Assessment of Global Longitudinal and Circumferential Strain Using Computed Tomography Feature Tracking: Intra-Individual Comparison with CMR Feature Tracking and Myocardial Tagging in Patients with Severe Aortic Stenosis
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Cardiac Computed Tomography Acquisition
2.3. Cardiac Magnetic Resonance Acquisition
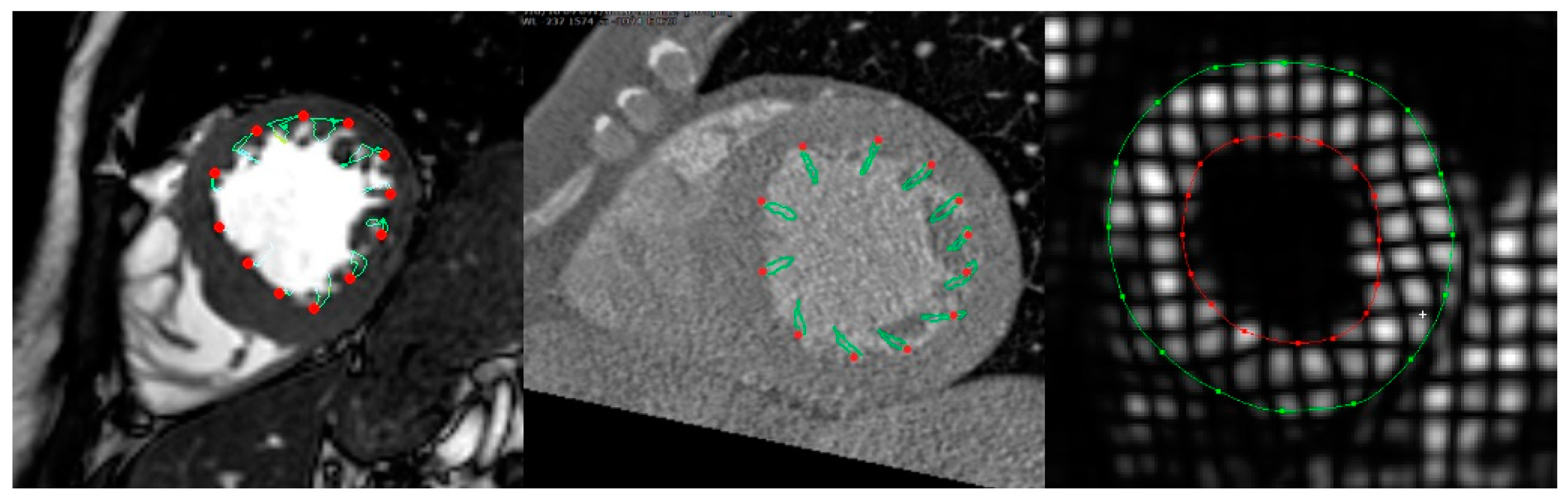
2.4. Cardiac CT Data Analysis
2.5. CMR Data Analysis
2.6. Statistics
3. Results
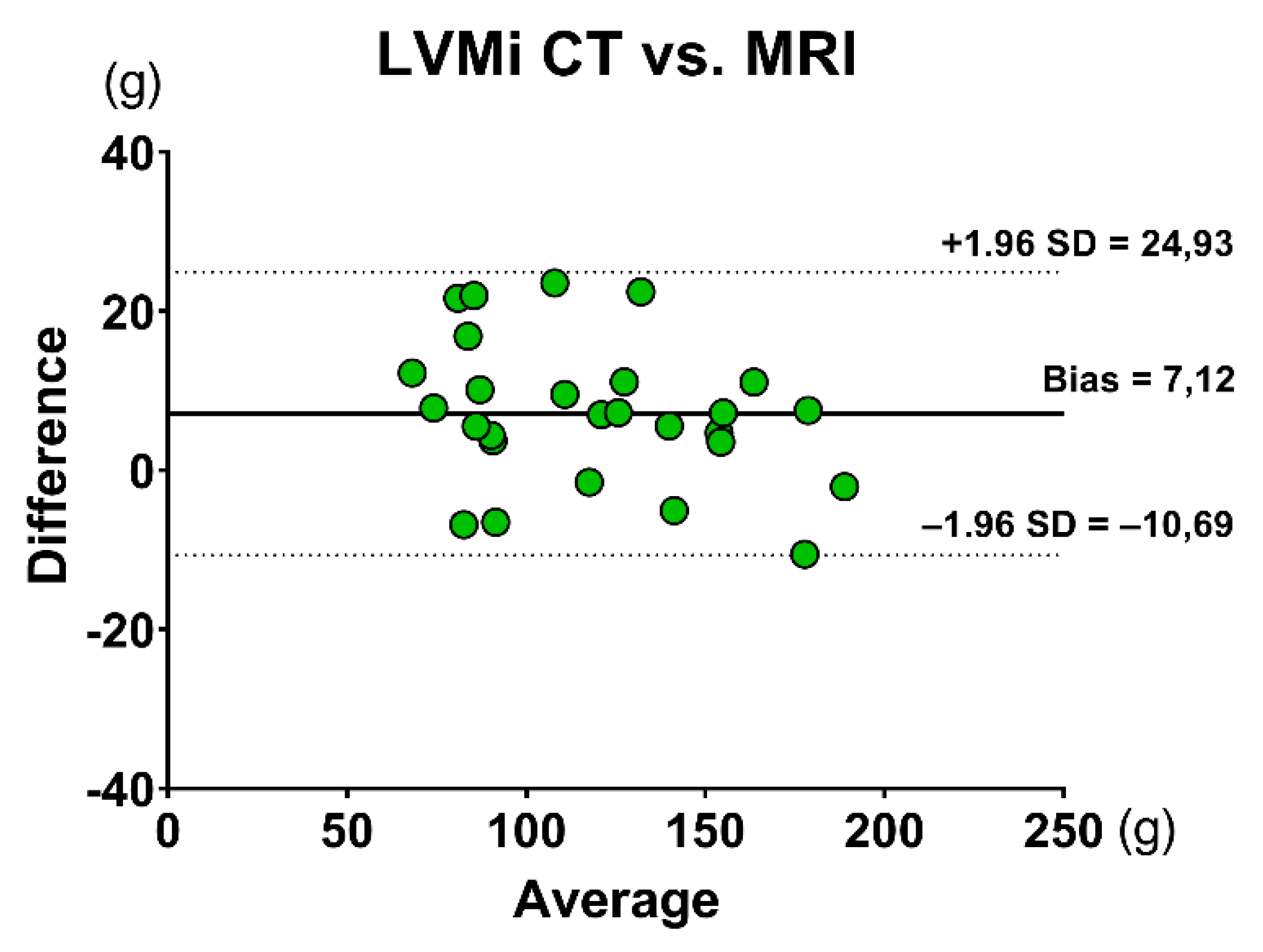
3.1. Volumetric Assessment
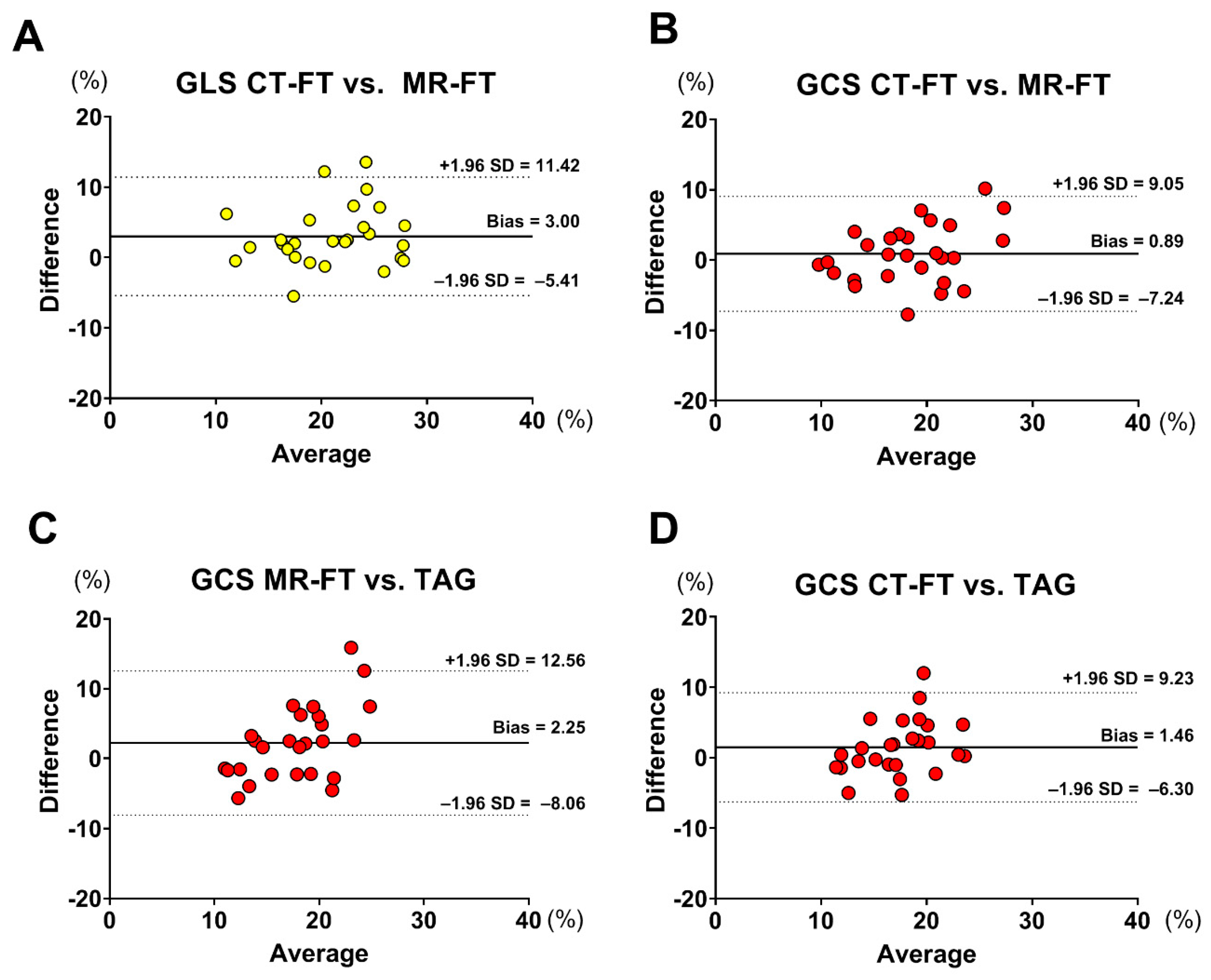
3.2. Strain Assessment
3.3. Intraobserver and Interobserver Reproducibility
4. Discussion
4.1. Main Findings
- There was good correlation between CMR-FT and CT-FT for GLS and GCS assessment, while GCS derived from both CMR-FT and CT-FT had a moderate correlation with TAG;
- The intra- and interobserver reproducibility of CMR-FT and CT-FT were excellent;
- There were no significant differences between cardiac CT and CMR for the volumetric assessment of the LV.
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caforio, A.L.P.; Adler, Y.; Agostini, C.; Allanore, Y.; Anastasakis, A.; Arad, M.; Böhm, M.; Charron, P.; Elliott, P.M.; Eriksson, U.; et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur. Heart J. 2017, 38, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Hermann, J.; Gabriel, S.E.; Weyand, C.M.; Mulvagh, S.; Mankad, R.; Oh, J.K.; Matteson, E.L.; Lerman, A. Cardiorheumatology: Cardiac involvement in systemic rheumatic disease. Nat. Rev. Cardiol. 2015, 12, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Lapinskas, T.; Tonti, G.; Stoiber, L.; Zaliunas, R.; Gebker, R.; Pieske, B.; Kelle, S. The Relationship Between EF and Strain Permits a More Accurate Assessment of LV Systolic Function. JACC Cardiovasc. Imaging 2019, 3033. [Google Scholar] [CrossRef] [PubMed]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Van Cleemput, J.; Delforge, M.; Bogaert, J.; Kuznetsova, T.; Voigt, J.-U. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: a head-to-head comparison of deformation and nondeformation parameters. Circ. Cardiovasc. Imaging 2017, 10, e005588. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Stronati, G.; Fischietti, C.; Ferrarini, A.; Zuliani, L.; Pomponio, G.; Capucci, A.; Danieli, M.G.; Gabrielli, A. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur. J. Prev. Cardiol. 2018, 25, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.M.; Crowson, C.S.; Lin, G.; Oh, J.K.; Villarraga, H.R.; Gabriel, S.E. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann. Rheum Dis. 2014, 73, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-J.; Lee, J.-W.; Lee, J.; Shin, Y.B. Evaluation of Early Left Ventricular Dysfunction in Patients with Duchenne Muscular Dystrophy Using Two-Dimensional Speckle Tracking Echocardiography and Tissue Doppler Imaging. Pediatr. Cardiol. 2018, 39, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Nagata, Y.; Wu, V.C.-C.; Kado, Y.; Kurabayashi, M.; Otsuji, Y.; Takeuchi, M. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 525–532. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Markousis-Mavrogenis, G.; Koutsogeorgopoulou, L.; Kolovou, G. Cardiovascular magnetic resonance imaging: Clinical implications in the evaluation of connective tissue diseases. J. Inflamm. Res. 2017, 10, 55–61. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Fukui, M.; Xu, J.; Abdelkarim, I.; Sharbaugh, M.S.; Thoma, F.W.; Althouse, A.D.; Pedrizzetti, G.; Cavalcante, J.L. Global longitudinal strain assessment by computed tomography in severe aortic stenosis patients—Feasibility using feature tracking analysis. J. Cardiovasc. Comput. Tomogr. 2019, 13, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Schulz, F.; Mereles, D.; Hosch, W.; Galuschky, C.; Schummers, G.; Stapf, D.; Hofmann, N.; Giannitsis, E.; Hardt, S.E.; et al. Quantitative analysis of left ventricular strain using cardiac computed tomography. Eur. J. Radiol. 2014, 83, e123–e130. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Lung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.; Schuster, A.; Jogiya, R.; Kutty, S.; Beerbaum, P.; Nagel, E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J. Cardiovasc. Magn. Reson. 2012, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Oppo, K.; Leen, E.; Angerson, W.J.; Cooke, T.G.; McArdle, C.S. Doppler perfusion index: An interobserver and intraobserver reproducibility study. Radiology 1998, 208, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Helle-Valle, T.M.; Yu, W.C.; Fernandes, V.R.S.; Rosen, B.D.; Lima, J.A.C. Usefulness of radial strain mapping by multidetector computer tomography to quantify regional myocardial function in patients with healed myocardial infarction. Am. J. Cardiol. 2010, 106, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Kido, T.; Kurata, A.; Sawada, S.; Suekuni, H.; Kido, T.; Yokoi, T.; Uetani, T.; Inoue, K.; Miyagawa, M.; et al. Three-dimensional maximum principal strain using cardiac computed tomography for identification of myocardial infarction. Eur. Radiol. 2017, 27, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Marwan, M.; Ammon, F.; Bittner, D.; Röther, J.; Mekkhala, N.; Hell, M.; Schuhbaeck, A.; Gitsioudis, G.; Feryrer, R.; Schlundt, C.; et al. CT-derived left ventricular global strain in aortic valve stenosis patients: A comparative analysis pre and post transcatheter aortic valve implantation. J. Cardiovasc. Comput. Tomogr. 2018, 12, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Ammon, F.; Bittner, D.; Hell, M.; Mansour, H.; Achenbach, S.; Arnold, M.; Marwan, M. CT-derived left ventricular global strain: A head-to-head comparison with speckle tracking echocardiography. Int. J. Cardiovasc. Imaging 2019, 35, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J. Cardiovasc. Magn. Reson. 2013, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Greupner, J.; Zimmermann, E.; Grohmann, A.; Dübel, H.-P.; Althoff, T.; Borges, A.C.; Rutsch, W.; Schlattmann, P.; Hamm, B.; Dewey, M. Head-to-Head Comparison of Left Ventricular Function Assessment with 64-Row Computed Tomography, Biplane Left Cineventriculography, and Both 2- and 3-Dimensional Transthoracic Echocardiography. J. Am. Coll. Cardiol. 2012, 59, 1897–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-W.; Tadamura, E.; Yamamuro, M.; Kanao, S.; Okayama, S.; Ozasa, N.; Toma, M.; Kimura, T.; Komeda, M.; Togashi, K. Estimation of global and regional cardiac function using 64-slice computed tomography: A comparison study with echocardiography, gated-SPECT and cardiovascular magnetic resonance. Int. J. Cardiol. 2008, 128, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Shapiro, M.D.; Nasir, K.; Nieman, K.; Nomura, C.H.; Brady, T.J.; Cury, R.C. Evaluating global and regional left ventricular function in patients with reperfused acute myocardial infarction by 64-slice multidetector CT: A comparison to magnetic resonance imaging. J. Cardiovasc. Comput. Tomogr. 2009, 3, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Ngai, N.; Duncanson, L.; Cheng, J.; Gliganic, K.; Chen, Q. A comparison of both DENSE and feature tracking techniques with tagging for the cardiovascular magnetic resonance assessment of myocardial strain. J. Cardiovasc. Magn. Reson. 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed]


| Variables. | n = 27 Mean ± SD or n (%) |
|---|---|
| Age | 22.40 ± 5.69 |
| Male | 18.91 ± 5.97 |
| Body mass index (kg/m2) | 26.60 ± 3.60 |
| Heart rate | 67.59 ± 10.27 |
| Clinical history | |
| Hypertension | 25 (92.56%) |
| CAD | 16 (59.25%) |
| Myocardial infarction | 6 (22.22%) |
| History of CABG | 5 (18.51%) |
| Stroke | 4 (14.81%) |
| Diabetes mellitus type 2 | 6 (22.22%) |
| COPD | 5 (18.51%) |
| Measurement | CMR | CCT | p-Value |
|---|---|---|---|
| LVEF (%) | 64.57 ± 14.55 | 59.15 ± 14.82 | 0.181 |
| LVEDVi (mL/m2) | 72.60 ± 27.22 | 80.35 ± 26.42 | 0.374 |
| LVESVi (mL/m2) | 28.62 ± 21.21 | 35.79 ± 23.39 | 0.293 |
| LVSVi (mL/m2) | 43.98 ± 11.65 | 44.56 ± 8.13 | 0.933 |
| LVMi (g/m2) | 62.13 ± 20.51 | 66.04 ± 19.42 | 0.471 |
| Measurement | CMR-FT | CT-FT | TAG |
|---|---|---|---|
| GLS (%) | 22.40 ± 5.69 | 19.4 ± 5.04 | N/A |
| GCS (%) | 18.91 ± 5.97 | 18.13 ± 4.63 | 16.66 ± 3.38 |
| Measurement | Comparison | Bias (%) | LOA (%) | Pearson’s R |
|---|---|---|---|---|
| GLS | CMR-FT vs. CT-FT | 3.003 | ±8.415 | 0.6860 |
| GCS GCS GCS | CMR-FT vs. CT-FT | 0.888 | ±8.16 | 0.7067 |
| CMR-FT vs. TAG | 2.250 | ±10.31 | 0.4799 | |
| CT-FT vs. TAG | 1.468 | ±7.77 | 0.5484 |
| Bias (%) | Limits of Agreement (±) | CoV (%) | ICC (95% CI) | |
|---|---|---|---|---|
| Intraobserver reproducibility | ||||
| CMR-FT | ||||
| GLS | 0.09 | 4.36 | 10.1 | 0.960 (0.837–0.990) |
| GCS | −2.44 | 4.8 | 13.1 | 0.931 (0.439–0.985) |
| CT-FT | ||||
| GLS | −0.08 | 2.4 | 6.8 | 0.983 (0.932–0.996) |
| GCS | −0.05 | 5.0 | 14.4 | 0.949 (0.801–0.987) |
| TAG | ||||
| GCS | −0.08 | 1.26 | 3.9 | 0.992 (0.969–0.998) |
| Interobserver reproducibility | ||||
| CMR-FT | ||||
| GLS | 0.03 | 3.16 | 7.4 | 0.982 (0.926–0.995) |
| GCS | −2.3 | 6.6 | 18.1 | 0.922 (0.629–0.981) |
| CT-FT | ||||
| GLS | 1.25 | 5.5 | 16.1 | 0.866 (0.501–0.966) |
| GCS | 0.39 | 5.1 | 14.6 | 0.940 (0.759–0.985) |
| TAG | ||||
| GCS | 0.48 | 1.72 | 5.4 | 0.981 (0.918–0.995) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miskinyte, E.; Bucius, P.; Erley, J.; Zamani, S.M.; Tanacli, R.; Stehning, C.; Schneeweis, C.; Lapinskas, T.; Pieske, B.; Falk, V.; et al. Assessment of Global Longitudinal and Circumferential Strain Using Computed Tomography Feature Tracking: Intra-Individual Comparison with CMR Feature Tracking and Myocardial Tagging in Patients with Severe Aortic Stenosis. J. Clin. Med. 2019, 8, 1423. https://doi.org/10.3390/jcm8091423
Miskinyte E, Bucius P, Erley J, Zamani SM, Tanacli R, Stehning C, Schneeweis C, Lapinskas T, Pieske B, Falk V, et al. Assessment of Global Longitudinal and Circumferential Strain Using Computed Tomography Feature Tracking: Intra-Individual Comparison with CMR Feature Tracking and Myocardial Tagging in Patients with Severe Aortic Stenosis. Journal of Clinical Medicine. 2019; 8(9):1423. https://doi.org/10.3390/jcm8091423
Chicago/Turabian StyleMiskinyte, Emilija, Paulius Bucius, Jennifer Erley, Seyedeh Mahsa Zamani, Radu Tanacli, Christian Stehning, Christopher Schneeweis, Tomas Lapinskas, Burkert Pieske, Volkmar Falk, and et al. 2019. "Assessment of Global Longitudinal and Circumferential Strain Using Computed Tomography Feature Tracking: Intra-Individual Comparison with CMR Feature Tracking and Myocardial Tagging in Patients with Severe Aortic Stenosis" Journal of Clinical Medicine 8, no. 9: 1423. https://doi.org/10.3390/jcm8091423





