The Risk for Glucose Intolerance after Gestational Diabetes Mellitus since the Introduction of the IADPSG Criteria: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Data Sources and Searches
2.3. Study Selection
2.4. Data Extraction
2.5. Outcomes
2.6. Data Synthesis and Analysis
2.7. Quality Assessment
3. Results
3.1. Search Results
3.2. Study Characteristics
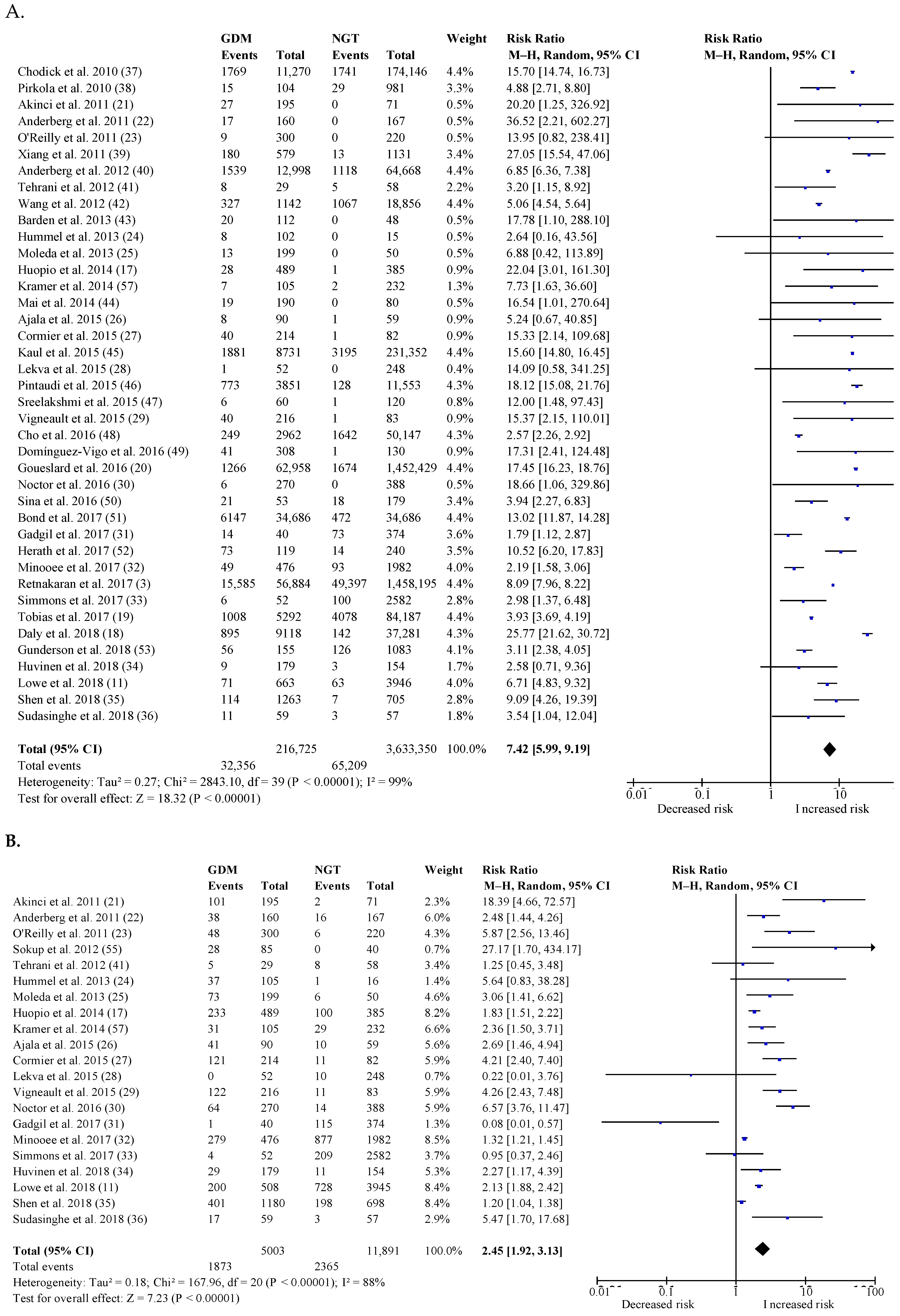
3.3. Postpartum Risk of Type 2 Diabetes
3.4. Postpartum Risk of Impaired Glucose Tolerance
3.5. Sensitivity Analyses
3.6. Postpartum Risk of Cardiovascular Events
3.7. Publication Bias
3.8. Assessment of Quality
4. Discussion
4.1. Summary of Findings
4.2. Results in Relation to What We Already Know
4.3. Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S165–S172. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shah, B.R. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women With Previous Gestational Diabetes Mellitus. Diabetes Care 2017, 40, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; de Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30 (Suppl. 2), S251–S260. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- International Association of Diabetes; Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar]
- Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pr. 2014, 103, 341–363. [CrossRef]
- Song, C.; Lyu, Y.; Li, C.; Liu, P.; Li, J.; Ma, R.C.; Yang, X. Long-term risk of diabetes in women at varying durations after gestational diabetes: A systematic review and meta-analysis with more than 2 million women. Obes. Rev. 2018, 19, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Jegers, K.; Devlieger, R.; Verhaeghe, J.; Mathieu, C. Glucose Intolerance after a Recent History of Gestational Diabetes Based on the 2013 WHO Criteria. PLoS ONE 2016, 11, e0157272. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; de Block, C.; et al. Prediction of Glucose Intolerance in Early Postpartum in Women with Gestational Diabetes Mellitus Based on the 2013 WHO Criteria. J. Clin. Med. 2019, 8, 383. [Google Scholar] [CrossRef]
- Lowe, W.L., Jr.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 October 2018).
- Huopio, H.; Hakkarainen, H.; Paakkonen, M.; Kuulasmaa, T.; Voutilainen, R.; Heinonen, S.; Cederberg, H. Long-term changes in glucose metabolism after gestational diabetes: A double cohort study. BMC Pregnancy Childbirth 2014, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Toulis, K.A.; Thomas, N.; Gokhale, K.; Martin, J.; Webber, J.; Keerthy, D.; Jolly, K.; Saravanan, P.; Nirantharakumar, K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018, 15, e1002488. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Stuart, J.J.; Li, S.; Chavarro, J.; Rimm, E.B.; Rich-Edwards, J.; Hu, F.B.; Manson, J.E.; Zhang, C. Association of History of Gestational Diabetes With Long-term Cardiovascular Disease Risk in a Large Prospective Cohort of US Women. JAMA Intern. Med. 2017, 177, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Goueslard, K.; Cottenet, J.; Mariet, A.S.; Giroud, M.; Cottin, Y.; Petit, J.M.; Quantin, C. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Celtik, A.; Genc, S.; Yener, S.; Demir, T.; Secil, M.; Kebapcilar, L.; Yesil, S. Evaluation of postpartum carbohydrate intolerance and cardiovascular risk factors in women with gestational diabetes. Gynecol. Endocrinol. 2011, 27, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, E.; Landin-Olsson, M.; Kalen, J.; Frid, A.; Ursing, D.; Berntorp, K. Prevalence of impaired glucose tolerance and diabetes after gestational diabetes mellitus comparing different cut-off criteria for abnormal glucose tolerance during pregnancy. Acta Obstet. Gynecol. Scand. 2011, 90, 1252–1258. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Avalos, G.; Dennedy, M.C.; O’Sullivan, E.P.; Dunne, F. Atlantic DIP: High prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus. Eur. J. Endocrinol. 2011, 165, 953–959. [Google Scholar] [CrossRef]
- Hummel, S.; Much, D.; Rossbauer, M.; Ziegler, A.G.; Beyerlein, A. Postpartum outcomes in women with gestational diabetes and their offspring: POGO study design and first-year results. Rev. Diabet. Stud. 2013, 10, 49–57. [Google Scholar] [CrossRef]
- Moleda, P.; Homa, K.; Safranow, K.; Celewicz, Z.; Fronczyk, A.; Majkowska, L. Women with normal glucose tolerance and a history of gestational diabetes show significant impairment of beta-cell function at normal insulin sensitivity. Diabetes Metab. 2013, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ajala, O.; Jensen, L.A.; Ryan, E.; Chik, C. Women with a history of gestational diabetes on long-term follow up have normal vascular function despite more dysglycemia, dyslipidemia and adiposity. Diabetes Res. Clin. Pract. 2015, 110, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cormier, H.; Vigneault, J.; Garneau, V.; Tchernof, A.; Vohl, M.C.; Weisnagel, S.J.; Robitaille, J. An explained variance-based genetic risk score associated with gestational diabetes antecedent and with progression to pre-diabetes and type 2 diabetes: A cohort study. BJOG Int. J. Obs. Gynaecol. 2015, 122, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lekva, T.; Bollerslev, J.; Godang, K.; Roland, M.C.; Friis, C.M.; Voldner, N.; Henriksen, T.; Ueland, T. beta-cell dysfunction in women with previous gestational diabetes is associated with visceral adipose tissue distribution. Eur. J. Endocrinol. 2015, 173, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, J.; Lemieux, S.; Garneau, V.; Weisnagel, S.J.; Tchernof, A.; Robitaille, J. Association between metabolic deteriorations and prior gestational diabetes according to weight status. Obesity 2015, 23, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Noctor, E.; Crowe, C.; Carmody, L.A.; Saunders, J.A.; Kirwan, B.; O’Dea, A.; Gillespie, P.; Glynn, L.G.; McGuire, B.E.; O’Neill, C.; et al. Abnormal glucose tolerance post-gestational diabetes mellitus as defined by the International Association of Diabetes and Pregnancy Study Groups criteria. Eur. J. Endocrinol. 2016, 175, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, M.D.; Oza-Frank, R.; Kandula, N.R.; Kanaya, A.M. Type 2 diabetes after gestational diabetes mellitus in South Asian women in the United States. Diabetes Metabzolism Res. Rev. 2017, 33, e2891. [Google Scholar] [CrossRef]
- Minooee, S.; Ramezani Tehrani, F.; Rahmati, M.; Mansournia, M.A.; Azizi, F. Diabetes incidence and influencing factors in women with and without gestational diabetes mellitus: A 15 year population-based follow-up cohort study. Diabetes Res. Clin. Pract. 2017, 128, 24–31. [Google Scholar] [CrossRef]
- Simmons, D.; Kumar, S.; Crook, N.; Rush, E. Diabetes among Maori women with self-reported past gestational diabetes mellitus in a New Zealand Maori community. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 599–603. [Google Scholar] [CrossRef]
- Huvinen, E.; Eriksson, J.G.; Koivusalo, S.B.; Grotenfelt, N.; Tiitinen, A.; Stach-Lempinen, B.; Rönö, K. Heterogeneity of gestational diabetes (GDM) and long-term risk of diabetes and metabolic syndrome: Findings from the RADIEL study follow-up. Acta Diabetol. 2018, 55, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, P.; Wang, L.; Zhang, S.; Liu, H.; Li, W.; Li, N.; Li, W.; Leng, J.; Wang, J.; et al. Gestational diabetes with diabetes and prediabetes risks: A large observational study. Eur. J. Endocrinol. 2018, 179, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sudasinghe, B.H.; Wijeyaratne, C.N.; Ginige, P.S. Long and short-term outcomes of Gestational Diabetes Mellitus (GDM) among South Asian women-A community-based study. Diabetes Res. Clin. Pract. 2018, 145, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chodick, G.; Elchalal, U.; Sella, T.; Heymann, A.D.; Porath, A.; Kokia, E.; Shalev, V. The risk of overt diabetes mellitus among women with gestational diabetes: A population-based study. Diabet. Med. J. Br. Diabet. Assoc. 2010, 27, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Pirkola, J.; Pouta, A.; Bloigu, A.; Miettola, S.; Hartikainen, A.L.; Jarvelin, M.R.; Vääräsmäki, M. Prepregnancy overweight and gestational diabetes as determinants of subsequent diabetes and hypertension after 20-year follow-up. J. Clin. Endocrinol. Metab. 2010, 95, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.H.; Li, B.H.; Black, M.H.; Sacks, D.A.; Buchanan, T.A.; Jacobsen, S.J.; Lawrence, J.M. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia 2011, 54, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, E.; Carlsson, K.S.; Berntorp, K. Use of healthcare resources after gestational diabetes mellitus: A longitudinal case-control analysis. Scand. J. Public Health 2012, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.R.; Hashemi, S.; Hasheminia, M.; Azizi, F. Follow-up of women with gestational diabetes in the Tehran Lipid and Glucose Study (TLGS): A population-based cohort study. J. Obstet. Gynaecol. Res. 2012, 38, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Horswell, R.; Xiao, K.; Besse, J.; Johnson, J.; Ryan, D.H.; Hu, G. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J. Women’s Health 2012, 21, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Singh, R.; Walters, B.; Phillips, M.; Beilin, L.J. A simple scoring method using cardiometabolic risk measurements in pregnancy to determine 10-year risk of type 2 diabetes in women with gestational diabetes. Nutr. Diabetes 2013, 3, e72. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.; Wang, B.; Wen, J.; Lin, X.; Niu, J. Lipoprotein-associated phospholipase A2 and AGEs are associated with cardiovascular risk factors in women with history of gestational diabetes mellitus. Gynecol. Endocrinol. 2014, 30, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Kaul, P.; Savu, A.; Nerenberg, K.A.; Donovan, L.E.; Chik, C.L.; Ryan, E.A.; Johnson, J.A. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: A population-level analysis. Diabet. Med. 2015, 32, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Pintaudi, B.; Lucisano, G.; Pellegrini, F.; D’Ettorre, A.; Lepore, V.; De Berardis, G.; Scardapane, M.; Di Vieste, G.; Rossi, M.C.; Sacco, M.; et al. The long-term effects of stillbirth on women with and without gestational diabetes: A population-based cohort study. Diabetologia 2015, 58, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sreelakshmi, P.R.; Nair, S.; Soman, B.; Alex, R.; Vijayakumar, K.; Kutty, V.R. Maternal and neonatal outcomes of gestational diabetes: A retrospective cohort study from Southern India. J. Fam. Med. Prim. Care 2015, 4, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.J.; Park, J.H.; Lee, H.; Yoo, S.; Shin, S.A.; Oh, M.J. Prepregnancy Factors as Determinants of the Development of Diabetes Mellitus After First Pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 2923–2930. [Google Scholar] [PubMed]
- Dominguez-Vigo, P.; Alvarez-Silvares, E.; Alves-Perez, M.T.; Dominguez-Sanchez, J.; Gonzalez-Gonzalez, A. Incidence and clinical risk factors for the development of diabetes mellitus in women with previous gestational diabetes. Ginecol. Obstet. Mex. 2016, 84, 228–242. [Google Scholar]
- Sina, M.; Hoy, W.E.; Wang, Z. Gestational diabetes and the risk of subsequent Type 2 diabetes in Australian Aboriginal women living in a remote community. Diabet. Med. 2016, 33, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Pace, R.; Rahme, E.; Dasgupta, K. Diabetes risk in women with gestational diabetes mellitus and a history of polycystic ovary syndrome: A retrospective cohort study. Diabet. Med. 2017, 34, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.; Herath, R.; Wickremasinghe, R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women-A community based retrospective cohort study. PLoS ONE 2017, 12, e0179647. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Lewis, C.E.; Lin, Y.; Sorel, M.; Gross, M.; Sidney, S.; Jacobs, D.R., Jr.; Shikany, J.M.; Quesenberry, C.P., Jr. Lactation Duration and Progression to Diabetes in Women Across the Childbearing Years: The 30-Year CARDIA Study. JAMA Intern. Med. 2018, 178, 328–337. [Google Scholar] [CrossRef]
- Freibert, S.M.; Mannino, D.M.; Bush, H.; Crofford, L.J. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J. Women’s Health 2011, 20, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sokup, A.; Ruszkowska, B.; Goralczyk, B.; Goralczyk, K.; Szymanski, M.; Grabiec, M.; Rość, D. Elevation of sE-Selectin Levels 2-24 Months following Gestational Diabetes Is Associated with Early Cardiometabolic Risk in Nondiabetic Women. Int. J. Endocrinol. 2012, 2012, 278050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKenzie-Sampson, S.; Paradis, G.; Healy-Profitos, J.; St-Pierre, F.; Auger, N. Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: A retrospective cohort study. Acta Diabetol. 2018, 55, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Swaminathan, B.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of beta-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care 2014, 37, 3262–3269. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Mathieu, C.; Damm, P.; Van Assche, A.; Devlieger, R.; Desoye, G.; Corcoy, R.; Mahmood, T.; Nizard, J.; Savona-Ventura, C.; et al. A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe: An opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG). Diabetologia 2015, 58, 1422–1429. [Google Scholar] [PubMed]


| A. | ||||||||
| Study Type, Country | Ethnic Origin | Age at Pregnancy, yr Age at Follow-Up, yr (± SD or IQR) | BMI at Pregnancy, kg/m2 BMI at Follow-Up, kg/m2 (± SD or IQR) | Definition NGT Group | Duration of Follow-Up (± SD or IQR) | |||
| Chodick et al. 2010 [37] | Retrospective cohort study, Israel | NR | NR 32.7 ± 5.5 | NR 63.2% < 25 | No matched controls | 5.7 (± 4.0) | ||
| Pirkola et al. 2010 [38] | Prospective cohort study, Finland | NR | NR NR | NR NR | No matched controls | 20.0 | ||
| Akinci et al. 2011 [21] | Case-control study, Turkey | Caucasian | NR 31.9 ± 5.3 | NR 27.1 ± 5.4 | Controls (hospital staff) matched for age, and time period of pregnancy | 3.4 (± 1.9) | ||
| Anderberg et al. 2011 [22] | Prospective cohort study, Sweden | Mixed | 33.1 ± 4.9 NR | NR NR | Controls matched by random sampling for residency | 1.3 (1.1–1.6) | ||
| Freibert et al. 2011 [54] | Cross-sectional study, USA (Kentucky) | NR | NR 57.1 ± 5.5 | NR NR | No matched controls, women ≥ 50 years | NR | ||
| O’Reilly et al. 2011 [23] | Prospective cohort study, Ireland | Caucasian | NR 33.5 ± 4.7 | NR NR | Controls matched for residency, and time period of pregnancy | 0.23 | ||
| Xiang et al. 2011 [39] | Retrospective cohort study, USA (California) | Mixed | NR 32.4 ± 5.2 | NR NR | Five matched controls for each GDM by random sampling for ethnicity, age, and calendar year of study entry | 3.9–5.2 (IQR NR) | ||
| Anderberg et al. 2012 [40] | Case-control study, Sweden | NR | NR NR | NR NR | Two matched controls for each GDM for year of birth, year of delivery, and municipality of residence | 8.0–14.0 | ||
| Sokup et al. 2012 [55] | Cross-sectional study, Poland | Caucasian | NR 29.0 (26.0–35.0) | NR 23.7 (21.0–27.5) | No matched controls | 0.17–2 | ||
| Tehrani et al. 2012 [41] | Case-control study, Iran | Middle-Eastern | NR 33.6 ± 7.5 | NR 30.0 ± 4.7 | Matched controls for age, and BMI from the TLGS cohort | 9.0 | ||
| Wang et al. 2012 [42] | Prospective cohort study, USA (Louisiana) | Mixed | NR 26.8 ± 0.2 | NR 48.2 ± 1.7 | Controls matched for age, and time period of pregnancy | 8.6 (± NR) | ||
| Barden et al. 2013 [43] | Case-control study, Australia | Mixed | 31.3 ± 0.4 (high risk), 33.8 ± 0.5 (low risk) NR | 35.5 ± 5.0 (high risk), 27.3 ± 4.1 (low risk) NR | No matched controls | 10.0 | ||
| Hummel et al. 2013 [24] | Prospective cohort study, Germany | NR | NR | NR NR | No matched controls | 5.5 (IQR NR) | ||
| Moleda et al. 2013 [25] | Prospective cohort study, Poland | Caucasian | 30.8 ± 5.7 NR | 22.4 ± 3.4 25.5 ± 5.6 | Controls matched for age, and time period of delivery | 7.4 (± 0.7) | ||
| Huopio et al. 2014 [17] | Prospective cohort study, Finland | Caucasian | 32.0 ± 5.9 NR | 26.4 ± 4.8 28.4 ± 5.5 | No matched controls | 7.3 (± 5.1) | ||
| McKenzie-Sampson et al. 2018 [56] | Retrospective cohort study, Canada | NR | NR NR | NR NR | No matched controls | NR | ||
| Kramer et al. 2014 [57] | Prospective cohort study, Canada | Mixed | 35 (33–38) NR | NR 25.4 (22.4–30.1) | No matched controls | 2.98 (± NR) | ||
| Mai et al. 2014 [44] | Case-control study, China | NR | NR 33.1 ± 4.8 | NR 22.7 ± 3.5 | No matched controls | 2.5 (± 1.8) | ||
| Ajala et al. 2015 [26] | Retrospective cohort study, Canada | Caucasian | NR 39.2 ± 4.1 | NR 28.9 ± 6.6 | No matched controls | 7.1 (± 1.6) | ||
| Cormier et al. 2015 [27] | Prospective cohort study, Canada | Caucasian | NR 36.4 ± 4.9 | NR 27.7 ± 6.5 | No matched controls | 3.5 (± 2.0) | ||
| Kaul et al. 2015 [45] | Retrospective cohort study, Canada | NR | NR NR | NR NR | No matched controls | 5.3 (2.2–8.4) | ||
| Lekva et al. 2015 [28] | Prospective cohort study, Norway | Caucasian | 33.4 ± 4.54 NR | 28.1 (26.7–30.1) 22.6 (22.4–27.9) | No matched controls | 5.0 | ||
| Pintaudi et al. 2015 [46] | Retrospective cohort study, Italy | NR | NR NR | NR NR | Three matched controls for each GDM for propensity scores | 5.4 (2.9–7.3) | ||
| Sreelakshmi et al. 2015 [47] | Retrospective cohort study, India | Asian | NR NR | NR 24.6 ± 3.9 | No matched controls | 4.0 | ||
| Vigneault et al. 2015 [29] | Cross-sectional study, Canada | Caucasian | NR NR | NR NR | No matched controls | 3.9 (± NR) | ||
| Cho et al. 2016 [48] | Retrospective cohort study, South Korea | NR | NR NR | NR NR | No matched controls | 8.0 | ||
| Domínguez-Vigo et al. 2016 [49] | Case-control study, Spain | NR | 33.8 ± 4.9 NR | NR NR | No matched controls | 12.9 (± 0.4) | ||
| Goueslard et al. 2016 [20] | Retrospective cohort study, France | NR | NR NR | NR NR | No matched controls | 7.0 | ||
| Noctor et al. 2016 [30] | Prospective cohort study, Ireland | Caucasian | 34.0 ± 5.0 NR | 31.3 ± 6.6 29.7 ± 6.9 | No matched controls | 2.6 (± NR) | ||
| Sina et al. 2016 [50] | Prospective cohort study, Australia | Pacific Islanders | 27.0 ± 6.7 NR | NR NR | No matched controls | 11.9 (7.3–17.0) | ||
| Bond et al. 2017 [51] | Retrospective cohort study, Canada | Mixed | NR NR | NR NR | Controls matched for age, birth year, and residency | 12.5 (± 5.6) | ||
| Gadgil et al. 2017 [31] | Cross-sectional study, USA | Asian | NR 51.1 ± 7.0 | NR 26.7 ± 3.8 | No matched controls, no self-reported GDM | NR | ||
| Herath et al. 2017 [52] | Retrospective cohort study, Sri Lanka | Asian | 31.7 ± 5.4 NR | NR NR | No matched controls, no self-reported GDM | 10.9 (± 0.35) | ||
| Minooee et al. 2017 [32] | Prospective cohort study, Iran | NR | NR 36.5 ± 8.0 | NR 28.4 ± 4.5 | No matched controls | 12.1 (8.1–13.5) | ||
| Retnakaran et al. 2017 [3] | Retrospective cohort study, Canada | NR | NR NR | NR NR | No matched controls | 10.0 (IQR NR) | ||
| Simmons et al. 2017 [33] | Cross-sectional study, New Zealand | Pacific Islanders | NR NR | NR NR | No matched controls, no self-reported GDM | NR | ||
| Tobias et al. 2017 [19] | Prospective cohort study, USA | Caucasian | 27.5 ± 4.8 NR | 21.5 ± 3.6 25.8 ± 5.9 | No matched controls, no self-reported GDM | 25.7 (± NR) | ||
| Daly et al. 2018 [18] | Retrospective cohort study, UK | NR | NR NR | NR NR | Four matched controls for each GDM for age, and timing of pregnancy | 2.9 (IQR NR) | ||
| Gunderson et al. 2018 [53] | Prospective cohort study, USA | NR | NR NR | NR NR | No matched controls, no self-reported GDM | 24.7 (± 6.6) | ||
| Huvinen et al. 2018 [34] | Prospective cohort study, Finland | NR | NR NR | NR NR | No matched controls | 5.0 (4.0–6.0) | ||
| Lowe et al. 2018 [11] | Prospective cohort study, International | Mixed | NR 43.6 ± 5.4 | 29.7 ± 5.2 28.9 ± 6.5 | No matched controls | 11.4 (10.6–2.2) | ||
| Shen et al. 2018 [35] | Prospective cohort study, China | NR | NR 30.1 ± 3.5 | NR 24.2 ± 3.9 | No matched controls | 3.5 (± NR) | ||
| Sudasinghe et al. 2018 [36] | Prospective cohort study, Sri Lanka | Asian | NR NR | NR NR | No matched controls | 1.0 | ||
| B. | ||||||||
| Screening Strategy for GDM | Criteria for GDM | Criteria for T2DM | Postpartum Screening Method for T2DM | GDM T2DM/Total NGT T2DM/Total (AR, %) | Criteria for Prediabetes | Postpartum Screening Method for Prediabetes | GDM Prediabetes/Total NGT Prediabetes/Total (AR, %) | |
| Chodick et al. 2010 [37] | Universal two-step screening | CC | NR | NR | 1769/11,270 (15.7) 1741/174,146 (1.0) | - | - | - |
| Pirkola et al. 2010 [38] | Selective screening based on risk factors | NR | NR | NR | 15/104 (14.4) 29/981 (3.0) | - | - | - |
| Akinci et al. 2011 [21] | Universal two-step screening | CC | ADA | OGTT | 27/195 (13.8) 0/71 (0.0) | ADA | OGTT | 101/195 (51.8) 2/71 (2.8) |
| Anderberg et al. 2011 [22] | NR | Local (Swedish) * | WHO | OGTT | WHO | OGTT | ||
| Freibert et al. 2011 [54] | NR | NR | - | - | - | - | - | - |
| O’Reilly et al. 2011 [23] | Universal one-step screening | IADPSG | ADA | OGTT | 9/300 (3.0) 0/220 (0.0) | ADA | OGTT | 48/300 (16.0) 6/220 (2.7) |
| Xiang et al. 2011 [39] | NR | CC | ADA | OGTT or HbA1c | 1539/12,998 (11.8) 1118/64,668 (1.7) | - | - | - |
| Anderberg et al. 2012 [40] | NR | NR | NR | NR | 180/579 (31.1) 13/1131 (1.1) | - | - | - |
| Sokup et al. 2012 [55] | Universal two-step screening | WHO, 1999 | - | - | - | WHO | OGTT | 28/85 (32.9) 0/40 (0.0) |
| Tehrani et al. 2012 [41] | Universal one-step screening | IADPSG | ADA | OGTT | 8/29 (27.6) 5/58 (8.6) | ADA | OGTT | 4/29 (13.8) 9/58 (15.5) |
| Wang et al. 2012 [42] | NR | WHO, 1999 | WHO | OGTT or FPG | 327/1142 (28.6) 1067/18,856 (5.7) | - | - | - |
| Barden et al. 2013 [43] | Universal one-step screening | ADIPS | NR | FPG | 20/112 (17.9) 0/48 (0.0) | - | - | - |
| Hummel et al. 2013 [24] | Selective screening based on risk factors | GDA | GDA | OGTT, FPG or HbA1c | 8/102 (7.8) 0/15 (0.0) | NR | OGTT | 37/105 (35.2) 1/16 (6.3) |
| Moleda et al. 2013 [25] | Universal one-step screening | Local (Polish) ** | WHO | OGTT | 13/199 (6.5) 0/50 (0.0) | WHO | OGTT | 73/199 (36.7) 6/50 (12.0) |
| Huopio et al. 2014 [17] | Universal one-step screening | Local (Finnish) *** | ADA | OGTT | 28/489 (5.7) 1/385 (0.3) | ADA | OGTT | 233/489 (47.6) 100/385 (26.0) |
| Kramer et al. 2014 [57] | Universal two-step screening | NDDG | CDA | OGTT | 7/105 (6.7) 2/232 (0.9) | CDA | OGTT | 31/105 (29.5) 29/232 (12.5) |
| Mai et al. 2014 [44] | NR | ADA | ADA | OGTT | 19/190 (10.0) 0/80 (0.0) | - | - | - |
| Ajala et al. 2015 [26] | Universal two-step screening | CDA | NR | OGTT or FPG | 8/90 (8.9) 1/59 (1.7) | NR | OGTT or FPG | 41/90 (45.6) 10/59 (16.9) |
| Cormier et al. 2015 [27] | NR | NR | CDA | OGTT | 40/214 (18.7) 1/82 (1.2) | CDA | OGTT | 121/214 (56.5) 11/82 (13.4) |
| Kaul et al. 2015 [45] | Universal two-step screening | CDA | NR | NR | 1881/8731 (13.5) 3195/231,352 (1.4) | - | - | - |
| Lekva et al. 2015 [28] | Universal one-step screening | IADPSG | ADA | OGTT | 1/52 (1.9) 0/248 (0.0) | ADA | OGTT | 8/52 (15.4) 10/248 (4.2) |
| Pintaudi et al. 2015 [46] | Selective screening based on risk factors | ADA | NR | NR | 773/3851 (20.1) 128/11,553 (1.1) | - | - | - |
| Sreelakshmi et al. 2015 [47] | NR | NR | NR | NR | 6/60 (10.0) 1/120 (0.8) | - | - | - |
| Vigneault et al. 2015 [29] | NR | NR | CDA | OGTT | 40/216 (10.5) 1/83 (1.2) | CDA | OGTT | 122/216 (56.5) 11/83 (13.3) |
| Cho et al. 2016 [48] | NR | NR | NR | NR | 249/2962 (8.4) 1642/50,147 (3.3) | - | - | - |
| Domínguez-Vigo et al. 2016 [49] | Universal two-step screening | NDDG, 1979 | ADA | NR | 41/308 (13.3) 1/130 (0.8) | - | - | - |
| Goueslard et al. 2016 [20] | NR | NR | NR | NR | 1266/62,958 (2.0) 1674/1,452,429 (0.1) | - | - | - |
| Noctor et al. 2016 [30] | Universal one-step screening | IADPSG | ADA | OGTT | 6/270 (2.2) 0/388 (0.0) | ADA | OGTT | 64/270 (23.7) 14/388 (3.6) |
| Sina et al. 2016 [50] | NR | NR | WHO | NR | 21/53 (39.6) 18/179 (10.1) | - | - | - |
| Bond et al. 2017 [51] | NR | CDA | NR | NR | 6147/34,686 (17.7) 472/34,686 (1.4) | - | - | - |
| Gadgil et al. 2017 [31] | NR | NR | NR | OGTT | 14/40 (35.0) 73/374 (19.5) | NR | OGTT | 11/40 (27.5) 115/374 (30.7) |
| Herath et al. 2017 [52] | NR | WHO, 1999 | WHO | OGTT or FPG | 73/119 (61.3) 14/240 (5.8) | - | - | - |
| Minooee et al. 2017 [32] | Universal one-step screening | WHO, 1999 | ADA | OGTT | 49/476 (10.3) 93/1982 (4.7) | ADA | OGTT | 279/476 (58.6) 877/1982 (44.2) |
| Retnakaran et al. 2017 [3] | Universal two-step screening | CDA | NR | NR | 15,585/56,884 (24.4) 49,397/1,458,195 (3.4) | - | - | - |
| Simmons et al. 2017 [33] | Universal one-step screening | ADIPS | WHO | OGTT | 6/52 (11.5) 100/2582 (3.9) | WHO | OGTT | 4/52 (7.9) 209/2582 (8.1) |
| Tobias et al. 2017 [19] | NR | NR | NR | NR | 1008/5292 (19.0) 4078/84,187 (4.8) | - | - | - |
| Daly et al. 2018 [18] | NR | NR | NR | NR | 895/9118 (9.8) 142/37,281 (0.4) | - | - | - |
| Gunderson et al. 2018 [53] | NR | NR | ADA | OGTT, FPG or HbA1c | 56/155 (36.1) 126/1083 (11.6) | - | - | - |
| Huvinen et al. 2018 [34] | Universal one-step screening | CC | NR | OGTT | 9/179 (5.0) 3/154 (1.9) | NR | OGTT | 29/179 (16.2) 11/154 (7.1) |
| Lowe et al. 2018 [11] | Universal one-step screening | IADPSG | ADA | OGTT | 71/663 (10.7) 63/3946 (1.6) | ADA | OGTT | 200/508 (39.4) 728/3945 (18.5) |
| McKenzie-Sampson et al. 2018 [56] | Universal two-step screening | NR | - | - | - | - | - | - |
| Shen et al. 2018 [35] | Universal two-step screening | WHO, 1999 | ADA | OGTT | 114/1263 (9.0) 7/705 (1.0) | ADA | OGTT | 401/1080 (34.0) 198/698 (28.4) |
| Sudasinghe et al. 2018 [36] | NR | WHO, 1999 | WHO | OGTT | 11/59 (18.6) 3/57 (5.3) | WHO | OGTT | 17/59 (28.9) 3/57 (5.3) |
| C. | ||||||||
| GDM Stroke/Total NGT Stroke/Total (AR, %) | GDM MI/Total NGT MI/Total (AR, %) | |||||||
| Freibert et al. 2011 [54] | - - | 5/146 (3.42) 46/2558 (1.80) | ||||||
| Goueslard et al. 2016 [20] | 71/62,958 (0.11) 1181/1,452,429 (0.08) | 26/62 958 (0.04) 257/1,452,429 (0.02) | ||||||
| Tobias et al. 2017 [19] | 33/5292 (0.62) 520/84,187 (0.62) | 49/5292 (0.93) 563/84,187 (0.67) | ||||||
| Daly et al. 2017 [18] | 14/9118 (0.15) 50/37,281 (0.13) | 14/9118 (0.15) 22/37,281 (0.06) | ||||||
| McKenzie-Sampson et al. 2018 [56] | 181/67,356 (0.27) 2207/1,003,311 (0.22) | 280/67 356 (0.42) 2219/1,003,311 (0.22) | ||||||
| A. | |||||||
| Subgroup | Number of studies | Weight of subjects (%) | Relative risk (95% CI) | p for RR | I2 (%) | τ2 | p for heterogeneity |
| Diagnostic criteria GDM | 0.17 * | ||||||
| IADPSG | 5 | 7.6 | 6.45 (4.74, 8.77) | <0.00001 | 0 | 0.00 | 0.57 |
| Other criteria | 23 | 53.4 | 9.08 (6.96, 11.85) | <0.00001 | 98 | 0.21 | <0.00001 |
| NR | 12 | 39.0 | 6.04 (3.61, 10.10) | <0.00001 | 99 | 0.68 | <0.00001 |
| Screening strategy GDM | 0.09 * | ||||||
| Universal two-step | 8 | 19.7 | 11.56 (7.50, 17.84) | <0.00001 | 99 | 0.21 | 0.0007 |
| Universal one-step | 11 | 18.0 | 4.66 (2.68, 8.10) | <0.00001 | 67 | 0.36 | <0.00001 |
| Selective | 3 | 8.1 | 8.17 (2.45, 27.23) | 0.0006 | 90 | 0.82 | <0.0001 |
| NR | 18 | 54.2 | 7.44 (4.96, 11.15) | <0.00001 | 99 | 0.58 | <0.00001 |
| Follow-up, yr | <0.00001 * | ||||||
| <3 | 7 | 9.5 | 13.15 (5.60, 30.85) | <0.00001 | 51 | 0.54 | 0.05 |
| 3–<6 | 12 | 25.2 | 15.95 (14.53, 17.52) | <0.00001 | 38 | 0.00 | 0.09 |
| 6–<10 | 7 | 17.6 | 6.23 (2.54, 15.26) | <0.0001 | 99 | 1.07 | <0.00001 |
| 10–<15 | 9 | 29.5 | 6.82 (5.33, 8.71) | <0.00001 | 96 | 0.09 | <0.00001 |
| ≥20 | 3 | 11.8 | 3.75 (3.14, 4.48) | <0.00001 | 42 | 0.01 | 0.18 |
| NR | 2 | 6.4 | 2.10 (1.32, 3.32) | 0.002 | 17 | 0.02 | 0.27 |
| Age during pregnancy, yr | <0.00001 * | ||||||
| <30 | 2 | 7.8 | 3.93 (3.69, 4.19) | <0.00001 | 0 | 0.00 | 0.99 |
| 30–<35 | 9 | 9.1 | 11.50 (7.39, 17.90) | <0.00001 | 0 | 0.00 | 0.97 |
| ≥35 | 0 | - | - | - | - | - | - |
| NR | 29 | 83.1 | 7.43 (5.92, 9.32) | <0.00001 | 99 | 0.25 | <0.00001 |
| Age postpartum, yr | <0.00001 * | ||||||
| <30 | 1 | 4.4 | 5.06 (4.54, 5.64) | <0.00001 | NA | NA | NA |
| 30–<35 | 7 | 14.4 | 12.98 (7.89, 21.35) | <0.00001 | 60 | 0.19 | 0.02 |
| ≥35 | 5 | 13.4 | 3.69 (1.76, 7.77) | 0.0006 | 88 | 0.49 | <0.00001 |
| NR | 27 | 67.8 | 7.87 (6.07, 10.21) | <0.00001 | 99 | 0.27 | <0.00001 |
| BMI in pregnancy, kg/m2 | <0.00001 * | ||||||
| <25 | 1 | 4.4 | 3.93 (3.69, 4.19) | <0.00001 | NA | NA | NA |
| 25–<30 | 3 | 5.3 | 6.97 (5.05, 9.63) | <0.00001 | 0 | 0.00 | 0.40 |
| ≥30 | 2 | 1.0 | 18.20 (2.46, 134.42) | 0.004 | 0 | 0.00 | 0.98 |
| NR | 34 | 89.3 | 7.51 (6.03, 9.36) | <0.00001 | 99 | 0.25 | <0.00001 |
| BMI follow-up, kg/m2 | <0.00001 * | ||||||
| <25 | 5 | 9.0 | 15.64 (14.68, 16.66) | <0.00001 | 0 | 0.00 | 0.69 |
| 25–<30 | 11 | 21.6 | 4.21 (2.84, 6.25) | <0.00001 | 76 | 0.18 | <0.00001 |
| ≥30 | 2 | 6.6 | 5.03 (4.52, 5.61) | <0.00001 | 0 | 0.00 | 0.38 |
| NR | 22 | 62.8 | 8.63 (6.65, 11.22) | <0.00001 | 99 | 0.25 | <0.00001 |
| Ethnicity | 0.02 * | ||||||
| Caucasian | 10 | 10.5 | 7.59 (4.06, 14.20) | <0.00001 | 23 | 0.22 | 0.23 |
| Asian | 4 | 9.8 | 4.87 (1.44, 16.41) | 0.01 | 89 | 1.22 | <0.00001 |
| Pacific Islander | 2 | 6.2 | 3.59 (2.29, 5.62) | <0.00001 | 0 | 0.00 | 0.56 |
| Middle Eastern | 1 | 2.2 | 3.20 (1.15, 8.92) | 0.03 | NA | NA | NA |
| Mixed | 7 | 18.5 | 10.76 (5.55, 20.85) | <0.00001 | 98 | 0.53 | <0.00001 |
| NR | 16 | 52.8 | 8.09 (6.06, 10.80) | <0.00001 | 99 | 0.26 | <0.00001 |
| Diagnostic criteria T2DM | 0.28 * | ||||||
| WHO | 7 | 16.9 | 5.27 (3.62, 7.66) | <0.00001 | 54 | 0.10 | 0.04 |
| ADA | 13 | 24.9 | 7.94 (4.22, 14.94) | <0.00001 | 88 | 0.79 | <0.00001 |
| CDA | 3 | 3.2 | 11.31 (4.01, 31.91) | <0.00001 | 0 | 0.00 | 0.78 |
| GDA | 1 | 0.5 | 2.64 (0.16, 43.56) | 0.50 | - | - | - |
| NR | 16 | 54.5 | 8.40 (6.29, 11.22) | <0.00001 | 99 | 0.27 | <0.00001 |
| Screening method T2DM | 0.23 * | ||||||
| OGTT | 19 | 30.6 | 5.42 (3.43, 8.55) | <0.00001 | 71 | 0.47 | <0.00001 |
| FGP | 1 | 0.5 | 17.78 (1.10, 288.10) | 0.04 | NA | NA | NA |
| HbA1c | 0 | - | - | - | - | - | - |
| Multiple methods | 6 | 16.8 | 7.23 (3.78, 13.84) | <0.00001 | 93 | 0.45 | <0.00001 |
| NR | 14 | 52.1 | 9.38 (6.99, 12.58) | <0.00001 | 99 | 0.27 | <0.00001 |
| B. | |||||||
| Subgroup | Number of studies | Weight of subjects (%) | Relative risk (95% CI) | p for RR | I2 (%) | τ2 | p for heterogeneity |
| Diagnostic criteria GDM | 0.73 * | ||||||
| IADPSG | 5 | 22.7 | 2.79 (1.30, 5.96) | 0.008 | 84 | 0.52 | <0.00001 |
| Other criteria | 12 | 59.5 | 2.03 (1.57, 2.62) | <0.00001 | 82 | 0.10 | <0.00001 |
| NR | 4 | 17.7 | 2.24 (0.86, 5.80) | 0.10 | 83 | 0.72 | 0.0004 |
| Screening strategy GDM | 0.63 * | ||||||
| Universal two-step | 5 | 23.6 | 3.22 (1.46, 7.10) | 0.004 | 89 | 0.57 | <0.00001 |
| Universal one-step | 10 | 53.0 | 2.18 (1.60, 2.97) | <0.00001 | 89 | 0.15 | <0.00001 |
| Selective | 1 | 1.4 | 5.64 (0.83, 38.28) | 0.08 | NA | NA | NA |
| NR | 5 | 22.0 | 2.63 (1.24, 5.57) | 0.01 | 79 | 0.52 | 0.0007 |
| Follow-up, yr | 0.04 * | ||||||
| <3 | 6 | 26.5 | 4.16 (2.46, 7.03) | <0.00001 | 66 | 0.25 | 0.01 |
| 3–<6 | 7 | 29.8 | 3.10 (1.40, 6.87) | 0.005 | 90 | 0.84 | <0.00001 |
| 6–<10 | 4 | 21.8 | 2.00 (1.55, 2.58) | <0.00001 | 15 | 0.01 | 0.32 |
| 10–<15 | 2 | 16.9 | 1.68 (1.05, 2.68) | 0.03 | 97 | 0.11 | <0.00001 |
| ≥20 | 0 | - | - | - | - | - | - |
| NR | 2 | 5.0 | 0.31 (0.02, 5.36) | 0.42 | 86 | 3.66 | 0.008 |
| Age during pregnancy, yr | 0.66 * | ||||||
| <30 | 0 | - | - | - | - | - | - |
| 30–<35 | 6 | 32.1 | 2.66 (1.68, 4.21) | <0.0001 | 77 | 0.21 | 0.0005 |
| ≥35 | 0 | - | - | - | - | - | - |
| NR | 15 | 67.9 | 2.36 (1.76, 3.16) | <0.00001 | 89 | 0.18 | <0.00001 |
| Age postpartum, yr | 0.20 * | ||||||
| <30 | 1 | 0.7 | 27.17 (1.70, 434.17) | 0.02 | NA | NA | NA |
| 30–<35 | 4 | 18.4 | 3.26 (0.94, 11.37) | 0.06 | 91 | 1.40 | <0.00001 |
| ≥35 | 5 | 29.8 | 1.93 (1.26, 2.96) | 0.002 | 93 | 0.16 | <0.00001 |
| NR | 11 | 51.2 | 2.73 (1.91, 3.88) | <0.00001 | 71 | 0.20 | 0.0002 |
| BMI in pregnancy, kg/m2 | 0.0003 * | ||||||
| <25 | 0 | - | - | - | - | - | - |
| 25–<30 | 3 | 17.3 | 1.98 (1.61, 2.42) | <0.00001 | 52 | 0.01 | 0.12 |
| ≥30 | 1 | 5.9 | 6.57 (3.76, 11.47) | <0.00001 | NA | NA | NA |
| NR | 17 | 76.8 | 2.46 (1.82, 3.33) | <0.00001 | 86 | 0.23 | <0.00001 |
| BMI follow-up, kg/m2 | 0.14 * | ||||||
| <25 | 2 | 9.1 | 0.95 (0.31, 2.96) | 0.93 | 27 | 0.38 | 0.24 |
| 25–<30 | 11 | 58.1 | 2.61 (1.88, 3.63) | <0.00001 | 91 | 0.19 | <0.00001 |
| ≥30 | 1 | 3.4 | 1.25 (0.45, 3.48) | 0.67 | NA | NA | NA |
| NR | 7 | 29.4 | 3.06 (1.97, 4.77) | <0.00001 | 52 | 0.17 | 0.05 |
| Ethnicity | <0.00001 * | ||||||
| Caucasian | 10 | 44.1 | 4.04 (2.43, 6.72) | <0.00001 | 83 | 0.45 | <0.00001 |
| Asian | 2 | 4.2 | 0.71 (0.01, 68.22) | 0.88 | 94 | 10.20 | <0.0001 |
| Pacific Islander | 1 | 3.7 | 0.95 (0.37, 2.46) | 0.92 | NA | NA | NA |
| Middle Eastern | 1 | 3.4 | 1.25 (0.45, 3.48) | 0.67 | NA | NA | NA |
| Mixed | 3 | 21.1 | 2.16 (1.92, 2.44) | <0.00001 | 0 | 0.00 | 0.80 |
| NR | 4 | 23.5 | 1.32 (1.12, 1.56) | 0.001 | 53 | 0.01 | 0.09 |
| Diagnostic criteria prediabetes | 0.33 * | ||||||
| WHO | 5 | 17.9 | 2.76 (1.42, 5.36) | 0.003 | 58 | 0.30 | 0.05 |
| ADA | 9 | 50.1 | 2.19 (1.60, 2.98) | <0.00001 | 92 | 0.14 | <0.00001 |
| CDA | 3 | 18.4 | 3.39 (2.22, 5.17) | <0.00001 | 49 | 0.07 | 0.14 |
| GDA | 0 | - | - | - | - | - | - |
| NR | 4 | 13.5 | 1.55 (0.48, 5.04) | 0.46 | 81 | 1.02 | 0.001 |
| Screening method prediabetes | 0.63 * | ||||||
| OGTT | 20 | 94.4 | 2.44 (1.90, 3.13) | <0.00001 | 88 | 0.18 | <0.00001 |
| FGP | 0 | - | - | - | - | - | - |
| HbA1c | 0 | - | - | - | - | - | - |
| Multiple methods | 1 | 5.6 | 2.69 (1.46, 4.94) | 0.001 | NA | NA | NA |
| NR | 0 | - | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhalima, K.; Lens, K.; Bosteels, J.; Chantal, M. The Risk for Glucose Intolerance after Gestational Diabetes Mellitus since the Introduction of the IADPSG Criteria: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1431. https://doi.org/10.3390/jcm8091431
Benhalima K, Lens K, Bosteels J, Chantal M. The Risk for Glucose Intolerance after Gestational Diabetes Mellitus since the Introduction of the IADPSG Criteria: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(9):1431. https://doi.org/10.3390/jcm8091431
Chicago/Turabian StyleBenhalima, Katrien, Karen Lens, Jan Bosteels, and Mathieu Chantal. 2019. "The Risk for Glucose Intolerance after Gestational Diabetes Mellitus since the Introduction of the IADPSG Criteria: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 9: 1431. https://doi.org/10.3390/jcm8091431
APA StyleBenhalima, K., Lens, K., Bosteels, J., & Chantal, M. (2019). The Risk for Glucose Intolerance after Gestational Diabetes Mellitus since the Introduction of the IADPSG Criteria: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(9), 1431. https://doi.org/10.3390/jcm8091431






