Environmental Risk Factors of Pancreatic Cancer
Abstract
1. Introduction
2. Lifestyle Risk Factors of Pancreatic Cancer
2.1. Cigarette Smoking and Other Tobacco Products
2.2. Alcohol Drinking
2.3. Diet
2.4. Physical Activities
2.5. Obesity
2.6. Oral Health/Hygiene
2.7. Oral Microbiome
2.8. Gut Microbiome
2.9. Coffee
3. Clinical Risk Factors of Pancreatic Cancer
3.1. Diabetes
3.2. Chronic Pancreatitis
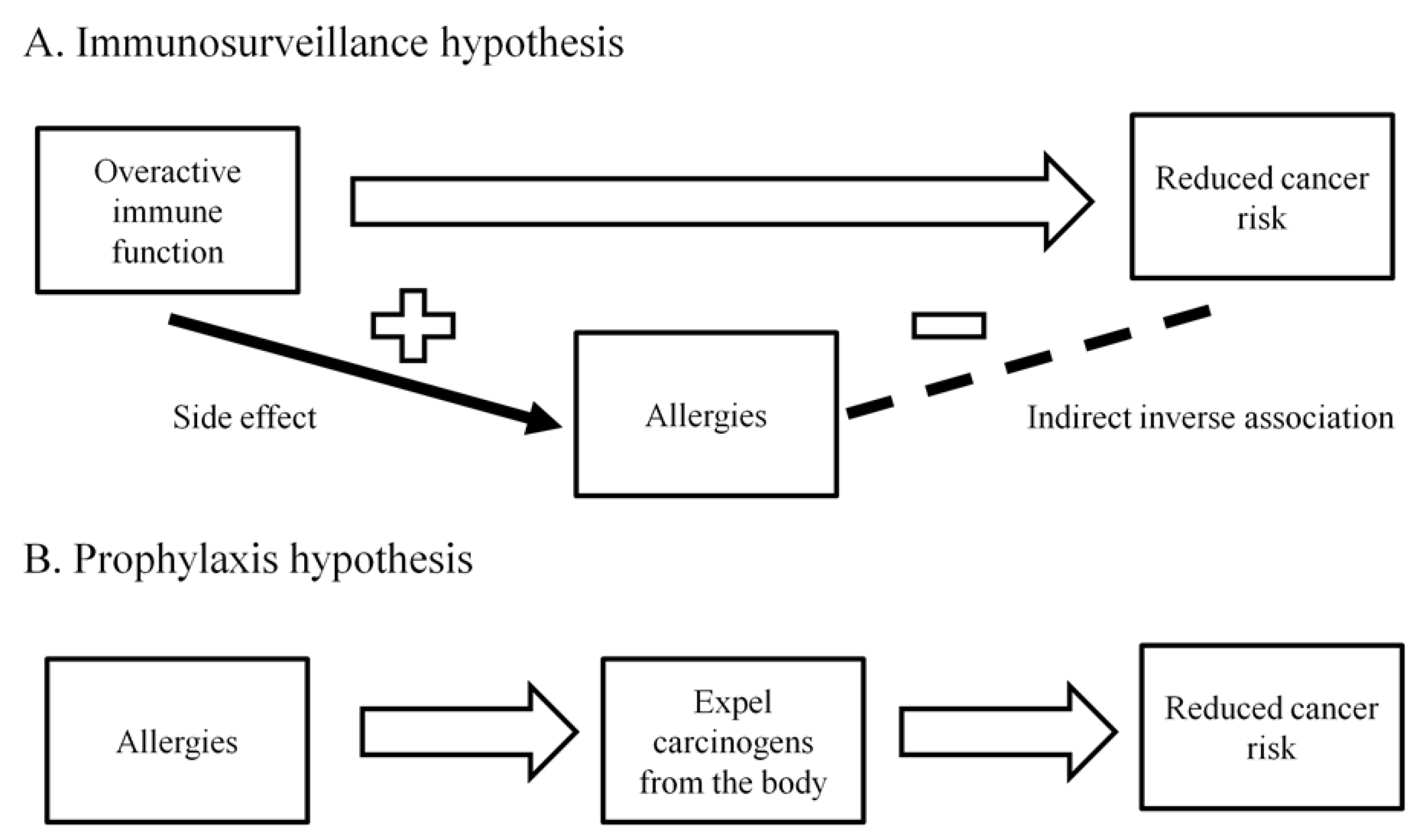
3.3. Allergies
3.4. Infections
4. Incorporation of Environmental Risk Factors for Constructing Pancreatic Cancer Risk Prediction Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CI | confidence interval; |
| IPMN | intraductal papillary mucinous neoplasm; |
| HR | hazard ratio; |
| OS | overall survival; |
| OR | odds ratio; |
| RR | relative risk; |
| SNP | Single nucleotide polymorphism |
References
- Cancer Today: Data visualization tool for exploring the global cancer burden in 2018. International Agency for Research on Cancer, Lyon France. 2018. Available online: http://gco.iarc.fr/today/home (accessed on 7 November 2018.).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D.; Sitzmann, J.V.; Hruban, R.H.; Goodman, S.N.; Dooley, W.C.; Coleman, J.; Pitt, H.A. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Annals. Surg. 1995, 221, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet (London, England) 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Ueno, H.; Ioka, T.; Ikeda, M.; Ohkawa, S.; Yanagimoto, H.; Boku, N.; Fukutomi, A.; Sugimori, K.; Baba, H.; Yamao, K.; et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J. clin. Oncol. : j. American Soc. Clin. Oncol. 2013, 31, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nature reviews. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Kalser, M.H.; Ellenberg, S.S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch. surg. (Chicago, Ill. : 1960) 1985, 120, 899–903. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. Jama 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Tudur Smith, C.; Bassi, C.; Ghaneh, P.; Owen, E.; Moore, M.; Padbury, R.; Doi, R.; Smith, D.; et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: Composite data from the ESPAC-1 and -3(v1) trials. Br. J. Cancer 2009, 100, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hurt, C.N.; Bridgewater, J.; Falk, S.; Cummins, S.; Wasan, H.; Crosby, T.; Jephcott, C.; Roy, R.; Radhakrishna, G.; et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): A multicentre, randomised, phase 2 trial. Lancet 2013, 14, 317–326. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Maggino, L.; Malleo, G.; Marchegiani, G.; Viviani, E.; Nessi, C.; Ciprani, D.; Esposito, A.; Landoni, L.; Casetti, L.; Tuveri, M.; et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA surg. 2019, 10.1001/jamasurg.2019.2277. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Kamei, K.; Omae, K.; Suzuki, S.; Matsuoka, H.; Mizuno, N.; Ozaka, M.; Ueno, H.; Kobayashi, S.; Uesugi, K.; et al. FOLFIRINOX for locally advanced pancreatic cancer: Results and prognostic factors of subset analysis from a nation-wide multicenter observational study in Japan. Pancreatology 2019, 19, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; Marsh Rde, W.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA surg. 2016, 151, e161137. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Stat Fact Sheets: Pancreas Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 6 September 2019).
- Pannala, R.; Basu, A.; Petersen, G.M.; Chari, S.T. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet. Oncol. 2009, 10, 88–95. [Google Scholar] [CrossRef]
- Raimondi, S.; Maisonneuve, P.; Lowenfels, A.B. Epidemiology of pancreatic cancer: An overview. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 699–708. [Google Scholar] [CrossRef]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef]
- Lynch, S.M.; Vrieling, A.; Lubin, J.H.; Kraft, P.; Mendelsohn, J.B.; Hartge, P.; Canzian, F.; Steplowski, E.; Arslan, A.A.; Gross, M.; et al. Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am. J. Epidemiol. 2009, 170, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.; Peveri, G.; Bosetti, C.; Bagnardi, V.; Crippa, A.; Orsini, N.; Rota, M.; Gallus, S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur. J. Cancer 2018, 104, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; La Vecchia, C.; Silverman, D.T.; Petersen, G.M.; Bracci, P.M.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; Gallinger, S.; et al. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 2011, 22, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wellenius, G.A.; Michaud, D.S. Environmental tobacco smoke and the risk of pancreatic cancer among non-smokers: A meta-analysis. Occup. Environ. Med. 2012, 69, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bergkvist, L.; Bernstein, L.; van den Brandt, P.A.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; Giles, G.G.; et al. Alcohol intake and pancreatic cancer risk: A pooled analysis of fourteen cohort studies. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Lucenteforte, E.; La Vecchia, C.; Silverman, D.; Petersen, G.M.; Bracci, P.M.; Ji, B.T.; Bosetti, C.; Li, D.; Gallinger, S.; Miller, A.B.; et al. Alcohol consumption and pancreatic cancer: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 2012, 23, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Gou, Y.W.; Jin, W.W.; Xiao, M.; Fang, H.Y. Association between alcohol intake and the risk of pancreatic cancer: A dose-response meta-analysis of cohort studies. BMC Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Hsiao, J.R.; Chen, C.H. ALDH2 polymorphism and alcohol-related cancers in Asians: A public health perspective. J. Biomed. Sci. 2017, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Kanda, J.; Matsuo, K.; Suzuki, T.; Kawase, T.; Hiraki, A.; Watanabe, M.; Mizuno, N.; Sawaki, A.; Yamao, K.; Tajima, K.; et al. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci. 2009, 100, 296–302. [Google Scholar] [CrossRef]
- Chan, J.M.; Gong, Z.; Holly, E.A.; Bracci, P.M. Dietary patterns and risk of pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area. Nutr. Cancer 2013, 65, 157–164. [Google Scholar] [CrossRef]
- Bosetti, C.; Bravi, F.; Turati, F.; Edefonti, V.; Polesel, J.; Decarli, A.; Negri, E.; Talamini, R.; Franceschi, S.; La Vecchia, C.; et al. Nutrient-based dietary patterns and pancreatic cancer risk. Ann. Epidemiol. 2013, 23, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; Sinha, R.; et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J. Gastrointest. Cancer 2013, 44, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Wu, L.; Zheng, L.Q.; Xu, X.; Ji, C.; Gong, T.T. Consumption of fruit and vegetables reduces risk of pancreatic cancer: Evidence from epidemiological studies. Eur. J. Cancer Prev. 2016, 25, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Taunk, P.; Hecht, E.; Stolzenberg-Solomon, R. Are meat and heme iron intake associated with pancreatic cancer? Results from the NIH-AARP diet and health cohort. Int. J. Cancer 2016, 138, 2172–2189. [Google Scholar] [CrossRef] [PubMed]
- Casari, I.; Falasca, M. Diet and Pancreatic Cancer Prevention. Cancers 2015, 7, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M.; Casari, I.; Maffucci, T. Cancer chemoprevention with nuts. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Lin, Y.W.; Chen, H.; Qin, J.; Zheng, X.Y.; Xu, X.; Xie, L.P. Dietary fiber intake is inversely associated with risk of pancreatic cancer: A meta-analysis. Asia Pac. J. Clin. Nutr. 2017, 26, 89–96. [Google Scholar] [CrossRef] [PubMed]
- O'Rorke, M.A.; Cantwell, M.M.; Cardwell, C.R.; Mulholland, H.G.; Murray, L.J. Can physical activity modulate pancreatic cancer risk? a systematic review and meta-analysis. Int. J. Cancer 2010, 126, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Farris, M.S.; Mosli, M.H.; McFadden, A.A.; Friedenreich, C.M.; Brenner, D.R. The Association between Leisure Time Physical Activity and Pancreatic Cancer Risk in Adults: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1462–1473. [Google Scholar] [CrossRef]
- Keum, N.; Bao, Y.; Smith-Warner, S.A.; Orav, J.; Wu, K.; Fuchs, C.S.; Giovannucci, E.L. Association of Physical Activity by Type and Intensity With Digestive System Cancer Risk. JAMA oncology 2016, 2, 1146–1153. [Google Scholar] [CrossRef]
- Noor, N.M.; Banim, P.J.; Luben, R.N.; Khaw, K.T.; Hart, A.R. Investigating Physical Activity in the Etiology of Pancreatic Cancer: The Age at Which This Is Measured Is Important and Is Independent of Body Mass Index. Pancreas 2016, 45, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zheng, W.; Xiang, Y.B.; Gao, Y.T.; Li, H.L.; Cai, H.; Shu, X.O. Physical Activity and Pancreatic Cancer Risk among Urban Chinese: Results from Two Prospective Cohort Studies. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.A.; Helzlsouer, K.J.; Kooperberg, C.; Shu, X.O.; Steplowski, E.; Bueno-de-Mesquita, H.B.; Fuchs, C.S.; Gross, M.D.; Jacobs, E.J.; Lacroix, A.Z.; et al. Anthropometric measures, body mass index, and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch. Intern. Med. 2010, 170, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Greenwood, D.C.; Chan, D.S.; Vieira, R.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Cade, J.E.; Burley, V.J.; Norat, T. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Kitahara, C.M.; Bernstein, L.; Berrington de Gonzalez, A.; Brotzman, M.; Elena, J.W.; Giles, G.G.; Hartge, P.; Singh, P.N.; Stolzenberg-Solomon, R.Z.; et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann. Oncol. 2015, 26, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, Y.N.; Matsuo, K.; Ito, H.; Tamakoshi, A.; Sugawara, Y.; Hidaka, A.; Wada, K.; Oze, I.; Kitamura, Y.; Liu, R.; et al. Body-Mass Index and Pancreatic Cancer Incidence: A Pooled Analysis of Nine Population-Based Cohort Studies With More Than 340,000 Japanese Subjects. J. Epidemiol. 2018, 28, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zohar, L.; Rottenberg, Y.; Twig, G.; Katz, L.; Leiba, A.; Derazne, E.; Tzur, D.; Eizenstein, S.; Keinan-Boker, L.; Afek, A.; et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: A nationwide study of 1.79 million Israeli adolescents. Cancer 2019, 125, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Thandra, K.C.; Sunkara, T. Pancreatic cancer and obesity: Epidemiology, mechanism, and preventive strategies. Clin. J. Gastroenterol. 2019. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Dodd, K.W.; Blaser, M.J.; Virtamo, J.; Taylor, P.R.; Albanes, D. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am. J. Clin. Nutr. 2003, 78, 176–181. [Google Scholar] [CrossRef]
- Gerlovin, H.; Michaud, D.S.; Cozier, Y.C.; Palmer, J.R. Oral Health in Relation to Pancreatic Cancer Risk in African American Women. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjonneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2016. [Google Scholar]
- Olson, S.H.; Satagopan, J.; Xu, Y.; Ling, L.; Leong, S.; Orlow, I.; Saldia, A.; Li, P.; Nunes, P.; Madonia, V.; et al. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: A pilot study. Cancer Causes Control. 2017, 28, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, C.; Gudino, C.V.; Gibson, F.C., 3rd; Genco, C.A. Review: Pathogen-induced inflammation at sites distant from oral infection: Bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol. Oral. Microbiol. 2010, 25, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Mendez, R.; Kesh, K.; Arora, N.; Di Martino, L.; McAllister, F.; Merchant, N.; Banerjee, S.; Banerjee, S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis 2019, 10.1093/carcin/bgz116. [Google Scholar] [CrossRef]
- Archibugi, L.; Signoretti, M.; Capurso, G. The Microbiome and Pancreatic Cancer: An Evidence-based Association? J. Clin. Gastroenterol. 2018. [Google Scholar] [CrossRef]
- Zhou, C.D.; Kuan, A.S.; Reeves, G.K.; Green, J.; Floud, S.; Beral, V.; Yang, T.O. Coffee and pancreatic cancer risk among never-smokers in the UK prospective Million Women Study. Int. J. Cancer 2019, 145, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Guertin, K.A.; Freedman, N.D.; Loftfield, E.; Stolzenberg-Solomon, R.Z.; Graubard, B.I.; Sinha, R. A prospective study of coffee intake and pancreatic cancer: Results from the NIH-AARP Diet and Health Study. Br. J. Cancer 2015, 113, 1081–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bosetti, C.; Rosato, V.; Li, D.; Silverman, D.; Petersen, G.M.; Bracci, P.M.; Neale, R.E.; Muscat, J.; Anderson, K.; Gallinger, S.; et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2015, 25, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Bragg, F.; Yang, L.; Bian, Z.; Chen, Y.; Iona, A.; Millwood, I.Y.; Lv, J.; et al. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int. J. Cancer 2017, 140, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M. Pancreatic cancer-associated diabetes mellitus: Prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008, 134, 95–101. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Stram, D.O.; Porcel, J.; Chari, S.T.; Maskarinec, G.; Le Marchand, L.; Wilkens, L.R.; Haiman, C.A.; Pandol, S.J.; Monroe, K.R. Pancreatic Cancer Following Incident Diabetes in African Americans and Latinos: The Multiethnic Cohort. J. Natl. Cancer Inst. 2019, 111, 27–33. [Google Scholar] [CrossRef]
- Kirkegard, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Gandini, S.; Lowenfels, A.B.; Jaffee, E.M.; Armstrong, T.D.; Maisonneuve, P. Allergies and the risk of pancreatic cancer: A meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1908–1916. [Google Scholar] [CrossRef]
- Olson, S.H.; Hsu, M.; Satagopan, J.M.; Maisonneuve, P.; Silverman, D.T.; Lucenteforte, E.; Anderson, K.E.; Borgida, A.; Bracci, P.M.; Bueno-de-Mesquita, H.B.; et al. Allergies and risk of pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case-Control Consortium. Am. J. Epidemiol. 2013, 178, 691–700. [Google Scholar] [CrossRef]
- Huang, B.Z.; Le Marchand, L.; Haiman, C.A.; Monroe, K.R.; Wilkens, L.R.; Zhang, Z.F.; Setiawan, V.W. Atopic allergic conditions and pancreatic cancer risk: Results from the Multiethnic Cohort Study. Int. J. Cancer 2018, 142, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Sherman, P.W.; Holland, E.; Sherman, J.S. Allergies: Their role in cancer prevention. Q. Rev. Biol. 2008, 83, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Profet, M. The function of allergy: Immunological defense against toxins. Q. Rev. Biol. 1991, 66, 23–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Fu, J.J.; Wang, X.L.; Zhu, J.Y.; Ye, X.H.; Chen, S.D. Hepatitis B or C viral infection and risk of pancreatic cancer: A meta-analysis of observational studies. World J. Gastroenterol. 2013, 19, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Krull Abe, S.; Inoue, M.; Sawada, N.; Iwasaki, M.; Shimazu, T.; Yamaji, T.; Sasazuki, S.; Saito, E.; Tanaka, Y.; Mizokami, M.; et al. Hepatitis B and C Virus Infection and Risk of Pancreatic Cancer: A Population-Based Cohort Study (JPHC Study Cohort II). Cancer Epidemiol. Biomarkers Prev. 2016, 25, 555–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamiza, A.B.; Su, F.H.; Wang, W.C.; Sung, F.C.; Chang, S.N.; Yeh, C.C. Chronic hepatitis infection is associated with extrahepatic cancer development: A nationwide population-based study in Taiwan. BMC Cancer 2016, 16, 861. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chen, C.H.; Liang, J.D.; Tien, Y.W.; Hsu, C.; Wong, J.M.; Chang, Y.T. Hepatitis B and C viruses are not risks for pancreatic adenocarcinoma. World J. Gastroenterol. 2014, 20, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.D.; Tong, X.; Moorman, A.C.; Ly, K.N.; Rupp, L.; Xu, F.; Gordon, S.C.; Holmberg, S.D. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J. Hepatol. 2015, 63, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Pandeya, N.; Fawcett, J.; Fritschi, L.; Risch, H.A.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Association between Helicobacter pylori and pancreatic cancer risk: A meta-analysis. Cancer Causes Control. 2015, 26, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zagai, U.; Hallmans, G.; Nyren, O.; Engstrand, L.; Stolzenberg-Solomon, R.; Duell, E.J.; Overvad, K.; Katzke, V.A.; Kaaks, R.; et al. Helicobacter pylori infection, chronic corpus atrophic gastritis and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort: A nested case-control study. Int. J. Cancer 2017, 140, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P.; Lindstrom, S.; Mendelsohn, J.B.; Steplowski, E.; Arslan, A.A.; Bueno-de-Mesquita, H.B.; Fuchs, C.S.; Gallinger, S.; Gross, M.; Helzlsouer, K.; et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS ONE 2013, 8, e72311. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A.; Yu, H.; Lu, L.; Kidd, M.S. Detectable Symptomatology Preceding the Diagnosis of Pancreatic Cancer and Absolute Risk of Pancreatic Cancer Diagnosis. Am. J. Epidemiol. 2015, 182, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Woo, S.M.; Joo, J.; Yang, H.R.; Lee, W.J.; Park, S.J.; Nam, B.H. Development and Validation of a Prediction Model to Estimate Individual Risk of Pancreatic Cancer. PLoS ONE 2016, 11, e0146473. [Google Scholar] [CrossRef] [PubMed]
- Nakatochi, M.; Lin, Y.; Ito, H.; Hara, K.; Kinoshita, F.; Kobayashi, Y.; Ishii, H.; Ozaka, M.; Sasaki, T.; Sasahira, N.; et al. Prediction model for pancreatic cancer risk in the general Japanese population. PLoS ONE 2018, 13, e0203386. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Finkelman, B.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.X. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients With New-Onset Diabetes. Gastroenterology 2017, 152, 840–850 e843. [Google Scholar] [CrossRef] [PubMed]


| The Level of Evidence | Risk Factors |
|---|---|
| Very strong | Cigarette smoking, chronic diabetes, obesity |
| Strong | Poor oral health/oral hygiene, chronic pancreatitis, no history of allergies, heavy alcohol drinking, dietary patterns with low amount of vegetables and fruits |
| Moderate, although further investigations are required | Dietary patterns rich in meat and animal products, oral/gut microbiome, hepatitis B or C infection |
| The level of evidence is unclear, further investigations are required | Environmental tobacco smoke, light to moderate alcohol drinking, physical inactivity, coffee, Helicobacer pylori infection |
| Environmental Factors | Key Points |
|---|---|
| Cigarette smoking | Cigarette smoking is an established risk factor of pancreatic cancer. Smoking cessation can reverse the increased risk of pancreatic cancer associated with cigarette smoking |
| Alcohol drinking | Heavy alcohol use is associated with an increased pancreatic cancer risk. The association between low to moderate level of alcohol use and pancreatic cancer is unclear. |
| Diet | Diet rich in fruits and vegetables and other plant-based foods has been associated with a reduced pancreatic cancer risk |
| Physical activities | The role of physical activities in preventing the occurrence of pancreatic cancer is inconclusive, although they may possibly reduce the pancreatic cancer risk by preventing obesity. |
| Obesity | Obesity is an established risk factor of pancreatic cancer, although biological mechanisms are yet to be delineated |
| Oral health/hygiene and oral microbiome | Poor oral health/hygiene has consistently been associated with an increased pancreatic cancer risk. Poor oral health/hygiene may affect oral microbiome by promoting the growth of pathogenic oral bacteria, which may increased the risk of pancreatic cancer by inducing inflammation and other mechanisms. More investigations are needed for the role of oral microbiome in the development of pancreatic cancer |
| Gut microbiome | The current information regarding the association between gut microbiome and pancreatic cancer is very limited and more investigations are required |
| Coffee | The association between coffee and pancreatic cancer is unclear |
| Diabetes | Diabetes can be both a risk factor and a consequence of pancreatic cancer |
| Chronic pancreatitis | Chronic pancreatitis is a known risk factor of pancreatic cancer with a lifetime risk of 5% |
| Allergies | Allergies are associated with a reduced pancreatic cancer risk, although the biological mechanisms are unclear |
| Infections | The majority of studies suggested that hepatitis B or C infection may be associated with an increased pancreatic cancer risk. More studies on the association between the different strains of H. Pylori and pancreatic cancer are needed to confirm the strain-specific association between H. Pylori and pancreatic cancer. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-J.; Chang, J.S. Environmental Risk Factors of Pancreatic Cancer. J. Clin. Med. 2019, 8, 1427. https://doi.org/10.3390/jcm8091427
Tsai H-J, Chang JS. Environmental Risk Factors of Pancreatic Cancer. Journal of Clinical Medicine. 2019; 8(9):1427. https://doi.org/10.3390/jcm8091427
Chicago/Turabian StyleTsai, Hui-Jen, and Jeffrey S. Chang. 2019. "Environmental Risk Factors of Pancreatic Cancer" Journal of Clinical Medicine 8, no. 9: 1427. https://doi.org/10.3390/jcm8091427
APA StyleTsai, H.-J., & Chang, J. S. (2019). Environmental Risk Factors of Pancreatic Cancer. Journal of Clinical Medicine, 8(9), 1427. https://doi.org/10.3390/jcm8091427




