.; Mayer, S.A.; Rordorf, G.A. External Ventricular Drains After Subarachnoid Hemorrhage: Is Less More? Neurocrit. Care 2018, 28, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Griesdale, D.E.; McEwen, J.; Kurth, T.; Chittock, D.R. External ventricular drains and mortality in patients with severe traumatic brain injury. Can. J. Neurol. Sci. Le J. Canadien des Sci. Neurologiques 2010, 37, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.C.C.; Mediratta, S.; Gregson, B.; Tülü, S.; Kolias, A.; Hutchinson, P. Optimal timing of external ventricular drainage in traumatic brain injury: A systematic review and meta-analysis: PROSPERO protocol. PROSPERO Int. Prospect. Regist. Syst. Rev. 2019. [Google Scholar]
- Ullman, J.S.; Raksin, P.B. 7 Invasive Neuromonitoring Techniques; Thieme: Stuttgart, Germany, 2015. [Google Scholar]
- Ghajar, J.B. A guide for ventricular catheter placement. Technical note. J. Neurosurg. 1985, 63, 985–986. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Adeeb, N.; Griessenauer, C.J.; Sheikh, H.; Shahidi, S.; Tubbs, R.I.; Tubbs, R.S. The ventricular system of the brain: A comprehensive review of its history, anatomy, histology, embryology, and surgical considerations. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatric Neurosurg. 2014, 30, 19–35. [Google Scholar] [CrossRef]
- Winn, H.R. Youmans & Winn Neurological Surgery; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Muirhead, W.R.; Basu, S. Trajectories for frontal external ventricular drain placement: Virtual cannulation of adults with acute hydrocephalus. Br. J. Neurosurg. 2012, 26, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.D.E.; Hartley, J.C.; Chakraborty, A.; Thompson, D.N.P. Long subcutaneous tunnelling reduces infection rates in paediatric external ventricular drains. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatric Neurosurg. 2014, 30, 1671–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, M.Z.; Sobani, Z.A.; Murtaza, M.; Enam, S.A. Long-tunneled versus short-tunneled external ventricular drainage: Prospective experience from a developing country. Asian J. Neurosurg. 2016, 11, 114–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamjoom, A.A.B.; Joannides, A.J.; Poon, M.T.; Chari, A.; Zaben, M.; Abdulla, M.A.H.; Roach, J.; Glancz, L.J.; Solth, A.; Duddy, J.; et al. Prospective, multicentre study of external ventricular drainage-related infections in the UK and Ireland. J. Neurol. Neurosurg. Psychiatry 2018, 89, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.; Gaastra, B.; Bulters, D.; Shtaya, A. Safety, Accuracy, and Cost Effectiveness of Bedside Bolt External Ventricular Drains (EVDs) in Comparison with Tunneled EVDs Inserted in Theaters. World Neurosurg. 2019, 125, e473–e478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergdal, O.; Springborg, J.B.; Holst, A.V.; Hauerberg, J.; Way, S.; Breum, P.; Romner, B. Accuracy of tunnelated vs. bolt-connected external ventricular drains. Clin. Neurol. Neurosurg. 2013, 115, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Asaad, S.K.; Bjarkam, C.R. The Aalborg Bolt-Connected Drain (ABCD) study: A prospective comparison of tunnelled and bolt-connected external ventricular drains. Acta Neurochir. 2019, 161, 33–39. [Google Scholar] [CrossRef]
- Jensen, T.S.; Carlsen, J.G.; Sørensen, J.C.; Poulsen, F.R. Fewer complications with bolt-connected than tunneled external ventricular drainage. Acta Neurochir. 2016, 158, 1491–1494. [Google Scholar] [CrossRef]
- Huyette, D.R.; Turnbow, B.J.; Kaufman, C.; Vaslow, D.F.; Whiting, B.B.; Oh, M.Y. Accuracy of the freehand pass technique for ventriculostomy catheter placement: Retrospective assessment using computed tomography scans. J. Neurosurg. 2008, 108, 88–91. [Google Scholar] [CrossRef]
- Toma, A.K.; Camp, S.; Watkins, L.D.; Grieve, J.; Kitchen, N.D. External ventricular drain insertion accuracy: Is there a need for change in practice? Neurosurgery 2009, 65, 1197–1201. [Google Scholar] [CrossRef]
- AlAzri, A.; Mok, K.; Chankowsky, J.; Mullah, M.; Marcoux, J. Placement accuracy of external ventricular drain when comparing freehand insertion to neuronavigation guidance in severe traumatic brain injury. Acta Neurochir. 2017, 159, 1399–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.T.; Chen, G.J.; Ma, H.I.; Chang, C.F.; Cheng, C.M.; Su, Y.H.; Ju, D.T.; Hsia, C.C.; Chen, Y.H.; Wu, H.Y.; et al. The misplacement of external ventricular drain by freehand method in emergent neurosurgery. Acta Neurol. Belg. 2011, 111, 22–28. [Google Scholar] [PubMed]
- Candanedo, C.; Doron, O.; Hemphill, J.C., 3rd; Ramirez de Noriega, F.; Manley, G.T.; Patal, R.; Rosenthal, G. Characterizing the Response to Cerebrospinal Fluid Drainage in Patients with an External Ventricular Drain: The Pressure Equalization Ratio. Neurocrit. Care 2018, 30, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kakarla, U.K.; Kim, L.J.; Chang, S.W.; Theodore, N.; Spetzler, R.F. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery 2008, 63, ONS162–ONS166; discussion ONS166–ONS167. [Google Scholar] [CrossRef]
- O’Leary, S.T.; Kole, M.K.; Hoover, D.A.; Hysell, S.E.; Thomas, A.; Shaffrey, C.I. Efficacy of the Ghajar Guide revisited: A prospective study. J. Neurosurg. 2000, 92, 801–803. [Google Scholar] [CrossRef]
- Wilson, T.J.; Stetler, W.R., Jr.; Al-Holou, W.N.; Sullivan, S.E. Comparison of the accuracy of ventricular catheter placement using freehand placement, ultrasonic guidance, and stereotactic neuronavigation. J. Neurosurg. 2013, 119, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neurodynamics, Inc. (NY). Catheter guide apparatus for perpendicular insertion into a cranium orifice. US4931056A, 5 June 1990. [Google Scholar]
- Nowacki, A.; Wagner, F.; Soll, N.; Hakim, A.; Beck, J.; Raabe, A.; Z’Graggen, W.J. Preliminary Results of Emergency Computed Tomography-Guided Ventricular Drain Placement-Precision for the Most Difficult Cases. World Neurosurg. 2018, 114, e1290–e1296. [Google Scholar] [CrossRef]
- Patil, V.; Lacson, R.; Vosburgh, K.G.; Wong, J.M.; Prevedello, L.; Andriole, K.; Mukundan, S.; Popp, A.J.; Khorasani, R. Factors associated with external ventricular drain placement accuracy: Data from an electronic health record repository. Acta Neurochir. 2013, 155, 1773–1779. [Google Scholar] [CrossRef]
- Raabe, C.; Fichtner, J.; Beck, J.; Gralla, J.; Raabe, A. Revisiting the rules for freehand ventriculostomy: A virtual reality analysis. J. Neurosurg. 2018, 128, 1250–1257. [Google Scholar] [CrossRef]
- Fried, H.I.; Nathan, B.R.; Rowe, A.S.; Zabramski, J.M.; Andaluz, N.; Bhimraj, A.; Guanci, M.M.; Seder, D.B.; Singh, J.M. The Insertion and Management of External Ventricular Drains: An Evidence-Based Consensus Statement: A Statement for Healthcare Professionals from the Neurocritical Care Society. Neurocrit. Care 2016, 24, 61–81. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.; Smoll, N.; Schaller, K. Guided (VENTRI-GUIDE) versus freehand ventriculostomy: Study protocol for a randomized controlled trial. Trials 2014, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Lipman, J.; Shorr, A.; Shankar, A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect. Dis. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Holloway, K.L.; Barnes, T.; Choi, S.; Bullock, R.; Marshall, L.F.; Eisenberg, H.M.; Jane, J.A.; Ward, J.D.; Young, H.F.; Marmarou, A. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J. Neurosurg. 1996, 85, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lozier, A.P.; Sciacca, R.R.; Romagnoli, M.F.; Connolly, E.S., Jr. Ventriculostomy-related infections: A critical review of the literature. Neurosurgery 2002, 51, 170–181; discussion 181–182. [Google Scholar] [CrossRef] [PubMed]
- Lyke, K.E.; Obasanjo, O.O.; Williams, M.A.; O’Brien, M.; Chotani, R.; Perl, T.M. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.P.; Ong, V.; Lagman, C.; Udawatta, M.; Duong, C.; Nguyen, T.; Prashant, G.N.; Plurad, D.S.; Kim, D.Y.; Yang, I. Systemic Antimicrobial Prophylaxis and Antimicrobial-Coated External Ventricular Drain Catheters for Preventing Ventriculostomy-Related Infections: A Meta-Analysis of 5242 Cases. Neurosurgery 2018. [Google Scholar] [CrossRef] [PubMed]
- Lele, A.V.; Hoefnagel, A.L.; Schloemerkemper, N.; Wyler, D.A.; Chaikittisilpa, N.; Vavilala, M.S.; Naik, B.I.; Williams, J.H.; Venkat Raghavan, L.; Koerner, I.P. Perioperative Management of Adult Patients With External Ventricular and Lumbar Drains: Guidelines From the Society for Neuroscience in Anesthesiology and Critical Care. J. Neurosurg. Anesthesiol. 2017, 29, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Talibi, S.; Tarnaris, A.; Shaw, S.A. Has the introduction of antibiotic-impregnated external ventricular drain catheters changed the nature of the microorganisms cultured in patients with drain-related infection? A single neurosurgical centre’s experience. Br. J. Neurosurg. 2016, 30, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, B.; Zhong, Z.; Sun, Y.; Sun, Q.; Yang, G.; Bian, L. Impact of antibiotic- and silver-impregnated external ventricular drains on the risk of infections: A systematic review and meta-analysis. Am. J. Infect. Control 2015, 43, e23–e32. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Korenfeld, Y.; Crisman, C.; Badjatia, N.; Mayer, S.A.; Connolly, E.S., Jr. Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: A systematic review. Neurosurgery 2011, 68, 996–1005. [Google Scholar] [CrossRef]
- Wiesmann, M.; Mayer, T.E. Intracranial bleeding rates associated with two methods of external ventricular drainage. J. Clin. Neurosci. 2001, 8, 126–128. [Google Scholar] [CrossRef]
- Binz, D.D.; Toussaint, L.G.; Friedman, J.A. Hemorrhagic Complications of Ventriculostomy Placement: A Meta-Analysis. Neurocrit. Care 2009, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Tummala, R.P. Risk factors for hemorrhage associated with external ventricular drain placement and removal. J. Neurosurg. 2017, 126, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, D.F.; McGwin, G., Jr.; Melton, S.M.; George, R.L.; Markert, J.M. The relationship between INR and development of hemorrhage with placement of ventriculostomy. J. Trauma 2011, 70, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Maniker, A.H.; Vaynman, A.Y.; Karimi, R.J.; Sabit, A.O.; Holland, B. Hemorrhagic complications of external ventricular drainage. Neurosurgery 2006, 59, ONS419–ONS424; discussion ONS424–ONS425. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Cha, S.H.; Choi, B.K.; Lee, J.I.; Yun, E.Y.; Choi, C.H. Hemorrhage rates associated with two methods of ventriculostomy: External ventricular drainage vs. ventriculoperitoneal shunt procedure. Neurologia Medico-Chirurgica 2014, 54, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.A.; Piñero, G.R. Intensive Care in Neurology and Neurosurgery: Pathophysiological Basis for the Management of Acute Cerebral Injury; SEEd: Torino, Italy, 2013. [Google Scholar]
- Loftus, C.M. Neurosurgical Emergencies; Thieme: Stuttgart, Germany, 2011. [Google Scholar]
- Rowell, S.E.; Barbosa, R.R.; Lennox, T.C.; Fair, K.A.; Rao, A.J.; Underwood, S.J.; Schreiber, M.A. Moderate elevations in international normalized ratio should not lead to delays in neurosurgical intervention in patients with traumatic brain injury. J. Trauma Acute Care Surg. 2014, 77, 846–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochanek, P.M.; Tasker, R.C.; Carney, N.; Totten, A.M.; Adelson, P.D.; Selden, N.R.; Davis-O’Reilly, C.; Hart, E.L.; Bell, M.J.; Bratton, S.L.; et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines. Pediatric Crit. Care Med. 2019, 20, 1169–1178. [Google Scholar] [CrossRef]
- Keenan, H.T.; Nocera, M.; Bratton, S.L. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatric Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatric Intensive Crit. Care Soc. 2005, 6, 537–541. [Google Scholar]
- Richard, C.E.A.; Peter, K.; Paul, K.; Douglas, L.B.; Marion, L.W.; John, R.W.K. Complications of intracranial pressure monitoring in children with head trauma. J. Neurosurg. Pediatrics 2004, 101, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Morris, K.P.; Forsyth, R.J.; Parslow, R.C.; Tasker, R.C.; Hawley, C.A. UK Paediatric Traumatic Brain Injury Study Group; Paediatric Intensive Care Society Study Group. Intracranial pressure complicating severe traumatic brain injury in children: Monitoring and management. Intensive Care Med. 2006, 32, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Riva-Cambrin, J.; Keenan, H.T.; Korgenski, E.K.; Bratton, S.L. Variation in Intracranial Pressure Monitoring and Outcomes in Pediatric Traumatic Brain InjuryTBI Intracranial Pressure Monitoring and Outcomes. Arch. Pediatrics Adolesc. Med. 2012, 166, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Alkhoury, F.; Kyriakides, T.C. Intracranial Pressure Monitoring in Children with Severe Traumatic Brain Injury: National Trauma Data Bank–Based Review of Outcomes. JAMA Surg. 2014, 149, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Van Cleve, W.; Kernic, M.A.; Ellenbogen, R.G.; Wang, J.; Zatzick, D.F.; Bell, M.J.; Wainwright, M.S.; Groner, J.I.; Mink, R.B.; Giza, C.C.; et al. National variability in intracranial pressure monitoring and craniotomy for children with moderate to severe traumatic brain injury. Neurosurgery 2013, 73, 746–752; discussion 752, quiz 752. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Tasker, R.C.; Bell, M.J.; Adelson, P.D.; Carney, N.; Vavilala, M.S.; Selden, N.R.; Bratton, S.L.; Grant, G.A.; Kissoon, N.; et al. Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatric Crit. Care Med. 2019, 20, 269–279. [Google Scholar] [CrossRef]
- Kenneth, S.; Anthony, M. Clinical applications of the pressure-volume index in treatment of pediatric head injuries. J. Neurosurg. 1982, 56, 819–825. [Google Scholar] [CrossRef]
- Jay, J.; David, O.O.; Hian Kwang, Y.; Aaron, S.D.; Dwight, S.; Julie, H.; Jeffrey, T.B.; John, A.J.; John, A.J. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J. Neurosurg. Pediatrics PED 2008, 2, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Andrade, A.F.; Paiva, W.S.; Amorim, R.L.; Figueiredo, E.G.; Almeida, A.N.; Brock, R.S.; Bor-Seng-Shu, E.; Teixeira, M.J. Continuous ventricular cerebrospinal fluid drainage with intracranial pressure monitoring for management of posttraumatic diffuse brain swelling. Arquivos de Neuro-Psiquiatria 2011, 69, 79–84. [Google Scholar] [CrossRef]
- Giza, C.C.; Mink, R.B.; Madikians, A. Pediatric traumatic brain injury: Not just little adults. Curr. Opin. Crit. Care 2007, 13, 143–152. [Google Scholar] [CrossRef]
- Dorothy, A.L.; Graham, M.T.; Peter, M.; Audrey, L. Diffuse brain swelling after head injury: More often malignant in adults than children? J. Neurosurg. 1994, 80, 675–680. [Google Scholar] [CrossRef]
- Grant, R.; Condon, B.; Lawrence, A.; Hadley, D.M.; Patterson, J.; Bone, I.; Teasdale, G.M. Human cranial CSF volumes measured by MRI: Sex and age influences. Magn. Reson. Imaging 1987, 5, 465–468. [Google Scholar] [CrossRef]
- Mitsunori, M.; Ron, K.; István, A.M.; Antonio, V.L.; Tamás, S.; Marilyn, S.A.; Peter Mc, L.B.; Ferenc, A.J. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J. Neurosurg. 1996, 84, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.J.; Adelson, P.D.; Wisniewski, S.R.; Agrawal, S.; Mahoney, S.; Gupta, D.; Beca, J.; Loftis, L.; Morris, K.; Piper, L.; et al. Challenges and opportunities for pediatric severe TBI—Review of the evidence and exploring a way forward. Child’s Nerv. Syst. 2017, 33, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Asha, M.A.; Bankole, O.B.; Kanu, O.O. Improvised external ventricular drain in neurosurgery: A Nigerian tertiary hospital experience. J. Neurosci. Rural Pract. 2015, 6, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Kammoun, B.; Kolsi, F.; Borni, M.; Abdelhedi, A.; Abdelmouleh, S.; Jarraya, F.; Bouhamed, O.; Kammoun, O.; Elleuch, E.; Boudawara, M.Z. Applicability, Safety, and Cost-Effectiveness of Improvised External Ventricular Drainage: An Observational Study of Tunisian Neurosurgery Inpatients. World Neurosurg. 2018, 119, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M. Intracranial pressure monitoring in a resource-constrained environment: A technical note. Neurol. India 2003, 51, 333–335. [Google Scholar] [PubMed]
- Warf, B.C. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: A prospective study in 195 children. J. Neurosurg. 2005, 102, 358–362. [Google Scholar] [CrossRef]
- Rosenfeld, J.V.; Maas, A.I.; Bragge, P.; Morganti-Kossmann, M.C.; Manley, G.T.; Gruen, R.L. Early management of severe traumatic brain injury. Lancet 2012, 380, 1088–1098. [Google Scholar] [CrossRef]
- Edwards, N.C.; Engelhart, L.; Casamento, E.M.; McGirt, M.J. Cost-consequence analysis of antibiotic-impregnated shunts and external ventricular drains in hydrocephalus. J. Neurosurg. 2015, 122, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Fargen, K.M.; Hoh, B.L.; Neal, D.; O’Connor, T.; Rivera-Zengotita, M.; Murad, G.J. The burden and risk factors of ventriculostomy occlusion in a high-volume cerebrovascular practice: Results of an ongoing prospective database. J. Neurosurg. 2016, 124, 1805–1812. [Google Scholar] [CrossRef]
- Whitmore, R.G.; Thawani, J.P.; Grady, M.S.; Levine, J.M.; Sanborn, M.R.; Stein, S.C. Is aggressive treatment of traumatic brain injury cost-effective? J. Neurosurg. 2012, 116, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, C.Y.C.; Craven, C.L.; Rubiano, A.M.; Adams, H.; Tülü, S.; Czosnyka, M.; Servadei, F.; Ercole, A.; Hutchinson, P.J.; Kolias, A.G. The Evolution of the Role of External Ventricular Drainage in Traumatic Brain Injury. J. Clin. Med. 2019, 8, 1422. https://doi.org/10.3390/jcm8091422
Chau CYC, Craven CL, Rubiano AM, Adams H, Tülü S, Czosnyka M, Servadei F, Ercole A, Hutchinson PJ, Kolias AG. The Evolution of the Role of External Ventricular Drainage in Traumatic Brain Injury. Journal of Clinical Medicine. 2019; 8(9):1422. https://doi.org/10.3390/jcm8091422
Chicago/Turabian StyleChau, Charlene Y. C., Claudia L. Craven, Andres M. Rubiano, Hadie Adams, Selma Tülü, Marek Czosnyka, Franco Servadei, Ari Ercole, Peter J. Hutchinson, and Angelos G. Kolias. 2019. "The Evolution of the Role of External Ventricular Drainage in Traumatic Brain Injury" Journal of Clinical Medicine 8, no. 9: 1422. https://doi.org/10.3390/jcm8091422




