Exercise Training in Patients with Chronic Respiratory Diseases: Are Cardiovascular Comorbidities and Outcomes Taken into Account?—A Systematic Review
Abstract
:1. Background
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Quality Assessment and Data Extraction
2.4. Data Analysis and Synthesis
3. Results
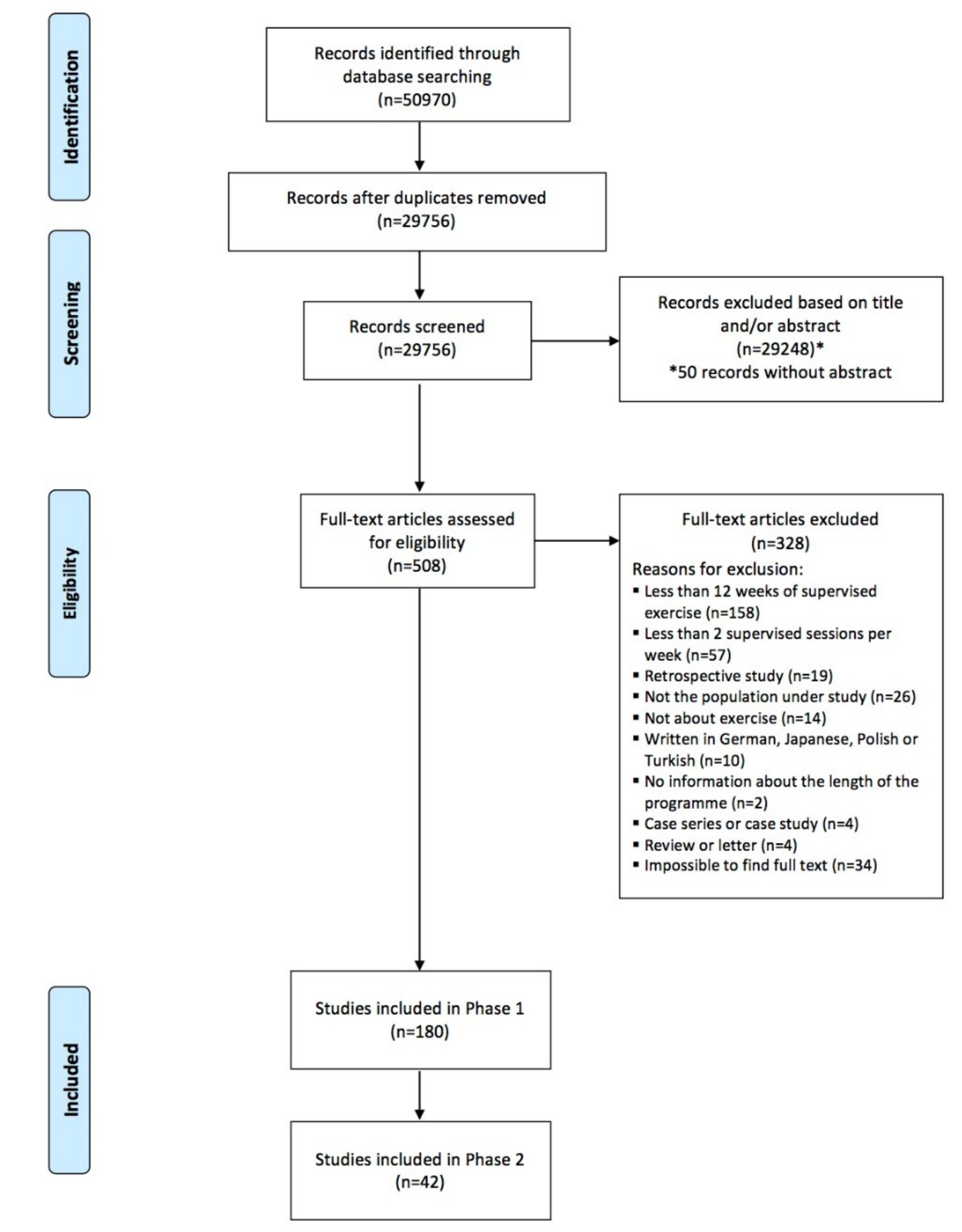
3.1. Study Selection
3.2. Phase 1: Criteria Used to Exclude Patients with Cardiovascular Comorbidities from Exercise Programmes
3.3. Phase 2: Impact of Exercise Training on Cardiovascular Outcomes and Design of the Exercise Programmes
3.3.1. Quality Assessment
3.3.2. Study Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategy
A1. PubMed
A2. Cochrane, Scopus and Web of Science
References
- Armstrong, M.; Vogiatzis, I. Personalized exercise training in chronic lung diseases. Respirology 2019, 24, 854–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, J.; Kiley, J.; Bateman, E.; Viegi, G.; Cruz, A.; Khaltaev, N.; Khaled, N.A.; Baena-Cagnani, C.; Barreto, M.; Billo, N. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur. Respir. J. 2010, 36, 995–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.J.; Wu, F.; Guo, Y.; Robledo, L.M.G.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulet, L.-P.; Boulay, M.-È. Asthma-related comorbidities. Expert Rev. Respir. Med. 2011, 5, 377–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franssen, F.M.; Rochester, C.L. Comorbidities in patients with COPD and pulmonary rehabilitation: Do they matter? Eur Respir. Soc 2014, 23, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Spruit, M.A.; Uszko-Lencer, N.H.; Otkinska, G.; Vanfleteren, L.E.; Jones, P.W.; Wouters, E.F.; Franssen, F.M. Echocardiographic abnormalities and their impact on health status in patients with COPD referred for pulmonary rehabilitation. Respirology 2017, 22, 928–934. [Google Scholar] [CrossRef]
- Hyldgaard, C.; Hilberg, O.; Bendstrup, E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir. Med. 2014, 108, 647–653. [Google Scholar] [CrossRef] [Green Version]
- King, C.; Nathan, S.D. Identification and treatment of comorbidities in idiopathic pulmonary fibrosis and other fibrotic lung diseases. Curr. Opin. Pulm. Med. 2013, 19, 466–473. [Google Scholar] [CrossRef]
- Soriano, J.B.; Visick, G.T.; Muellerova, H.; Payvandi, N.; Hansell, A.L. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005, 128, 2099–2107. [Google Scholar] [CrossRef]
- Triest, F.J.; Singh, S.J.; Vanfleteren, L.E. Cardiovascular risk, chronic obstructive pulmonary disease and pulmonary rehabilitation: Can we learn from cardiac rehabilitation? Chronic Respir. Dis. 2016, 13, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Triest, F.J.; Franssen, F.M.; Janssen, D.J.; Wouters, E.F.; Vanfleteren, L.E. Revealing methodological challenges in chronic obstructive pulmonary disease studies assessing comorbidities: A narrative review. Chronic Obstr. Pulm. Dis. J. Copd. Found. 2019, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Edwards, L.D.; Agustí, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; Lomas, D.A.; Miller, B.E. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir. Med. 2013, 107, 1376–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzano, C.; Conti, V.; Di Stefano, F.; Petroianni, A.; Ceccarelli, D.; Graziani, E.; Mariotta, S.; Ricci, A.; Vitarelli, A.; Puglisi, G. Comorbidity, hospitalization, and mortality in COPD: Results from a longitudinal study. Lung 2010, 188, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Bettoncelli, G.; Cricelli, C.; Romeo, F.; Matera, M.G.; Rogliani, P. Cardiovascular disease in asthma and COPD: A population-based retrospective cross-sectional study. Respir. Med. 2012, 106, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Crisafulli, E.; Costi, S.; Luppi, F.; Cirelli, G.; Cilione, C.; Coletti, O.; Fabbri, L.M.; Clini, E.M. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008, 63, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Margaritopoulos, G.A.; Antoniou, K.M.; Wells, A.U. Comorbidities in interstitial lung diseases. Eur. Respir. Rev. 2017, 26, 160027. [Google Scholar] [CrossRef]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur. J. Prev. Cardiol. 2016, 23, 1715–1733. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Hornikx, M.; Van Remoortel, H.; Lehouck, A.; Mathieu, C.; Maes, K.; Gayan-Ramirez, G.; Decramer, M.; Troosters, T.; Janssens, W. Vitamin D supplementation during rehabilitation in COPD: A secondary analysis of a randomized trial. Respir. Res. 2012, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Man, W.D.; Chowdhury, F.; Taylor, R.S.; Evans, R.A.; Doherty, P.; Singh, S.J.; Booth, S.; Thomason, D.; Andrews, D.; Lee, C. Building consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure. Chronic Respir. Dis. 2016, 13, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M. An official American Thoracic Society/European Respiratory Society policy statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef]
- Thomas, B.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. World Views Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Morris, S.B.; DeShon, R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 2002, 7, 105. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Exercise alleviates depression related systemic inflammation in chronic obstructive pulmonary disease patients. Afr. Health Sci. 2016, 16, 1078–1088. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H.; Al-Jiffri, H.O. Aerobic exercise training modulates bone mineral status in patients with chronic obstructive pulmonary disease. Eur. J. Gen. Med. 2016, 13, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Almadana Pacheco, V.; Pavon Masa, M.; Gomez-Bastero Fernandez, A.P.; Muniz Rodriguez, A.M.; Tallon Moreno, R.; Montemayor Rubio, T. Patient Profile of Drop-Outs From a Pulmonary Rehabilitation Program. Arch. De Bronconeumol. 2017, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, W.A.; Duiverman, M.L.; Ten Hacken, N.H.; Kerstjens, H.A.; de Greef, M.H.; Wijkstra, P.J.; Wempe, J.B. Changes in the endurance shuttle walk test in COPD patients with chronic respiratory failure after pulmonary rehabilitation: The minimal important difference obtained with anchor- and distribution-based method. Respir. Res. 2015, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Abrazado, M.; Quinn, M.; Storer, T.W.; Tseng, C.H.; Cooper, C.B. A controlled study of community-based exercise training in patients with moderate COPD. BMC Pulm. Med. 2014, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Arnardóttir, R.H.; Boman, G.; Larsson, K.; Hedenström, H.; Emtner, M. Interval training compared with continuous training in patients with COPD. Respir. Med. 2007, 101, 1196–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averna, T.; Brunelli, S.; Delussu, A.S.; Porcacchia, P.; Lucarelli, E.; Polidori, L.; Traballesi, M. Effects of a moderately intensive, 12-week training program on participants over 60 years of age with chronic obstructive pulmonary disease. Med. Dello Sport 2009, 62, 299–313. [Google Scholar]
- Barakat, S.; Michele, G.; George, P.; Nicole, V.; Guy, A. Outpatient pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 155–162. [Google Scholar]
- Bendstrup, K.E.; Jensen, J.I.; Holm, S.; Bengtsson, B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. Eur. Respir. J. 1997, 10, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Whittom, F.; Leblanc, P.; Jobin, J.; Belleau, R.; Berube, C.; Carrier, G.; Maltais, F. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 159, 896–901. [Google Scholar] [CrossRef]
- Berry, M.J.; Adair, N.E.; Sevensky, K.S.; Quinby, A.; Lever, H.M. Inspiratory muscle training and whole-body reconditioning in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 153, 1812–1816. [Google Scholar] [CrossRef]
- Berry, M.J.; Jack Rejeski, W.; Adair, N.E.; Zaccaro, D. Exercise rehabilitation and chronic obstructive pulmonary disease stage. Am. J. Respir. Crit. Care Med. 1999, 160, 1248–1253. [Google Scholar] [CrossRef]
- Berry, M.J.; Rejeski, W.J.; Adair, N.E.; Ettinger Jr, W.H.; Zaccaro, D.J.; Sevick, M.A. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2003, 23, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Rejeski, W.J.; Miller, M.E.; Adair, N.E.; Lang, W.; Foy, C.G.; Katula, J.A. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir. Med. 2010, 104, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, M.J.; Sheilds, K.L.; Adair, N.E. Comparison of Effects of Endurance and Strength Training Programs in Patients with COPD. COPD 2018, 15, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Bingisser, R.M.; Joos, L.; Frühauf, B.; Caravatti, M.; Knoblauch, A.; Villiger, P.M. Pulmonary rehabilitation in outpatients with asthma or chronic obstructive lung disease. Swiss Med Wkly. 2001, 131, 407–411. [Google Scholar] [PubMed]
- Bisca, G.W.; Proenca, M.; Salomao, A.; Hernandes, N.A.; Pitta, F. Minimal detectable change of the London chest activity of daily living scale in patients with COPD. J. Cardiopulm. Rehabil. Prev. 2014, 34, 213–216. [Google Scholar] [CrossRef]
- Boeselt, T.; Nell, C.; Lutteken, L.; Kehr, K.; Koepke, J.; Apelt, S.; Veith, M.; Beutel, B.; Spielmanns, M.; Greulich, T.; et al. Benefits of High-Intensity Exercise Training to Patients with Chronic Obstructive Pulmonary Disease: A Controlled Study. Respir. Int. Rev. Thorac. Dis. 2017, 93, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Borghi-Silva, A.; Mendes, R.G.; Trimer, R.; Oliveira, C.R.; Fregonezi, G.A.; Resqueti, V.R.; Arena, R.; Sampaio-Jorge, L.M.; Costa, D. Potential effect of 6 versus 12-weeks of physical training on cardiac autonomic function and exercise capacity in chronic obstructive pulmonary disease. Eur. J. Phys. Rehabil. Med. 2015, 51, 211–221. [Google Scholar]
- Burtin, C.; Langer, D.; van Remoortel, H.; Demeyer, H.; Gosselink, R.; Decramer, M.; Dobbels, F.; Janssens, W.; Troosters, T. Physical Activity Counselling during Pulmonary Rehabilitation in Patients with COPD: A Randomised Controlled Trial. PLoS ONE 2015, 10, e0144989. [Google Scholar] [CrossRef]
- Burtin, C.; Saey, D.; Saglam, M.; Langer, D.; Gosselink, R.; Janssens, W.; Decramer, M.; Maltais, F.; Troosters, T. Effectiveness of exercise training in patients with COPD: The role of muscle fatigue. Eur. Respir. J. 2012, 40, 338–344. [Google Scholar] [CrossRef]
- Cambach, W.; Chadwick-Straver, R.V.; Wagenaar, R.C.; van Keimpema, A.R.; Kemper, H.C. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: A randomized controlled trial. Eur. Respir. J. 1997, 10, 104–113. [Google Scholar] [CrossRef]
- Camillo, C.A.; Laburu, V.D.M.; Gonalves, N.S.; Cavalheri, V.; Tomasi, F.P.; Hernandes, N.A.; Ramos, D.; Marquez Vanderlei, L.C.; Cipulo Ramos, E.M.; Probst, V.S.; et al. Improvement of heart rate variability after exercise training and its predictors in COPD. Respir. Med. 2011, 105, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, A.A.; Cabrera, R.O.; Arancibia, H.F. Respiratory rehabilitation in COPD patients: Experience in a rural primary health care center. Rev. Chil. De Enferm. Respir. 2015, 31, 77–85. [Google Scholar]
- Charikiopoulou, M.; Nikolaidis, P.T.; Knechtle, B.; Rosemann, T.; Rapti, A.; Trakada, G. Subjective and Objective Outcomes in Patients With COPD After Pulmonary Rehabilitation—The Impact of Comorbidities. Front. Physiol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, R.; Chen, X.; Chen, L. Effect of endurance training on expiratory flow limitation and dynamic hyperinflation in patients with stable chronic obstructive pulmonary disease. Intern. Med. J. 2014, 44, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.T.; Wu, Y.K.; Yang, M.C.; Huang, C.Y.; Huang, H.C.; Chu, W.H.; Lan, C.C. Pulmonary rehabilitation improves heart rate variability at peak exercise, exercise capacity and health-related quality of life in chronic obstructive pulmonary disease. Heart Lung J. Crit. Care 2014, 43, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; Cochrane, L.M.; Mackay, E.; Paton, B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur. Respir. J. 2000, 15, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corhay, J.L.; Nguyen, D.; Duysinx, B.; Graas, C.; Pirnay, F.; Bury, T.; Louis, R. Should we exclude elderly patients with chronic obstructive pulmonary disease from a long-time ambulatory pulmonary rehabilitation programme? J. Rehabil. Med. 2012, 44, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortopassi, F.; Castro, A.A.; Porto, E.F.; Colucci, M.; Fonseca, G.; Torre-Bouscoulet, L.; Iamonti, V.; Jardim, J.R. Comprehensive exercise training improves ventilatory muscle function and reduces dyspnea perception in patients with COPD. Monaldi Arch. Chest Dis. Arch. Monaldi Per Le Mal. Del Torace 2009, 71, 106–112. [Google Scholar] [CrossRef]
- Covey, M.K.; Collins, E.G.; Reynertson, S.I.; Dilling, D.F. Resistance training as a preconditioning strategy for enhancing aerobic exercise training outcomes in COPD. Respir. Med. 2014, 108, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Covey, M.K.; McAuley, E.; Kapella, M.C.; Collins, E.G.; Alex, C.G.; Berbaum, M.L.; Larson, J.L. Upper-Body Resistance Training and Self-Efficacy Enhancement in COPD. J. Pulm. Respir. Med. 2012, 1 (Suppl. 9.). [Google Scholar] [CrossRef]
- Cox, N.J.M.; Hendricks, J.C.; Binkhorst, R.A.; van Herwaarden, C.L.A. A pulmonary rehabilitation program for patients with asthma and mild chronic obstructive pulmonary diseases (COPD). Lung Int. J. Lungs Airw. Breath 1993, 171, 235–244. [Google Scholar] [CrossRef]
- Crimi, C.; Heffler, E.; Augelletti, T.; Campisi, R.; Noto, A.; Vancheri, C.; Crimi, N. Utility of ultrasound assessment of diaphragmatic function before and after pulmonary rehabilitation in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Brooks, D.; Marques, A. Impact of feedback on physical activity levels of individuals with chronic obstructive pulmonary disease during pulmonary rehabilitation: A feasibility study. Chron. Respir. Dis. 2014, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Brooks, D.; Marques, A. Walk2Bactive: A randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron. Respir. Dis. 2016, 13, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.C.; de Azeredo Lermen, C.; Colombo, C.; Canterle, D.B.; Machado, M.L.L.; Kessler, A.; Teixeira, P.J.Z. Effect of a Pulmonary Rehabilitation Program on the levels of anxiety and depression and on the quality of life of patients with chronic obstructive pulmonary disease. Rev. Port. Pneumol. 2014, 20, 299–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Cardoso, M.C.; Sayão, L.B.; Souza, R.M.P.; De Melo Marinho, P.E. Pulmonary rehabilitation and whole-body vibration in chronic obstructive pulmonary disease. Mot. Rev. De Educ. Fis. 2016, 22, 44–50. [Google Scholar] [CrossRef]
- da Silva, G.P.; Nascimento, F.A.; Macedo, T.P.; Morano, M.T.; Mesquita, R.; Pereira, E.D. Religious coping and religiosity in patients with COPD following pulmonary rehabilitation. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 175–181. [Google Scholar] [CrossRef] [PubMed]
- De Alencar Silva, B.S.; Gobbo, L.A.; Freire, A.P.C.F.; Trevisan, I.B.; Silva, I.G.; Ramos, E.M.C. Effects of a resistance training with elastic tubing in strength, quality of life and dypsnea in patients with chronic obstructive pulmonary disease. J. Phys. Educ. 2016, 27. [Google Scholar] [CrossRef]
- De Oliveira, J.C.M.; Leitao, F.S.S.; Sampaio, L.M.M.; de Oliveira, A.C.N.; Hirata, R.P.; Costa, D.; Donner, C.F.; de Oliveira, L.V.F. Outpatient vs. home-based pulmonary rehabilitation in COPD: A randomized controlled trial. Multidiscip. Respir. Med. 2010, 5, 401–408. [Google Scholar] [CrossRef]
- De Souza, Y.; da Silva, K.M.; Condesso, D.; Figueira, B.; Filho, A.J.N.; Rufino, R.; Gosselink, R.; da Costa, C.H. Use of a home-based manual as part of a pulmonary rehabilitation program. Respir. Care 2018, 63, 1485–1491. [Google Scholar] [CrossRef]
- Demeyer, H.; Burtin, C.; Van Remoortel, H.; Hornikx, M.; Langer, D.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Standardizing the Analysis of Physical Activity in Patients With COPD Following a Pulmonary Rehabilitation Program. Chest 2014, 146, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dourado, V.S.; Tanni, S.E.; Antunes, L.C.O.; Paiva, S.A.R.; Campana, A.O.; Renno, A.C.M.; Godoy, I. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Braz. J. Med Biol. Res. 2009, 42, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dourado, V.Z.; Antunes, L.C.O.; Tanni, S.E.; Godoy, I. Factors associated with the minimal clinically important difference for health-related quality of life after physical conditioning in patients with COPD. J. Bras. De Pneumol. 2009, 35, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Effing, T.; Zielhuis, G.; Kerstjens, H.; van der Valk, P.; van der Palen, J. Community based physiotherapeutic exercise in COPD self-management: A randomised controlled trial. Respir. Med. 2011, 105, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emtner, M.; Hallin, R.; Arnardottir, R.H.; Janson, C. Effect of physical training on fat-free mass in patients with chronic obstructive pulmonary disease (COPD). Upsala J. Med. Sci. 2015, 120, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Dobashi, K.; Uga, D.; Kato, D.; Nakazawa, R.; Sakamoto, M.; Fueki, M.; Makino, S. Effect of 12-month rehabilitation with low loading program on chronic respiratory disease. J. Phys. Ther. Sci. 2016, 28, 1032–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enea, C.; Schmitt, N.; Dugú, B.; Boisseau, N.; Le Creff, C.; Denjean, A. Oxidative stress in patients with chronic obstructive pulmonary disease after aerobic exercise training. Sci. Sports 2005, 20, 48–50. [Google Scholar] [CrossRef]
- Engel, R.M.; Gonski, P.; Beath, K.; Vemulpad, S. Medium term effects of including manual therapy in a pulmonary rehabilitation program for chronic obstructive pulmonary disease (COPD): A randomized controlled pilot trial. J. Man. Manip. Ther. 2016, 24, 80–89. [Google Scholar] [CrossRef]
- Etnier, J.L.; Berry, M. Fluid intelligence in an older COPD sample after short- or long-term exercise. Med. Sci. Sports Exerc. 2001, 33, 1620–1628. [Google Scholar] [CrossRef]
- Felcar, J.M.; Probst, V.S.; de Carvalho, D.R.; Merli, M.F.; Mesquita, R.; Vidotto, L.S.; Ribeiro, L.R.G.; Pitta, F. Effects of exercise training in water and on land in patients with COPD: A randomised clinical trial. Physiotherapy 2018, 104, 408–416. [Google Scholar] [CrossRef]
- Fernandes, J.R.; da Silva, C.; da Silva, A.G.; Pinto, R.M.D.; Duarte, A.J.D.; Carvalho, C.R.; Benard, G. Effect of an Exercise Program on Lymphocyte Proliferative Responses of COPD Patients. Lung 2018, 196, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Scharloo, M.; Abbink, J.; van ’t Hul, A.; van Ranst, D.; Rudolphus, A.; Weinman, J.; Rabe, K.F.; Kaptein, A.A. Concerns about exercise are related to walk test results in pulmonary rehabilitation for patients with COPD. Int. J. Behav. Med. 2012, 19, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.G.; Rejeski, W.J.; Berry, M.J.; Zaccaro, D.; Woodard, C.M. Gender moderates the effects of exercise therapy on health-related quality of life among COPD patients. Chest 2001, 119, 70–76. [Google Scholar] [CrossRef]
- Fuld, J.P.; Kilduff, L.P.; Neder, J.A.; Pitsiladis, Y.; Lean, M.E.J.; Ward, S.A.; Cotton, M.M. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005, 60, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, L.M.; Button, B.; Tarrant, B.; Steward, R.; Bennett, L.; Snell, G.; Holland, A.E. Longer Versus Shorter Duration of Supervised Rehabilitation After Lung Transplantation: A Randomized Trial. Arch. Phys. Med. Rehabil. 2017, 98, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gayle, R.C.; Spitler, D.L.; Karper, W.B.; Jaeger, R.M.; Rice, S.N. Psychological changes in exercising COPD patients. Int. J. Rehabil. Res. Int. Z. Fur Rehabil. Rev. Int. De Rech. De Readapt. 1988, 11, 335–342. [Google Scholar] [CrossRef]
- Georgiadou, O.; Vogiatzis, I.; Stratakos, G.; Koutsoukou, A.; Golemati, S.; Aliverti, A.; Roussos, C.; Zakynthinos, S. Effects of rehabilitation on chest wall volume regulation during exercise in COPD patients. Eur. Respir. J. 2007, 29, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulou, V.V.; Dimopoulos, S.; Sakellariou, D.; Papazachou, O.; Gerovasili, V.; Tasoulis, A.; Agapitou, V.; Vogiatzis, I.; Roussos, C.; Nanas, S. Cardiopulmonary rehabilitation enhances heart rate recovery in patients with COPD. Respir. Care 2012, 57, 2095–2103. [Google Scholar] [CrossRef]
- Godoy, R.F.; Teixeira, P.J.; Becker Junior, B.; Michelli, M.; Godoy, D.V. Long-term repercussions of a pulmonary rehabilitation program on the indices of anxiety, depression, quality of life and physical performance in patients with COPD. J. Bras. De Pneumol. Publ. Da Soc. Bras. De Pneumol. E Tisilogia 2009, 35, 129–136. [Google Scholar]
- Goldstein, R.S.; Gort, E.H.; Avendano, M.A.; Stubbing, D.; Guyatt, G.H. Randomised controlled trial of respiratory rehabilitation. Lancet 1994, 344, 1394–1397. [Google Scholar] [CrossRef]
- Grosbois, J.M.; Lamblin, C.; Lemaire, B.; Chekroud, H.; Dernis, J.M.; Douay, B.; Fortin, F. Long-term benefits of exercise maintenance after outpatient rehabilitation program in patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 1999, 19, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Guell, R.; Casan, P.; Belda, J.; Sangenis, M.; Morante, F.; Guyatt, G.H.; Sanchis, J. Long-term effects of outpatient rehabilitation of COPD—A randomized trial. Chest 2000, 117, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Beauchamp, M.K.; Sibley, K.; Araujo, T.; Romano, J.; Goldstein, R.S.; Brooks, D. Minimizing the evidence-practice gap—A prospective cohort study incorporating balance training into pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease. BMC Pulm. Med. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, M.; van Wetering, C.R.; Schols, A.M.; Rutten-van Molken, M.P. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? Eur. Respir. J. 2010, 35, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Janssen, D.J.A.; Franssen, F.M.E.; Vanfleteren, L.; Wouters, E.F.M.; Spruit, M.A. Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores. Health Qual. Life Outcomes 2018, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Jacome, C.; Marques, A. Short- and Long-term Effects of Pulmonary Rehabilitation in Patients With Mild COPD: A COMPARISON WITH PATIENTS WITH MODERATE TO SEVERE COPD. J. Cardiopulm. Rehabil. Prev. 2016, 36, 445–453. [Google Scholar] [CrossRef]
- Jácome, C.; Marques, A. Impact of pulmonary rehabilitation in subjects with mild COPD. Respir. Care 2014, 59, 1577–1582. [Google Scholar] [CrossRef]
- Kamal, M.A.W.; Nambi, S.G.; Mahmoud, M.Z. Impact of Resisted Exercise on Chronic Obstructive Pulmonary Disease (COPD) in Elderly Patients in Alkharj, Saudi Arabia. Int. J. Med Res. Health Sci. 2016, 5, 187–195. [Google Scholar]
- Kanao, K.; Shiraishi, M.; Higashimoto, Y.; Maeda, K.; Sugiya, R.; Okajima, S.; Chiba, Y.; Yamagata, T.; Terada, K.; Fukuda, K.; et al. Factors associated with the effect of pulmonary rehabilitation on physical activity in patients with chronic obstructive pulmonary disease. Geriatr. Gerontol. Int. 2017, 17, 17–23. [Google Scholar] [CrossRef]
- Kavoura, P.; Kostikas, K.; Tselebis, A.; Bratis, D.; Kosmas, E.; Alchanatis, M.; Koulouris, N.G.; Bakakos, P.; Loukides, S. Changes in BODE Quartiles After Pulmonary Rehabilitation Do Not Predict 2-Year Survival in Patients With COPD. J. Cardiopulm. Rehabil. Prev. 2016, 36, 62–67. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Backer, V.; Jorgensen, K.; Kjaer, M.; Beyer, N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients—A pilot study. Respir. Med. 2004, 98, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Chu, W.H.; Yang, M.C.; Lee, C.H.; Wu, Y.K.; Wu, C.P. Benefits of pulmonary rehabilitation in patients with COPD and normal exercise capacity. Respir. Care 2013, 58, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Huang, H.C.; Yang, M.C.; Lee, C.H.; Huang, C.Y.; Wu, Y.K. Pulmonary rehabilitation improves subjective sleep quality in COPD. Respir. Care 2014, 59, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Yang, M.C.; Huang, H.C.; Wu, C.W.; Su, W.L.; Tzeng, I.S.; Wu, Y.K. Serial changes in exercise capacity, quality of life and cardiopulmonary responses after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Heart Lung J. Crit. Care 2018, 47, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Yang, M.C.; Lee, C.H.; Huang, Y.C.; Huang, C.Y.; Huang, K.L.; Wu, Y.K. Pulmonary rehabilitation improves exercise capacity and quality of life in underweight patients with chronic obstructive pulmonary disease. Respirology 2011, 16, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.R.; Ramos, E.M.; Kalva-Filho, C.A.; Freire, A.P.; de Alencar Silva, B.S.; Nicolino, J.; de Toledo-Arruda, A.C.; Papoti, M.; Vanderlei, L.C.; Ramos, D. Effects of 12 weeks of aerobic training on autonomic modulation, mucociliary clearance, and aerobic parameters in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- López Varela, M.V.; Anido, T.; Larrosa, M. Functional status and survival in patients with chronic obstructive pulmonary disease following pulmonary rehabilitation. Arch. De Bronconeumol. 2006, 42, 434–439. [Google Scholar] [CrossRef]
- Lox, C.L.; Freehill, A.J. Impact of pulmonary rehabilitation on self-efficacy, quality of life, and exercise tolerance. Rehabil. Psychol. 1999, 44, 208–221. [Google Scholar] [CrossRef]
- Maa, S.H.; Gauthier, D.; Turner, M. Acupressure as an adjunct to a pulmonary rehabilitation program. J. Cardiopulm. Rehabil. 1997, 17, 268–276. [Google Scholar] [CrossRef]
- Magadle, R.; McConnell, A.K.; Beckerman, M.; Weiner, P. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir. Med. 2007, 101, 1500–1505. [Google Scholar] [CrossRef] [Green Version]
- Maltais, F.; LeBlanc, P.; Jobin, J.; Berube, C.; Bruneau, J.; Carrier, L.; Breton, M.J.; Falardeau, G.; Belleau, R. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 155, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; LeBlanc, P.; Simard, C.; Jobin, J.; Berube, C.; Bruneau, J.; Carrier, L.; Belleau, R. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 154, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Mandigout, S.; Antonini, M.T.; Laforge, Q.; Lemaire, F.; Dalmay, E.; Bouteille, B. Effects of training rehabilitation on the physical capacity of patients suffering from chronic obstructive pulmonary disease. Sci. Sports 2007, 22, 300–301. [Google Scholar] [CrossRef]
- Marques, A.; Gabriel, R.; Jácome, C.; Cruz, J.; Brooks, D.; Figueiredo, D. Development of a family-based pulmonary rehabilitation programme: An exploratory study. Disabil. Rehabil. 2015, 37, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Jacome, C.; Cruz, J.; Gabriel, R.; Brooks, D.; Figueiredo, D. Family-based psychosocial support and education as part of pulmonary rehabilitation in COPD: A randomized controlled trial. Chest 2015, 147, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Jacome, C.; Cruz, J.; Gabriel, R.; Figueiredo, D. Effects of a pulmonary rehabilitation program with balance training on patients with COPD. J. Cardiopulm. Rehabil. Prev. 2015, 35, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Martín, E.; Ruiz, F.O.; Ramos, P.C.; López-Campos, J.L.; Azcona, B.V.; Cortés, E.B. Randomized trial of non-invasive ventilation combined with exercise training in patients with chronic hypercapnic failure due to chronic obstructive pulmonary disease. Respir. Med. 2014, 108, 1741–1751. [Google Scholar] [CrossRef] [Green Version]
- Marquis, K.; Maltais, F.; Lacasse, Y.; Lacourciere, Y.; Fortin, C.; Poirier, P. Effects of aerobic exercise training and irbesartan on blood pressure and heart rate variability in patients with chronic obstructive pulmonary disease. Can. Respir. J. 2008, 15, 355–360. [Google Scholar] [CrossRef]
- Mekki, M.; Paillard, T.; Sahli, S.; Tabka, Z.; Trabelsi, Y. Effect of adding neuromuscular electrical stimulation training to pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: Randomized clinical trial. Clin. Rehabil. 2019, 33, 195–206. [Google Scholar] [CrossRef]
- Milani, R.V.; Lavie, C.J. Disparate effects of out-patient cardiac and pulmonary rehabilitation programs on work efficiency and peak aerobic capacity in patients with coronary disease or severe obstructive pulmonary disease. J. Cardiopulm. Rehabil. 1998, 18, 17–22. [Google Scholar] [CrossRef]
- Mkacher, W.; Mekki, M.; Tabka, Z.; Trabelsi, Y. Effect of 6 Months of Balance Training During Pulmonary Rehabilitation in Patients With COPD. J. Cardiopulm. Rehabil. Prev. 2015, 35, 207–213. [Google Scholar] [CrossRef]
- Moezy, A.; Erfani, A.; Mazaherinezhad, A.; Mousavi, S.A.J. Downhill walking influence on physical condition and quality of life in patients with COPD: A randomized controlled trial. Med. J. Islam. Repub. Iran 2018, 32, 49. [Google Scholar] [CrossRef]
- Neunhauserer, D.; Steidle-Kloc, E.; Weiss, G.; Kaiser, B.; Niederseer, D.; Hartl, S.; Tschentscher, M.; Egger, A.; Schonfelder, M.; Lamprecht, B.; et al. Supplemental Oxygen During High-Intensity Exercise Training in Nonhypoxemic Chronic Obstructive Pulmonary Disease. Am. J. Med. 2016, 129, 1185–1193. [Google Scholar] [CrossRef]
- Ortega, F.; Toral, J.; Cejudo, P.; Villagomez, R.; Sanchez, H.; Castillo, J.; Montemayor, T. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 669–674. [Google Scholar] [CrossRef]
- Osadnik, C.R.; Loeckx, M.; Louvaris, Z.; Demeyer, H.; Langer, D.; Rodrigues, F.M.; Janssens, W.; Vogiatzis, I.; Troosters, T. The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3515–3527. [Google Scholar] [CrossRef]
- Panton, L.B.; Golden, J.; Broeder, C.E.; Browder, K.D.; Cestaro-Seifer, D.J.; Seifer, F.D. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. Eur. J. Appl. Physiol. 2004, 91, 443–449. [Google Scholar] [CrossRef]
- Papp, M.E.; Wändell, P.E.; Lindfors, P.; Nygren-Bonnier, M. Effects of yogic exercises on functional capacity, lung function and quality of life in participants with obstructive pulmonary disease: A randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 447–461. [Google Scholar]
- Pereira, E.D.B.; Viana, C.S.; Taunay, T.C.E.; Sales, P.U.; Lima, J.W.O.; Holanda, M.A. Improvement of Cognitive Function After a Three-Month Pulmonary Rehabilitation Program for COPD Patients. Lung 2011, 189, 279–285. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Langer, D.; Decramer, M.; Gosselink, R. Are patients with COPD more active after pulmonary rehabilitation? Chest 2008, 134, 273–280. [Google Scholar] [CrossRef]
- Polkey, M.I.; Qiu, Z.H.; Zhou, L.; Zhu, M.D.; Wu, Y.X.; Chen, Y.Y.; Ye, S.P.; He, Y.S.; Jiang, M.; He, B.T.; et al. Tai Chi and Pulmonary Rehabilitation Compared for Treatment-Naive Patients With COPD: A Randomized Controlled Trial. Chest 2018, 153, 1116–1124. [Google Scholar] [CrossRef]
- Probst, V.S.; Kovelis, D.; Hernandes, N.A.; Camillo, C.A.; Cavalheri, V.; Pitta, F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir. Care 2011, 56, 1799–1807. [Google Scholar] [CrossRef]
- Probst, V.S.; Troosters, T.; Pitta, F.; Decramer, M.; Gosselink, R. Cardiopulmonary stress during exercise training in patients with COPD. Eur. Respir. J. 2006, 27, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Radom-Aizik, S.; Kaminski, N.; Hayek, S.; Halkin, H.; Cooper, D.M.; Ben-Dov, I. Effects of exercise training on quadriceps muscle gene expression in chronic obstructive pulmonary disease. J. Appl. Physiol. 2007, 102, 1976–1984. [Google Scholar] [CrossRef] [Green Version]
- Rejbi, I.B.; Trabelsi, Y.; Chouchene, A.; Ben Turkia, W.; Ben Saad, H.; Zbidi, A.; Kerken, A.; Tabka, Z. Changes in six-minute walking distance during pulmonary rehabilitation in patients with COPD and in healthy subjects. Int. J. Chronic Obstr. Pulm. Dis. 2010, 5, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Rinaldo, N.; Bacchi, E.; Coratella, G.; Vitali, F.; Milanese, C.; Rossi, A.; Schena, F.; Lanza, M. Effects of Combined Aerobic-Strength Training vs Fitness Education Program in COPD Patients. Int. J. Sports Med. 2017, 38, 1001–1008. [Google Scholar] [CrossRef]
- Rizk, A.K.; Wardini, R.; Chan-Thim, E.; Bacon, S.L.; Lavoie, K.L.; Pepin, V. Acute responses to exercise training and relationship with exercise adherence in moderate chronic obstructive pulmonary disease. Chron. Respir. Dis. 2015, 12, 329–339. [Google Scholar] [CrossRef]
- Roman, M.; Larraz, C.; Gomez, A.; Ripoll, J.; Mir, I.; Miranda, E.Z.; Macho, A.; Thomas, V.; Esteva, M. Efficacy of pulmonary rehabilitation in patients with moderate chronic obstructive pulmonary disease: A randomized controlled trial. BMC Fam. Pract. 2013, 14, 21. [Google Scholar] [CrossRef]
- Santana, V.T.S.; Squassoni, S.D.; Neder, J.A.; Fiss, E. Influence of current smoking on adherence and responses to pulmonary rehabilitation in patients with COPD. Braz. J. Phys. Ther. 2010, 14, 16–23. [Google Scholar] [CrossRef]
- Scherer, Y.K.; Schmieder, L.E. The effect of a pulmonary rehabilitation program on self-efficacy, perception of dyspnea, and physical endurance. Heart Lung J. Crit. Care 1997, 26, 15–22. [Google Scholar] [CrossRef]
- Scherer, Y.K.; Schmieder, L.E.; Shimmel, S. The effects of education alone and in combination with pulmonary rehabilitation on self-efficacy in patients with COPD. Rehabil. Nurs. Off. J. Assoc. Rehabil. Nurses 1998, 23, 71–77. [Google Scholar] [CrossRef]
- Sciriha, A.; Lungaro-Mifsud, S.; Bonello, A.; Agius, T.; Scerri, J.; Ellul, B.; Fenech, A.; Camilleri, L.; Montefort, S. Systemic inflammation in COPD is not influenced by pulmonary rehabilitation. Eur. J. Physiother. 2017, 19, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Sciriha, A.; Lungaro-Mifsud, S.; Scerri, J.; Bilocca, D.; Fsadni, C.; Fsadni, P.; Gerada, E.; Gouder, C.; Camilleri, L.; Montefort, S. Pulmonary rehabilitation in chronic obstructive pulmonary disease: Outcomes in a 12 week programme. Eur. J. Physiother. 2015, 17, 215–223. [Google Scholar] [CrossRef]
- Sciriha, A.; Lungaro-Mifsud, S.; Scerri, J.; Magro, R.; Camilleri, L.; Montefort, S. Health status of COPD patients undergoing pulmonary rehabilitation: A comparative responsiveness of the CAT and SGRQ. Chron. Respir. Dis. 2017, 14, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.S.A.; Lira, F.S.; Rossi, F.E.; Ramos, D.; Uzeloto, J.S.; Freire, A.; de Lima, F.F.; Gobbo, L.A.; Ramos, E.M.C. Inflammatory and Metabolic Responses to Different Resistance Training on Chronic Obstructive Pulmonary Disease: A Randomized Control Trial. Front. Physiol. 2018, 9, 262. [Google Scholar] [CrossRef]
- Silva, B.S.A.; Ramos, D.; Bertolini, G.N.; Freire, A.; Leite, M.R.; Camillo, C.A.; Gobbo, L.A.; Ramos, E.M.C. Resistance exercise training improves mucociliary clearance in subjects with COPD: A randomized clinical trial. Pulmonology 2019. [Google Scholar] [CrossRef]
- Skumlien, S.; Aure Skogedal, E.; Skrede Ryg, M.; Bjortuft, O. Endurance or resistance training in primary care after in-patient rehabilitation for COPD? Respir. Med. 2008, 102, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Solanes, I.; Guell, R.; Casan, P.; Sotomayor, C.; Gonzalez, A.; Feixas, T.; Gonzalez, M.; Guyatt, G. Duration of pulmonary rehabilitation to achieve a plateau in quality of life and walk test in COPD. Respir. Med. 2009, 103, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Spencer, L.M.; Alison, J.A.; McKeough, Z.J. A Survey of Opinions and Attitudes Toward Exercise Following a 12-month Maintenance Exercise Program for People with COPD. Cardiopulm. Phys. Ther. J. 2013, 24, 30–35. [Google Scholar] [CrossRef]
- Spielmanns, M.; Fuchs-Bergsma, C.; Winkler, A.; Fox, G.; Krüger, S.; Baum, K. Effects of oxygen supply during training on subjects with COPD who are normoxemic at rest and during exercise: A blinded randomized controlled trial. Respir. Care 2015, 60, 540–548. [Google Scholar] [CrossRef]
- Spruit, M.A.; Gosselink, R.; Troosters, T.; De Paepe, K.; Decramer, M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur. Respir. J. 2002, 19, 1072–1078. [Google Scholar] [CrossRef]
- Spruit, M.A.; Gosselink, R.; Troosters, T.; Kasran, A.; Van Vliet, M.; Decramer, M. Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest 2005, 128, 3183–3190. [Google Scholar] [CrossRef]
- Stav, D.; Raz, M.; Shpirer, I. Three years of pulmonary rehabilitation: Inhibit the decline in airflow obstruction, improves exercise endurance time, and body-mass index, in chronic obstructive pulmonary disease. BMC Pulm. Med. 2009, 9, 26. [Google Scholar] [CrossRef]
- Steinsbekk, A.; Lomundal, B.K. Three-year follow-up after a two-year comprehensive pulmonary rehabilitation program. Chron. Respir. Dis. 2009, 6, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Strijbos, J.H.; Postma, D.S.; van Altena, R.; Gimeno, F.; Koeter, G.H. Feasibility and effects of a home-care rehabilitation program in patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 1996, 16, 386–393. [Google Scholar] [CrossRef]
- Strijbos, J.H.; Postma, D.S.; vanAltena, R.; Gimeno, F.; Koeter, G.H. A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD - A follow-up of 18 months. Chest 1996, 109, 366–372. [Google Scholar] [CrossRef]
- Theander, K.; Jakobsson, P.; Jorgensen, N.; Unosson, M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: A randomized controlled trial. Clin. Rehabil. 2009, 23, 125–136. [Google Scholar] [CrossRef]
- Theodorakopoulou, E.P.; Gennimata, S.A.; Harikiopoulou, M.; Kaltsakas, G.; Palamidas, A.; Koutsoukou, A.; Roussos, C.; Kosmas, E.N.; Bakakos, P.; Koulouris, N.G. Effect of pulmonary rehabilitation on tidal expiratory flow limitation at rest and during exercise in COPD patients. Respir. Physiol. Neurobiol. 2017, 238, 47–54. [Google Scholar] [CrossRef]
- Toledo, A.; Borghi-Silva, A.; Sampaio, L.M.; Ribeiro, K.P.; Baldissera, V.; Costa, D. The impact of noninvasive ventilation during the physical training in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Clinics 2007, 62, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Trappenburg, J.C.; Troosters, T.; Spruit, M.A.; Vandebrouck, N.; Decramer, M.; Gosselink, R. Psychosocial conditions do not affect short-term outcome of multidisciplinary rehabilitation in chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2005, 86, 1788–1792. [Google Scholar] [CrossRef]
- Troosters, T.; Gosselink, R.; Decramer, M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: A randomized trial. Am. J. Med. 2000, 109, 207–212. [Google Scholar] [CrossRef]
- Troosters, T.; Gosselink, R.; Decramer, M. Exercise training in COPD: How to distinguish responders from nonresponders. J. Cardiopulm. Rehabil. 2001, 21, 10–17. [Google Scholar] [CrossRef]
- Tselebis, A.; Bratis, D.; Pachi, A.; Moussas, G.; Ilias, I.; Harikiopoulou, M.; Theodorakopoulou, E.; Dumitru, S.; Kosmas, E.; Vgontzas, A.; et al. A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidiscip. Respir. Med. 2013, 8. [Google Scholar] [CrossRef]
- Van de Bool, C.; Rutten, E.P.A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Van Helvoort, H.A.; de Boer, R.C.; van de Broek, L.; Dekhuijzen, R.; Heijdra, Y.F. Exercises commonly used in rehabilitation of patients with chronic obstructive pulmonary disease: Cardiopulmonary responses and effect over time. Arch. Phys. Med. Rehabil. 2011, 92, 111–117. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Papaioannou, A.I.; Kaltsakas, G.; Louvaris, Z.; Chynkiamis, N.; Spetsioti, S.; Kortianou, E.; Genimata, S.A.; Palamidas, A.; Kostikas, K.; et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Voduc, N.; Tessier, C.; Sabri, E.; Fergusson, D.; Lavallee, L.; Aaron, S.D. Effects of oxygen on exercise duration in chronic obstructive pulmonary disease patients before and after pulmonary rehabilitation. Can. Respir. J. 2010, 17, e14–e19. [Google Scholar] [CrossRef] [Green Version]
- Vogiatzis, I.; Nanas, S.; Roussos, C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur. Respir. J. 2002, 20, 12–19. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Williamson, A.F.; Miles, J.; Taylor, I.K. Physiological response to moderate exercise workloads in a pulmonary rehabilitation program in patients with varying degrees of airflow obstruction. Chest 1999, 116, 1200–1207. [Google Scholar] [CrossRef]
- Vonbank, K.; Strasser, B.; Mondrzyk, J.; Marzluf, B.A.; Richter, B.; Losch, S.; Nell, H.; Petkov, V.; Haber, P. Strength training increases maximum working capacity in patients with COPD - Randomized clinical trial comparing three training modalities. Respir. Med. 2012, 106, 557–563. [Google Scholar] [CrossRef]
- Wada, J.T.; Borges-Santos, E.; Porras, D.C.; Paisani, D.M.; Cukier, A.; Lunardi, A.C.; Carvalho, C.R. Effects of aerobic training combined with respiratory muscle stretching on the functional exercise capacity and thoracoabdominal kinematics in patients with COPD: A randomized and controlled trial. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2691–2700. [Google Scholar] [CrossRef]
- Wadell, K.; Henriksson-Larsen, K.; Lundgren, R.; Sundelin, G. Group training in patients with COPD—Long-term effects after decreased training frequency. Disabil. Rehabil. 2005, 27, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Wadell, K.; Sundelin, G.; Henriksson-Larsen, K.; Lundgren, R. High intensity physical group training in water—An effective training modality for patients with COPD. Respir. Med. 2004, 98, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Weiner, P.; Azgad, Y.; Ganam, R. Inspiratory muscle training combined with general exercise reconditioning in patients with COPD. Chest 1992, 102, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Wijkstra, P.J.; Van Altena, R.; Kraan, J.; Otten, V.; Postma, D.S.; Koeter, G.H. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur. Respir. J. 1994, 7, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijkstra, P.J.; van der Mark, T.W.; Kraan, J.; van Altena, R.; Koeter, G.H.; Postma, D.S. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). Eur. Respir. J. 1996, 9, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, P.R.; Heck, H.; Langenkamp, H. Effects of a resistance training on pulmonary function and performance measurements in patients with chronic obstructive pulmonary disease. Eur. J. Sport Sci. 2003, 3, 1–10. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Liu, J.; Li, P.; Wang, Z. Effectiveness of water-based Liuzijue exercise on respiratory muscle strength and peripheral skeletal muscle function in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1713–1726. [Google Scholar] [CrossRef]
- Xu, J.; He, S.; Han, Y.; Pan, J.; Cao, L. Effects of modified pulmonary rehabilitation on patients with moderate to severe chronic obstructive pulmonary disease: A randomized controlled trail. Int. J. Nurs. Sci. 2017, 4, 219–224. [Google Scholar] [CrossRef]
- Zambom-Ferraresi, F.; Cebollero, P.; Gorostiaga, E.M.; Hernandez, M.; Hueto, J.; Cascante, J.; Rezusta, L.; Val, L.; Anton, M.M. Effects of Combined Resistance and Endurance Training Versus Resistance Training Alone on Strength, Exercise Capacity, and Quality of Life in Patients With COPD. J. Cardiopulm. Rehabil. Prev. 2015, 35, 446–453. [Google Scholar] [CrossRef]
- ZuWallack, R.; Hashim, A.; McCusker, C.; Normandin, E.; Benoit-Connors, M.L.; Lahiri, B. The trajectory of change over multiple outcome areas during comprehensive outpatient pulmonary rehabilitation. Chron. Respir. Dis. 2006, 3, 11–18. [Google Scholar] [CrossRef]
- Zwerink, M.; Effing, T.; Kerstjens, H.A.; van der Valk, P.; Brusse-Keizer, M.; Zielhuis, G.; van der Palen, J. Cost-Effectiveness of a Community-Based Exercise Programme in COPD Self-Management. COPD 2016, 13, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Zwerink, M.; van der Palen, J.; Kerstjens, H.A.; van der Valk, P.; Brusse-Keizer, M.; Zielhuis, G.; Effing, T. A community-based exercise programme in COPD self-management: Two years follow-up of the COPE-II study. Respir. Med. 2014, 108, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrill, D.E.; Barton, C.; Beasley, W.; Lippard, W.M. The effects of short-term and long-term pulmonary rehabilitation on functional capacity, perceived dyspnea, and quality of life. Chest 2005, 128, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Jiffri, O.H.; Ashmawy, E.M.; Gaowgzeh, R.A. Treadmill walking exercise modulates bone mineral status and inflammatory cytokines in obese asthmatic patients with long term intake of corticosteroids. Afr. Health Sci. 2016, 16, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Arandelovic, M.; Stankovic, I.; Nikolic, M. Swimming and persons with mild persistant asthma. Sci. World J. 2007, 7, 1182–1188. [Google Scholar] [CrossRef]
- Boyd, A.; Yang, C.T.; Estell, K.; Ms, C.T.; Gerald, L.B.; Dransfield, M.; Bamman, M.; Bonner, J.; Atkinson, T.P.; Schwiebert, L.M. Feasibility of exercising adults with asthma: A randomized pilot study. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2012, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, L.M.; Clark, C.J. Benefits and problems of a physical training programme for asthmatic patients. Thorax 1990, 45, 345–351. [Google Scholar] [CrossRef]
- Dogra, S.; Kuk, J.L.; Baker, J.; Jamnik, V. Exercise is associated with improved asthma control in adults. Eur. Respir. J. 2011, 37, 318–323. [Google Scholar] [CrossRef]
- Franca-Pinto, A.; Mendes, F.A.; de Carvalho-Pinto, R.M.; Agondi, R.C.; Cukier, A.; Stelmach, R.; Saraiva-Romanholo, B.M.; Kalil, J.; Martins, M.A.; Giavina-Bianchi, P.; et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: A randomised controlled trial. Thorax 2015, 70, 732–739. [Google Scholar] [CrossRef]
- Goncalves, R.C.; Nunes, M.P.T.; Cukier, A.; Stelmach, R.; Martins, M.A.; Carvalho, C.R.F. Effects of an aerobic physical training program on psychosocial characteristics, quality-of-life, symptoms and exhaled nitric oxide in individuals with moderate or severe persistent asthma. Braz. J. Phys. Ther. 2008, 12, 127–135. [Google Scholar]
- Haas, F.; Pasierski, S.; Levine, N.; Bishop, M.; Axen, K.; Pineda, H.; Haas, A. Effect of aerobic training on forced expiratory airflow in exercising asthmatic humans. J. Appl. Physiol. 1987, 63, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.A.; Almeida, F.M.; Cukier, A.; Stelmach, R.; Jacob-Filho, W.; Martins, M.A.; Carvalho, C.R. Effects of aerobic training on airway inflammation in asthmatic patients. Med. Sci. Sports Exerc. 2011, 43, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.A.R.; Goncalves, R.C.; Nunes, M.P.T.; Saraiva-Romanholo, B.M.; Cukier, A.; Stelmach, R.; Jacob-Filho, W.; Martins, M.A.; Carvalho, C.R.F. Effects of Aerobic Training on Psychosocial Morbidity and Symptoms in Patients With Asthma A Randomized Clinical Trial. Chest 2010, 138, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, C.; Elfving, B.; Dupre, B.; Opava, C.H.; Lundberg, I.E.; Jansson, E. Effects of a one-year physical activity programme for women with systemic lupus erythematosus—A randomized controlled study. Lupus 2016, 25, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Florian, J.; Rubin, A.; Mattiello, R.; da Fontoura, F.F.; Camargo, J.D.P.; Teixeira, P.J.Z. Impact of pulmonary rehabilitation on quality of life and functional capacity in patients on waiting lists for lung transplantation. J. Bras. De Pneumol. 2013, 39, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Gaunaurd, I.A.; Gomez-Marin, O.W.; Ramos, C.F.; Sol, C.M.; Cohen, M.I.; Cahalin, L.P.; Cardenas, D.D.; Jackson, R.M. Physical activity and quality of life improvements of patients with idiopathic pulmonary fibrosis completing a pulmonary rehabilitation program. Respir. Care 2014, 59, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Iwanami, Y.; Sugino, K.; Gocho, K.; Homma, S.; Ebihara, S. Using 6-Min Walk Distance Expressed as a Percentage of Reference to Evaluate the Effect of Pulmonary Rehabilitation in Elderly Patients With Interstitial Lung Disease. J. Cardiopulm. Rehabil. Prev. 2018, 38, 342–347. [Google Scholar] [CrossRef]
- Jackson, R.M.; Gómez-Marín, O.W.; Ramos, C.F.; Sol, C.M.; Cohen, M.I.; Gaunaurd, I.A.; Cahalin, L.P.; Cardenas, D.D. Exercise limitation in IPF patients: A randomized trial of pulmonary rehabilitation. Lung 2014, 192, 367–376. [Google Scholar] [CrossRef]
- Marcellis, R.; Van der Veeke, M.; Mesters, I.; Drent, M.; De Bie, R.; De Vries, G.; Lenssen, A. Does physical training reduce fatigue in sarcoidosis? Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. Wasog 2015, 32, 53–62. [Google Scholar]
- Naz, I.; Ozalevli, S.; Ozkan, S.; Sahin, H. Efficacy of a Structured Exercise Program for Improving Functional Capacity and Quality of Life in Patients With Stage 3 and 4 Sarcoidosis: A RANDOMIZED CONTROLLED TRIAL. J. Cardiopulm. Rehabil. Prev. 2018, 38, 124–130. [Google Scholar] [CrossRef]
- Naz, I.; Sahin, H.; Uçsular, F.D.; Yalniz, E. A comparison trial of eight weeks versus twelve weeks of exercise program in interstitial lung diseases. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 299–307. [Google Scholar]
- Perez-Bogerd, S.; Wuyts, W.; Barbier, V.; Demeyer, H.; Van Muylem, A.; Janssens, W.; Troosters, T. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: A randomised controlled trial. Respir. Res. 2018, 19, 182. [Google Scholar] [CrossRef]
- Sciriha, A.; Lungaro-Mifsud, S.; Fsadni, P.; Scerri, J.; Montefort, S. Pulmonary Rehabilitation in patients with Interstitial Lung Disease: The effects of a 12-week programme. Respir. Med. 2019, 146, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Strookappe, B.; Elfferich, M.; Swigris, J.; Verschoof, A.; Veschakelen, J.; Knevel, T.; Drent, M. Benefits of physical training in patients with idiopathic or end-stage sarcoidosis-related pulmonary fibrosis: A pilot study. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2015, 32, 43–52. [Google Scholar]
- Vainshelboim, B.; Kramer, M.R.; Fox, B.D.; Izhakian, S.; Sagie, A.; Oliveira, J. Supervised exercise training improves exercise cardiovascular function in idiopathic pulmonary fibrosis. Eur. J. Phys. Rehabil. Med. 2017, 53, 209–218. [Google Scholar] [PubMed]
- Vainshelboim, B.; Oliveira, J.; Fox, B.D.; Soreck, Y.; Fruchter, O.; Kramer, M.R. Long-term effects of a 12-week exercise training program on clinical outcomes in idiopathic pulmonary fibrosis. Lung 2015, 193, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Vainshelboim, B.; Oliveira, J.; Yehoshua, L.; Weiss, I.; Fox, B.D.; Fruchter, O.; Kramer, M.R. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respir. Int. Rev. Thorac. Dis. 2014, 88, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Bettoncelli, G.; Novelli, L.; Cricelli, C.; Rogliani, P. Asthma and comorbid medical illness. Eur. Respir. J. 2011, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- King, C.S.; Nathan, S.D. Idiopathic pulmonary fibrosis: Effects and optimal management of comorbidities. Lancet Respir. Med. 2017, 5, 72–84. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Boyd, C.; Boschetto, P.; Rabe, K.F.; Buist, A.S.; Yawn, B.; Leff, B.; Kent, D.M.; Schünemann, H.J. How to integrate multiple comorbidities in guideline development: Article 10 in integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc. Am. Thorac. Soc. 2012, 9, 274–281. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Hsieh, P.-L.; Hsiao, S.-F.; Chien, M.-Y. Effects of exercise training on autonomic function in chronic heart failure: Systematic review. Biomed Res. Int. 2015, 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, N.; Nassen, J.; Buys, R.; Fourneau, I.; Cornelissen, V. The Impact of Supervised Exercise Training on Traditional Cardiovascular Risk Factors in Patients With Intermittent Claudication: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Kachur, S.; Chongthammakun, V.; Lavie, C.J.; De Schutter, A.; Arena, R.; Milani, R.V.; Franklin, B.A. Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog. Cardiovasc. Dis. 2017, 60, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.-c.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Hansen, D.; Dendale, P.; Coninx, K.; Vanhees, L.; Piepoli, M.F.; Niebauer, J.; Cornelissen, V.; Pedretti, R.; Geurts, E.; Ruiz, G.R. The European Association of Preventive Cardiology Exercise Prescription in Everyday Practice and Rehabilitative Training (EXPERT) tool: A digital training and decision support system for optimized exercise prescription in cardiovascular disease. Concept, definitions and construction methodology. Eur. J. Prev. Cardiol. 2017, 24, 1017–1031. [Google Scholar] [PubMed]
- American Association of Cardiovascular Pulmonary Rehabilitation. Guidelines for Pulmonary Rehabilitation Programs; Human Kinetics: Stanningley, UK, 2011. [Google Scholar]
- Troosters, T.; Van Remoortel, H. Pulmonary rehabilitation and cardiovascular disease. Semin. Respir. Crit. Care Med. 2009, 30, 675–683. [Google Scholar] [CrossRef]
- Hansen, D.; Piepoli, M.F.; Doehner, W. The importance of rehabilitation in the secondary prevention of cardiovascular disease. Eur. J. Prev. Cardiol. 2019, 26, 273–276. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Piepoli, M.F.; Corra, U.; Benzer, W.; Bjarnason-Wehrens, B.; Dendale, P.; Gaita, D.; McGee, H.; Mendes, M.; Niebauer, J.; Zwisler, A.-D.O. Secondary prevention through cardiac rehabilitation: From knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 1–17. [Google Scholar] [CrossRef]
- Wenger, N.K. Current status of cardiac rehabilitation. J. Am. Coll. Cardiol. 2008, 51, 1619–1631. [Google Scholar] [CrossRef]




| Study | Selection Bias | Study Design | Confounders | Blinding | Data Collection Method | Withdrawals and Drop-Outs | Global Rating |
|---|---|---|---|---|---|---|---|
| Cochrane et al., 1990 | 3 | 1 | 1 | 3 | 1 | 3 | 3 |
| Berry et al., 1999 | 2 | 2 | 3 | 3 | 1 | 1 | 3 |
| Foy et al., 2001 | 3 | 1 | 3 | 3 | 1 | 1 | 3 |
| Berry et al., 2003 | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
| Panton et al., 2004 | 2 | 1 | 3 | 3 | 1 | 1 | 3 |
| Marquis et al., 2008 | 2 | 1 | 3 | 1 | 1 | 3 | 3 |
| Averna et al., 2009 | 3 | 1 | 1 | 3 | 1 | 1 | 2 |
| Berry et al., 2010 | 3 | 1 | 1 | 2 | 1 | 1 | 2 |
| Rejbi et al., 2010 | 2 | 1 | 2 | 3 | 1 | 1 | 2 |
| Camillo et al., 2011 | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Lan et al., 2011 | 3 | 2 | 2 | 3 | 1 | 3 | 3 |
| Corhay et al., 2012 | 3 | 2 | 3 | 2 | 1 | 2 | 3 |
| Georgiopoulou et al., 2012 | 2 | 2 | NA | 3 | 1 | 1 | 2 |
| Lan et al., 2013 | 2 | 2 | NA | 3 | 1 | 3 | 3 |
| Cheng et al., 2014 | 3 | 2 | NA | 3 | 1 | 3 | 3 |
| Gaunaurd et al., 2014 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Vainshelboim et al., 2014 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Borghi-Silva et al., 2015 | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
| Campos et al., 2015 | 2 | 2 | NA | 3 | 1 | 1 | 2 |
| Leite et al., 2015 | 2 | 1 | 1 | 3 | 1 | 3 | 3 |
| Marcellis et al., 2015 | 2 | 2 | NA | 3 | 1 | 2 | 2 |
| Mkacher et al., 2015 | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Spielmanns et al., 2015 | 3 | 1 | 1 | 2 | 1 | 3 | 3 |
| Vainshelboim et al., 2015 | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Boström et al., 2016 | 3 | 1 | 1 | 2 | 1 | 1 | 2 |
| Cardoso et al., 2016 | 2 | 1 | 3 | 3 | 1 | 1 | 3 |
| El-Kader et al., 2016 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Engel et al., 2016 | 3 | 1 | 1 | 1 | 1 | 1 | 2 |
| Boeselt et al., 2017 | 3 | 1 | 1 | 3 | 1 | 2 | 3 |
| Kanao et al., 2017 | 3 | 2 | NA | 3 | 1 | 1 | 3 |
| Pacheco et al., 2017 | 2 | 3 | NA | 3 | 1 | 1 | 3 |
| Papp et al., 2017 | 3 | 1 | 3 | 3 | 1 | 2 | 3 |
| Vainshelboim et al., 2017 | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Vasilopoulou et al., 2017 | 3 | 1 | 3 | 3 | 1 | 1 | 3 |
| Lan et al., 2018 | 3 | 2 | NA | 3 | 1 | 1 | 3 |
| Moezy et al., 2018 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Naz et al., 2018a | 2 | 2 | NA | 3 | 1 | 1 | 2 |
| Naz et al., 2018b | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Silva et al., 2018 | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Charikiopoulou et al., 2019 | 2 | 2 | 3 | 3 | 1 | 1 | 3 |
| Mekki et al., 2019 | 2 | 1 | 1 | 2 | 1 | 2 | 2 |
| Silva et al., 2019 | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Study and Country | Study Design | Population | Intervention | Duration and Frequency | Cardiovascular Outcomes and Outcome Measures | Results on Cardiovascular Outcomes |
|---|---|---|---|---|---|---|
| Berry et al., 1999 United States of America | Non-controlled study | 151 patients with COPD Mild disease group: 99 (54♂; 67.4 ± 6.1 years; FEV1 68.0 ± 1.2%pred) Arterial hypertension: n = 44 Circulatory problems: n = 14 Coronary heart disease: n = 34 Moderate disease group: 36 (22♂; 68.3 ± 6.2 years; FEV1 41.9 ± 0.7%pred) Arterial hypertension: n = 16 Circulatory problems: n = 5 Coronary heart disease: n = 12 Severe disease group: 16 (10♂; 66.1 ± 5.6 years; FEV1 30.1 ± 0.9%pred) Arterial hypertension: n = 7 Circulatory problems: n = 2 Coronary heart disease: n = 8 | All groups: Aerobic and strength training Dyspnoea 3–4 in the mBorg | All groups: 12 weeks 3 sessions/week 1 h/session | ||
| Foy et al., 2001 United States of America | Randomized controlled trial | 140 patients with COPD Short-term intervention group: 70 (39♂; 66.9 ± 5.9 years; FEV1 59.1 ± 17.2%pred) Arterial hypertension: n = 29 Circulatory problems: n = 14 Cardiovascular disease: n = 27 Long-term intervention group: 70 (39♂; 68.4 ± 6.0 years; FEV1 57.6 ± 18.4%pred) Arterial hypertension: n = 32 Circulatory problems: n = 9 Cardiovascular disease: n = 24 | All groups: Aerobic and strength training Dyspnoea 3–4 in the mBorg | 3 sessions/week 55–65 min/session Short-term intervention group: 12 weeks Long-term intervention group: 72 weeks | ||
| Berry et al., 2003 United States of America | Randomized controlled trial | 140 patients with COPD Short-term intervention group: 70 (39♂; 66.9, 95%CI (65.5; 68.3) years; FEV1 59.1, 95%CI (55.0; 63.2)%pred) Arterial hypertension: n = 29 Circulatory problems: n = 14 Cardiovascular disease: n = 27 Long-term intervention group: 70 (39♂; 68.4, 95%CI (67.0; 69.8) years; FEV1 57.6, 95%CI (53.2; 62.0)%pred) Arterial hypertension: n = 32 Circulatory problems: n = 9 Cardiovascular disease: n = 24 | All groups: Aerobic and strength training Dyspnoea 3–4 in the mBorg | 3 sessions/week 1 h/session Short-term intervention group 1: 3 months Long-term intervention group: 18 months | ||
| Panton et al., 2004 United States of America | Non-randomized controlled trial | 17 patients with COPD Aerobic training group: 8 (2♂; 63.0 ± 8.0 years; FEV1 39.5 ± 31.9%pred) Aerobic+strength training group: 9 (6♂; 61.0 ± 7.0 years; FEV1 41.9 ± 16.0%pred) | Aerobic training group: Aerobic training 50%–70% of HR reserve Aerobic+strength training group: Aerobic and strength training 50%–70% of HR reserve | 12 weeks Aerobic training group: 2 sessions/week 60 min/session Aerobic + strength training group: 4 sessions/week (2 of each training) 45–60 min/session | Rate pressure product | Aerobic training group: Pre 177.0 ± 29.0 vs. Post 186.0 ± 30.0, p > 0.05 ES = 0.31 Aerobic+strength training group: Pre 195.0 ± 35.0 vs. Post 199.0 ± 35.0, p > 0.05 ES = 0.11 |
| Total blood cholesterol (mg/dl) | Aerobic training group: Pre 217.0 ± 46.0 vs. Post 217.0 ± 46.0, p > 0.05 ES = 0.00 Aerobic+strength training group: Pre 201.0 ± 34.0 vs. Post 193.0 ± 23.0, p > 0.05 ES = −0.28 | |||||
| Cholesterol – HDL (mg/dl) | Aerobic training group: Pre 62.0 ± 21.0 vs. Post 62.0 ± 20.0, p > 0.05 ES = 0.00 Aerobic+strength training group: Pre 55.0 ± 16.0 vs. Post 53.0 ± 12.0, p > 0.05 ES = −0.14 | |||||
| Cholesterol – LDL (mg/dl) | Aerobic training group: Pre 129.0 ± 34.0 vs. Post 132.0 ± 35.0, p > 0.05 ES = 0.09 Aerobic+strength training group: Pre 122.0 ± 21.0 vs. Post 118.0 ± 15.0, p > 0.05 ES = −0.22 | |||||
| Cholesterol – Triglycerides (mg/dl) | Aerobic training group: Pre 151.0 ± 65.0 vs. Post 185.0 ± 87.0, p < 0.05 ES = 0.44 Aerobic+strength training group: Pre 141.0 ± 132.0 vs. Post 135.0 ± 73.0, p > 0.05 ES = −0.06 | |||||
| Total cholesterol/HDL ratio | Aerobic training group: Pre 3.8 ± 1.1 vs. Post 3.9 ± 1.1, p > 0.05 ES = 0.09 Aerobic+strength training group: Pre 3.8 ± 0.8 vs. Post 3.8 ± 0.8, p > 0.05 ES = 0.00 | |||||
| Marquis et al., 2008 Canada | Randomized controlled trial | 16 patients with COPD Irbesartan+exercise group: 10 (7♂; 67.0 ± 7.0 years; FEV1 50.0 ± 19.0%pred; FVC 63.0 ± 16.0%pred; DLCO 80.0 ± 19.0%pred) Placebo+exercise group: 6 (1♂; 72.0 ± 5.0 years; FEV1 39.0 ± 9.0%pred; FVC 63.0 ± 15.0%pred; DLCO 63.0 ± 18.0%pred) | All groups: Aerobic training 80% of WRmax | All groups: 12 weeks 3 sessions/week 30 min/session | Systolic blood pressure at rest (mmHg) | Irbesartan+exercise group: Pre 151.0 ± 19.0 vs. Post 131.0 ± 18.0, p < 0.05 ES = −1.08 Placebo+exercise group: Pre 140.0 ± 15.0 vs. Post 136.0 ± 15.0, p > 0.05 ES = −0.27 |
| Mean systolic blood pressure during 24 h (mmHg) | Irbesartan+exercise group: Pre 135.0 ± 9.0 vs. Post 126.0 ± 12.0, p < 0.01 ES = −0.85 Placebo+exercise group: Pre 130.0 ± 14.0 vs. Post 128.0 ± 8.0, p > 0.05 ES = −0.18 | |||||
| Mean systolic blood pressure at daytime (mmHg) | Irbesartan+exercise group: Pre 139.0 ± 11.0 vs. Post 129.0 ± 15.0, p < 0.01 ES = −0.76 Placebo+exercise group: Pre 130.0 ± 14.0 vs. Post 131.0 ± 8.0, p > 0.05 ES = 0.09 | |||||
| Mean systolic blood pressure at nighttime (mmHg) | Irbesartan+exercise group: Pre 125.0 ± 8.0 vs. Post 121.0 ± 10.0, p > 0.05 ES = −0.44 Placebo+exercise group: Pre 128.0 ± 16.0 vs. Post 121.0 ± 9.0, p > 005 ES = −0.54 | |||||
| Diastolic blood pressure at rest (mmHg) | Irbesartan+exercise group: Pre 78.0 ± 8.0 vs. Post 71.0 ± 10.0, p < 0.05 ES = −0.77 Placebo+exercise group: Pre 72.0 ± 8.0 vs. Post 68.0 ± 10.0, p > 0.05 ES = −0.44 | |||||
| Mean diastolic blood pressure during 24 h (mmHg) | Irbesartan+exercise group: Pre 76.0 ± 9.0 vs. Post 72.0 ± 8.0, p < 0.05 ES = −0.47 Placebo+exercise group: Pre 70.0 ± 3.0 vs. Post 70.0 ± 8.0, p > 0.05 ES = 0.00 | |||||
| Mean diastolic blood pressure at daytime (mmHg) | Irbesartan+exercise group: Pre 80.0 ± 11.0 vs. Post 74.0 ± 10.0, p < 0.05 ES = −0.84 Placebo+exercise group: Pre 71.0 ± 2.0 vs. Post 72.0 ± 7.0, p > 0.05 ES = 0.19 | |||||
| Mean diastolic blood pressure at nighttime (mmHg) | Irbesartan+exercise group: Pre 68.0 ± 6.0 vs. Post 67.0 ± 7.0, p > 0.05 ES = −0.15 Placebo+exercise group: Pre 66.0 ± 6.0 vs. Post 65.0 ± 8.0, p > 0.05 ES = −0.14 | |||||
| Standard deviation of all NN intervals (ms) | Irbesartan+exercise group: Pre 102.0 ± 28.0 vs. Post 144.0 ± 36.0, p > 0.05 ES = 1.30 Placebo+exercise group: Pre 121.0 ± 27.0 vs. Post 113.0 ± 38.0, p > 0.05 ES = −0.24 | |||||
| Adjacent normal-to-normal (NN) intervals differing by more than 50 ms (%) | Irbesartan+exercise group: Pre 9.0 ± 9.0 vs. Post 9.0 ± 8.0, p > 0.05 ES = 0.00 Placebo+exercise group: Pre 10.0 ± 9.0 vs. Post 10.0 ± 8.0, p > 0.05 ES = 0.00 | |||||
| Square root of the mean squared differences of successive NN intervals (ms) | Irbesartan+exercise group: Pre 30.0 ± 12.0 vs. Post 29.0 ± 11.0, p > 0.05 ES = −0.09 Placebo+exercise group: Pre 31.0 ± 11.0 vs. Post 30.0 ± 10.0, p > 0.05 ES = −0.10 | |||||
| Very low frequency (ms) | Irbesartan+exercise group: Pre 3.3 ± 0.2 vs. Post 3.3 ± 0.2, p > 0.05 ES = 0.00 Placebo+exercise group: Pre 3.1 ± 0.2 vs. Post 3.1 ± 0.5, p > 0.05 ES = 0.07 | |||||
| Low frequency (ms) | Irbesartan+exercise group: Pre 2.9 ± 0.3 vs. Post 2.9 ± 0.3, p > 0.05 ES = −0.03 Placebo+exercise group: Pre 2.8 ± 0.4 vs. Post 2.8 ± 0.4, p > 0.05 ES = 0.02 | |||||
| High frequency (ms) | Irbesartan+exercise group: Pre 2.4 ± 0.3 vs. Post 2.4 ± 0.4, p > 0.05 ES = −0.03 Placebo+exercise group: Pre 2.4 ± 0.4 vs. Post 2.4 ± 0.5, p > 0.05 ES = −0.12 | |||||
| Low frequency/High frequency ratio | Irbesartan+exercise group: Pre 3.0 ± 1.3 vs. Post 3.1 ± 1.3, p > 0.05 ES = 0.08 Placebo+exercise group: Pre 2.1 ± 1.0 vs. Post 2.4 ± 1.0, p > 0.05 ES = 0.27 | |||||
| Averna et al., 2009 Italy | Randomized controlled trial | 56 patients with COPD (29♂; 69.0 ± 5.0 years; FEV1 82.0 ± 16.6%pred; FVC 91.0 ± 17.4%pred) | Aerobic and strength training 40%–50% of HR reserve 50% of 1 RM | 12 weeks 3 sessions/week 60 min/session | HR at rest (bpm) | Pre 65.0 ± 10.0 vs. Post 65.0 ± 9.0, p = 0.64 ES = 0.00 |
| Systolic blood pressure at rest (mmHg) | Pre 137.0 ± 12.0 vs. Post 131.0 ± 12.0, p = 0.001 ES = −0.70 | |||||
| Diastolic blood pressure at rest (mmHg) | Pre 84.0 ± 6.0 vs. Post 80.0 ± 7.0, p = 0.001 ES = −0.61 | |||||
| Berry et al., 2010 United States of America | Randomized controlled trial | 89 patients with COPD (48♂; 66.0 ± 10.0 years; FEV1 53.0 ± 18.5%pred) Arterial hypertension: n = 47 Circulatory problems: n = 17 Cardiovascular disease: n = 39 | Aerobic and strength training Dyspnoea 3–5 in the mBorg | 12 weeks 3 sessions/week 1 h/session | ||
| Rejbi et al., 2010 Tunisia | Non-randomized controlled trial | 26 patients with COPD (61.0 ± 4.0 years; FEV1 48.9 ± 11.3%pred; FVC 58.8 ± 9.8%pred) | Pulmonary rehabilitation HR of the gas exchange threshold | 3 months 3 sessions/week 45 min/session | HR at rest (bpm) | Pre 75.6 ± 13.9 vs. Post 76.5 ± 14.0, p > 0.05 ES = 0.06 |
| Camillo et al., 2011 Brazil | Randomized controlled trial | 40 patients with COPD High-intensity group: 20 (10♂; 67.0 ± 7.0 years; FEV1 40.0 ± 13.0%pred) Low-intensity group: 20 (11♂; 65.0 ± 10.0 years; FEV1 39.0 ± 14.0%pred) | High-intensity group: Aerobic and strength training 60% of WRmax 75% of average walking speed in the 6MWT 70% of 1RM Low-intensity group: Strength training | All groups: 12 weeks 3 sessions/week 1 h/session | Standard deviation of N-N intervals (ms) | High-intensity group: Pre 29.0 ± 15.0 vs. Post 36.0 ± 19.0, p < 0.05 ES = 0.41 Low-intensity group: Pre 25.0 ± 12.0 vs. Post 22.0 ± 10.0, p > 0.05 ES = −0.27 |
| Square root of the mean squared difference of the successive N-N intervals (ms) | High-intensity group: Pre 22.0 ± 14.0 vs. Post 28.0 ± 22.0, p < 0.05 ES = 0.33 Low-intensity group: Pre 22.0 ± 22.0 vs. Post 19.0 ± 14.0, p > 0.05 ES = −0.16 | |||||
| Low frequency in supine (%) | High-intensity group: Pre 44.0 ± 15.0 vs. Post 42.0 ± 24.0, p > 0.05 ES = −0.10 Low-intensity group: Pre 48.0 ± 19.0 vs. Post 43.0 ± 19.0, p > 0.05 ES = −0.26 | |||||
| Low frequency in orthostatic (%) | High-intensity group: Pre 55.0 ± 21.0 vs. Post 50.0 ± 20.0, p > 0.05 ES = −0.24 Low-intensity group: Pre 58.0 ± 15.0 vs. Post 62.0 ± 20.0, p > 0.05 ES = 0.23 | |||||
| High frequency in supine (%) | High-intensity group: Pre 56.0 ± 15.0 vs. Post 58.0 ± 24.0, p > 0.05 ES = 0.10 Low-intensity group: Pre 51.0 ± 19.0 vs. Post 56.0 ± 19.0, p > 0.05 ES = 0.26 | |||||
| High frequency in orthostatic (%) | High-intensity group: Pre 44.0 ± 21.0 vs. Post 50.0 ± 20.0, p > 0.05 ES = 0.29 Low-intensity group: Pre 41.0 ± 15.0 vs. Post 37.0 ± 20.0, p > 0.05 ES = −0.23 | |||||
| Low frequency/High frequency ratio in supine | High-intensity group: Pre 0.9 ± 0.8 vs. Post 1.3 ± 1.5, p > 0.05 ES = 0.60 Low-intensity group: Pre 1.2 ± 0.9 vs. Post 1.1 ± 1.2, p > 0.05 ES = −0.09 | |||||
| Low frequency/High frequency ratio in orthostatic | High-intensity group: Pre 2.3 ± 3.1 vs. Post 1.3 ± 0.9, p > 0.05 ES = −0.44 Low-intensity group: Pre 1.7 ± 1.0 vs. Post 2.8 ± 2.8, p > 0.05 ES = 0.52 | |||||
| Lan et al., 2011 Taiwan | Non-controlled study | 44 patients with COPD Underweight group: 22 (21♂; 69.1 ± 12.0 years; FEV1 52.8 ± 17.1%pred; FVC 79.5 ± 21.4%pred) Non-underweight group: 22 (21♂; 71.4 ± 7.5 years; FEV1 51.5 ± 13.3%pred; FVC 79.1 ± 15.1%pred) | All groups: Pulmonary rehabilitation 50%–75% of VO2peak | All groups: 12 weeks 2 sessions/week 40–50 min/session | HR at rest (bpm) | Underweight group: Pre 85.2 ± 13.0 vs. Post 83.1 ± 11.7, p = 0.315 ES = −0.17 Non-underweight group: Pre 88.2 ± 11.6 vs. Post 86.0 ± 10.8, p = 0.029 ES = −0.20 |
| Corhay et al., 2012 Belgium | Non-controlled study | 140 patients with COPD <65 years group: 69 (42♂; 57.6 ± 5.2 years; FEV1 38.1 ± 10.8%pred) Cardiovascular disease: n = 19 65–74 years group: 50 (36♂; 69.5 ± 2.6 years; FEV1 39.5 ± 11.7%pred) Cardiovascular disease: n = 23 ≥75 years group: 21 (17♂; 77.4 ± 2.5 years; FEV1 39.9 ± 9.2%pred) Cardiovascular disease: n = 14 | All groups: Pulmonary rehabilitation 50%–80% of WRmax 60% of maximal walking speed in the 6MWT 50% of 1RM | All groups: 6 months 2–3 sessions/week 2 h/session | ||
| Georgiopoulou et al., 2012 Greece | Pre-Post study | 45 patients with COPD (40♂; 66.5 ± 7.6 years; FEV1 45.7 ± 18.7%pred; FVC 78.3 ± 18.6%pred) | Pulmonary rehabilitation 60%–80% of WRmax | 12 weeks 3 sessions/week 40 min/session | HR at rest (bpm) | Pre 88.0 ± 10.7 vs. Post 83.3 ± 10.5, p = 0.004 ES = −0.63 |
| HR recovery (bpm) | Pre 16.2 ± 8.0 vs. Post 18.4 ± 8.4, p = 0.01 ES = 0.27 | |||||
| Lan et al., 2013 Taiwan | Pre-Post study | 26 patients with COPD (71.0 ± 10.7 years; FEV1 64.8 ± 23.0%pred; FVC 88.3 ± 34.5%pred) | Pulmonary rehabilitation 75%–100% of VO2max | 12 weeks 2 sessions/week 40 min/session | HR (bpm) | Pre 134.5 ± 14.9 vs. Post 137.4 ± 19.9, p = 0.36 ES = 0.16 |
| Mean blood pressure (mmHg) | Pre 109.6 ± 15.7 vs. Post 110.3 ± 15.1, p = 0.72 ES = 0.05 | |||||
| Oxygen pulse (ml/beat) | Pre 9.2 ± 2.5 vs. Post 9.8 ± 2.7, p = 0.02 ES = 0.23 | |||||
| Cheng et al., 2014 Taiwan | Pre-Post study | 64 patients with COPD (55♂; 70.1 ± 8.7 years; FEV1 44.9 ± 11.7%pred; FVC 78.2 ± 17.4%pred) | Pulmonary rehabilitation 60%–100% of VO2peak | 12 weeks 2 sessions/week 50 min/session | HR at rest (bpm) | Pre 87.2 ± 12.7 vs. Post 83.9 ± 13.5, p = 0.048 ES = −0.25 |
| Oxygen pulse (ml/beat) | Pre 7.2 ± 1.9 vs. Post 7.9 ± 2.2, p = 0.005 ES = 0.34 | |||||
| Oxygen pulse (%) | Pre 76.8 ± 18.4 vs. Post 85.2 ± 24.8, p = 0.003 ES = 0.38 | |||||
| Standard deviation of N-N | At rest: Pre vs. Post, p < 0.05 At exercise: Pre vs. Post, p < 0.05 | |||||
| Square root of the mean sum of the squares of the difference between adjacent normal R-R intervals | At rest: Pre vs. Post, p < 0.05 At exercise: Pre vs. Post, p < 0.05 | |||||
| Low frequency | At rest: Pre vs. Post, p < 0.05 At exercise: Pre vs. Post, p < 0.05 | |||||
| High frequency | At rest: Pre vs. Post, p < 0.05 At exercise: Pre vs. Post, p < 0.05 | |||||
| Low frequency/High frequency ratio | At rest: Pre vs. Post, p < 0.05 At exercise: Pre vs. Post, p < 0.05 | |||||
| Borghi-Silva et al., 2015 Brazil | Randomized controlled trial | 10 patients with COPD (7♂; 67.0 ± 7.0 years; FEV1 32.0 ± 11.0%pred; FVC 58.0 ± 15.0%pred) | Aerobic training 70% of peak speed in CPET | 12 weeks 3 sessions/week 30 min/session | Mean of RR and its standard deviation at rest (ms) | Pre 17.2 ± 7.3 vs. Post 25.4 ± 5.5, p < 0.05 ES = 1.27 |
| Mean of RR and its standard deviation at constant speed (ms) | Pre 12.7 ± 5.1 vs. Post 18.3 ± 4.7, p > 0.05 ES = 1.14 | |||||
| Square root of the mean squared differences of successive RRi at rest (ms) | Pre 11.7 ± 6.0 vs. Post 22.9 ± 0.2, p < 0.05 ES = 2.64 | |||||
| Square root of the mean squared differences of successive RRi at constant speed (ms) | Pre 3.5 ± 1.7 vs. Post 16.9 ± 7.0, p < 0.05 ES = 2.63 | |||||
| Nonlinear indices – SD1 at rest | Pre 7.1 ± 4.2 vs. Post 19.2 ± 11.8, p < 0.05 ES = 1.37 | |||||
| Nonlinear indices – SD1 at constant speed | Pre 3.7 ± 1.7 vs. Post 13.6 ± 8.8, p < 0.05 ES = 1.56 | |||||
| Nonlinear indices – SD2 at rest | Pre 31.2 ± 6.6 vs. Post 46.1 ± 22.0, p < 0.05 ES = 0.92 | |||||
| Nonlinear indices – SD2 at constant speed | Pre 17.3 ± 5.9 vs. Post 25.4 ± 6.5, p < 0.05 ES = 1.30 | |||||
| Low frequency (nu) | Pre 0.6 ± 0.2 vs. Post 0.5 ± 0.2, p > 0.05 ES = −0.60 | |||||
| High frequency (nu) | Pre 0.4 ± 0.2 vs. Post 0.5 ± 0.2, p > 0.05 ES = 0.60 | |||||
| Low frequency/High frequency ratio | Pre 2.4 ± 2.3 vs. Post 1.8 ± 1.7, p > 0.05 ES = −0.31 | |||||
| Sample entropy | Pre 0.7 ± 0.2 vs. Post 0.9 ± 0.2, p > 0.05 ES = 1.03 | |||||
| Campos et al., 2015 Chile | Pre-Post study | 39 patients with COPD (36%♂; 67.3 ± 8.5 years; FEV1 59.8 ± 21.0%pred; FVC 78.0 ± 20.3%pred) Arterial hypertension: n = 31 Dyslipidemia: n = 5 Congestive heart failure: n = 3 | Pulmonary rehabilitation 70%–80% of 6MWT | 12 weeks 2 sessions/week 90 min/session | ||
| Leite et al., 2015 Brazil | Non-randomized controlled trial | 10 patients with COPD (62.0 (60.3; 69.3) years; FEV1 55.0 (39.0; 70.0)%pred; FVC 78.0 (66.3; 83.5)%pred) | Aerobic training 60%–100% of VO2peak | 12 weeks 3 sessions/week 20–50 min/session | Standard deviation of the mean of all normal RR intervals (ms) | Pre 19.8 ± 6.2 vs. Post 24.9 ± 8.6, p > 0.05 ES = 0.67 |
| Root mean square of differences between adjacent normal RR intervals in a time interval (ms) | Pre 14.2 ± 5.7 vs. Post 18.3 ± 6.2, p > 0.05 ES = 0.69 | |||||
| Spectral component of low frequency (ms2) | Pre146.1 ± 118.9 vs. Post 177.7 ± 125.6, p > 0.05 ES = 0.26 | |||||
| Spectral component of low frequency (nu) | Pre 67.5 ± 16.0 vs. Post 58.5 ± 13.6, p > 0.05 ES = −0.61 | |||||
| Spectral component of high frequency (ms2) | Pre 62.3 ± 46.8 vs. Post 113.2 ± 62.2, p < 0.05 ES = 0.92 | |||||
| Spectral component of high frequency (nu) | Pre 32.6 ± 15.9 vs. Post 41.5 ± 13.6, p > 0.05 ES = 0.60 | |||||
| Low frequency/High frequency ratio | Pre 2.9 ± 2.2 vs. Post 1.6 ± 0.8, p > 0.05 ES = −0.78 | |||||
| Mkacher et al., 2015 Tunisia | Randomized controlled trial | 68 patients with COPD Pulmonary rehabilitation group: 33 (33♂; 61.2 ± 3.2 years; FEV1 38.6 ± 8.6%pred) Pulmonary rehabilitation+balance group: 35 (35♂; 58.3 ± 4.3 years; FEV1 39.4 ± 10.3%pred) | All groups: Pulmonary rehabilitation | All groups: 6 months 6 sessions/week (3 times/week, 2 sessions/day) | HR at rest (bpm) | Pulmonary rehabilitation group: Pre 72.7 ± 8.9 vs. Post 73.0 ± 4.3, p > 0.05 ES = 0.04 Pulmonary rehabilitation+balance group: Pre 75.3 ± 3.9 vs. Post 73.5 ± 4.5, p > 0.05 ES = −0.43 |
| Spielmanns et al., 2015 Germany | Randomized controlled trial | 36 patients with COPD Compressed air group: 17 (64.0 ± 8.4 years; FEV1 43.0 ± 12.0%pred) Arterial hypertension: n = 7 Cardiovascular disease: n = 2 Oxygen group: 19 (65.0 ± 8.7 years; FEV1 44.0 ± 10.0%pred) Arterial hypertension: n = 8 Cardiovascular disease: n = 4 | All groups: Continuous aerobic training 70%–85% of WRmax Interval aerobic training 110%–125% of WRmax | All groups: 24 weeks 3 sessions/week 30 min/session | ||
| Cardoso et al., 2016 Brazil | Non-randomized controlled trial | 10 patients with COPD (65.2 ± 4.2 years; FEV1 41.8 ± 21.3%pred; FVC 60.7 ± 18.0%pred) Arterial hypertension: n = 7 | Pulmonary rehabilitation 75% of WRmax 60% of 1RM | 12 weeks 3 sessions/week >30 min/session | ||
| Engel et al., 2016 Australia | Randomized controlled trial | 33 patients with COPD (10♂; 65.5 ± 4.0 years; FEV1 1.6 ± 0.5 L; FVC 2.3 ± 0.7 L) | Pulmonary rehabilitation | 16 weeks | Systolic blood pressure (mmHg) | Mean Pre/Post difference Group 1: −3.6, 95%CI (−13.5; 6.3) Group 2: −10.6, 95%CI (−19.6; −1.5) Group 3: −8.3, 95%CI (−20.5; 3.8) |
| Diastolic blood pressure (mmHg) | Mean Pre/Post difference Group 1: −3.5, 95%CI (−12.6; 5.6) Group 2: −7.7, 95%CI (−17.1; 1.8) Group 3: −4.7, 95%CI (−13.5; 4.2) | |||||
| Boeselt et al., 2017 Germany | Non-randomized controlled trial | 20 patients with COPD (16♂; 65.9 ± 8.2 years; FEV1 67.9 ± 29.2%pred) Arterial hypertension: n = 5 Cardiovascular disease: n = 2 | Strength training 35%–75% of 1RM | 3 months 2 sessions/week 90 min/session | ||
| Kanao et al., 2017 Japan | Pre-Post study | 29 patients with COPD (26♂; 73.2 ± 5 years; FEV1 51.0 ± 121.3%pred) Arterial hypertension: n = 10 Cardiovascular disease: n = 5 | Pulmonary rehabilitation 60% of WRpeak | 12 weeks 2 sessions/week | ||
| Pacheco et al., 2017 Spain | Observational study | 35 patients with COPD (88.6%♂; 65.1 ± 9.0 years; FEV1 42.2 ± 10.5; FVC 67.8 ± 13.3%pred; DLCO 47.9 ± 21.0%pred Arterial hypertension: n = 20 Dyslipidemia: n = 6 Congestive heart failure: n = 7 Ischemic cardiomyopathy: n = 3 | Pulmonary rehabilitation 70% of WRmax 75% of 1RM | 12 weeks 3 sessions/week >30 min/session | ||
| Papp et al., 2017 Sweden | Randomized controlled trial | 17 patients with COPD (7♂; 69.0 (62.0; 72.1) years; FEV1 64.3 ± 15.4%pred) | Aerobic and strength training 70% of 1RM Perceived exertion 12–14 in the Borg | 12 weeks 2 sessions/week 60–70 min/session | HR at rest (bpm) | Mean Pre/Post difference 0.6, p = 0.82 |
| Systolic blood pressure at rest (mmHg) | Mean Pre/Post difference 4.2 | |||||
| Diastolic blood pressure at rest (mmHg) | Mean Pre/Post difference 5.7, p = 0.04 | |||||
| Number of pairs of adjacent NN intervals differing by more than 50 ms in the 5 min recording divided by the total number of all NN intervals (%) | Mean Pre/Post difference 0.6, p = 0.56 | |||||
| Square root of the mean of the sum of the squares of differences between adjacent NN intervals | Mean Pre/Post difference −3.2, p = 0.27 | |||||
| Vasilopoulou et al., 2017 Greece | Randomized controlled trial | 50 patients with COPD (38♂; 66.7 ± 7.3 years; FEV1 51.8 ± 17.3%pred; FVC 78.4 ± 18.4%pred; DLCO 57.0 ± 20.4%pred) Cardiovascular disease: n = 15 | Pulmonary rehabilitation | 12 months 2 sessions/week | ||
| Lan et al., 2018 Taiwan | Pre-Post study | 43 patients with COPD (31♂; 69.7 ± 8.8 years; FEV1 49.5 ± 19.9%pred; FVC 76.5 ± 22.3%pred) | Pulmonary rehabilitation | 12 weeks 2 sessions/week 40 min/session | HR at rest | Pre vs. Post p > 0.05 |
| Mean blood pressure at rest | Pre vs. Post p < 0.05 | |||||
| Oxygen pulse | Pre vs. Post p < 0.05 | |||||
| Moezy et al., 2018 Iran | Randomized controlled trial | 14 patients with COPD (71.4%♂; 64.7 ± 7.5 years; FEV1 60.2 ± 14.0%pred) | Aerobic training Dyspnoea 3–4 in the mBorg | 12 weeks 3 sessions/week 15–60 min/session | HR at rest (bpm) | Pre 80.4 ± 12.6 vs. Post 77.8 ± 11.9, p = 0.968 ES = −0.21 |
| Silva et al., 2018 Brazil | Randomized controlled trial | 48 patients with COPD Elastic resistances group: 32 (69.4 ± 9.0 years; FEV1 50.7 ± 16.7%pred; FVC 72.5 ± 13.2%pred) Weight machines group: 16 (64.9 ± 11.2 years; FEV1 45.4 ± 15.2%pred; FVC 66.1 ± 14.0%pred) | All groups: Strength training | All groups: 12 weeks 3 sessions/week 60 min/session | Total cholesterol (mg/dL) | Elastic resistances group: Pre 108.4 ± 25.3 vs. Post 104.6 ± 14.3, p > 0.05 ES = −0.18 Weight machines group: Pre 84.6 ± 27.0 vs. Post 71.1 ± 32.0, p > 0.05 ES = −0.46 |
| Cholesterol – HDL (mg/dL) | Elastic resistances group: Pre 58.4 ± 23.2 vs. Post 63.4 ± 17.3, p > 0.05 ES = 0.24 Weight machines group: Pre 132.3 ± 43.6 vs. Post 150.3 ± 52.3, p > 0.05 ES = 0.37 | |||||
| Cholesterol – Triglycerides (mg/dL) | Elastic resistances group: Pre 154.2 ± 62.3 vs. Post 129.7 ± 40.3, p > 0.05 ES = −0.47 Weight machines group: Pre 104.8 ± 38.4 vs. Post 99.9 ± 32.9, p > 0.05 ES = −0.14 | |||||
| Total cholesterol/HDL ratio (mg/dL) | Elastic resistances group: Pre 50.7 ± 39.9 vs. Post 40.9 ± 25.8, p > 0.05 ES = −0.29 Weight machines group: Pre 71.9 ± 31.2 vs. Post 61.3 ± 15.4, p > 0.05 ES = −0.43 | |||||
| Charikiopoulou et al., 2019 Greece | Non-controlled study | 32 patients with COPD (25♂; 66.0 ± 6.0 years; FEV1 43.1 ± 15.1%pred; DLCO 38.2 ± 22.8%pred) Cardiovascular disease: n = 22 | Pulmonary rehabilitation 100% of WRmax | 13 weeks 2 sessions/week ≥1 h/session | ||
| Mekki et al., 2019 Tunisia | Randomized controlled trial | 45 patients with COPD Pulmonary rehabilitation+NMES group: 25 (25♂; 59.6 ± 4.8 years; FEV1 57.7 ± 14.4%pred; FVC 76.0 ± 13.2%pred) Pulmonary rehabilitation group: 20 (20♂; 59.5 ± 3.1 years; FEV1 57.1 ± 10.2%pred; FVC 75.9 ± 7.8%pred) | All groups: Pulmonary rehabilitation 60%–70% of HRmax in the 6MWT 50%–85% of 10RM | All groups: 6 months 3 sessions/week 80 min/session | HR at rest (bpm) | Pulmonary rehabilitation+NMES group: Pre 80.0 ± 9.0 vs. Post 78.0 ± 9.0, p < 0.001 ES = −0.22 Pulmonary rehabilitation group: Pre 80.0 ± 7.0 vs. Post 77.0 ± 7.0, p < 0.001 ES = −0.43 |
| Silva et al., 2019 Brazil | Randomized controlled trial | 19 patients with COPD Elastic resistances group: 9 (65.9 ± 8.9 years; FEV1 45.2 ± 16.2%pred; FVC 64.7 ± 19.0%pred) Weight machines group: 10 (65.5 ± 9.8 years; FEV1 57.6 ± 16.3%pred; FVC 79.8 ± 11.5%pred) | All groups: Strength training | All groups: 12 weeks 3 sessions/week 60 min/session | HR (bpm) | Elastic resistances group: Pre 74.1 ± 8.8 vs. Post 76.8 ± 8.9, p > 0.05 ES = 0.30 Weight machines group: Pre 71.4 ± 6.4 vs. Post 68.9 ± 9.9, p > 0.05 ES = −0.30 |
| Systolic blood pressure (mmHg) | Elastic resistances group: Pre 120.0 (105.0; 135.0) vs. Post 120.0 (110.0; 120.0), p > 0.05 Weight machines group: Pre 120.0 (117.5; 130.0) vs. Post 120.0 (110.0; 120.0), p > 0.05 | |||||
| Diastolic blood pressure (mmHg) | Elastic resistances group: Pre 70.0 (70.0; 80.0) vs. Post 70.0 (70.0; 75.0), p > 0.05 Weight machines group: Pre 80.0 (70.0; 90.0) vs. Post 75.0 (67.5; 80.0), p > 0.05 |
| Study and Country | Study design | Population | Intervention | Duration and Frequency | Cardiovascular Outcomes and Outcome measures | Results on Cardiovascular Outcomes |
|---|---|---|---|---|---|---|
| Cochrane et al., 1990 Scotland | Randomized controlled trial | 18 patients with Asthma (27.0 ± 17.0 years; FEV1 76.0 ± 12.0%pred) | Aerobic and muscle strength training 75% of HRmax | 3 months 3 sessions/week 30 min/session | Oxygen pulse (mL/beat) | Pre 8.8 ± 2.3 vs. Post 10.8 ± 2.4, p < 0.001 ES = 0.85 |
| Total blood cholesterol (mmol/L) | Pre 5.4 ± 1.1 vs. Post 5.3 ± 1.1, p > 0.05 ES = −0.09 | |||||
| Cholesterol – HDL (mmol/L) | Pre 1.7 ± 0.4 vs. Post 1.6 ± 0.3, p > 0.05 ES = −0.28 | |||||
| Cholesterol – LDL (mmol/L) | Pre 3.2 ± 1.2 vs. Post 2.9 ± 0.9, p > 0.05 ES = −0.28 | |||||
| El-Kader et al., 2016 Saudi Arabia | Randomized controlled trial | 40 patients with Asthma (23♂; 47.2 ± 6.5 years; FEV1 1.4 ± 0.7 L) | Aerobic training 60%–80% of HRmax | 6 months 3 sessions/week 30 min/session | Cholesterol – HDL (mg/dL) | Pre 34.7 ± 5.6 vs. Post 37.9 ± 4.6, p < 0.05 ES = 0.62 |
| Cholesterol – LDL (mg/dL) | Pre 133.7 ± 13.2 vs. Post 120.3 ± 11.5, p < 0.05 ES = −1.08 | |||||
| Cholesterol – Triglycerides (mg/dL) | Pre 155.4 ± 12.6 vs. Post 127.7 ± 11.3, p < 0.05 ES = −2.31 |
| Study and Country | Study Design | Population | Intervention | Duration and Frequency | Cardiovascular Outcomes and Outcome Measures | Results on Cardiovascular Outcomes |
|---|---|---|---|---|---|---|
| Gaunaurd et al., 2014 United States of America | Randomized controlled trial | 11 patients with IPF (71.0 ± 6.0 years; FVC 60.0 ± 11.0%pred; DLCO 44.0 ± 11.0%pred) History of heart disease: n = 1 | Pulmonary rehabilitation 70%–80% of HRmax | 12 weeks 2 sessions/week 90 min/session | ||
| Vainshelboim et al., 2014 Israel | Randomized controlled trial | 15 patients with IPF (10♂; 68.8 ± 6 years; FEV1 68.5 ± 15.8%pred; FVC 66.1 ± 14.8%pred; DLCO 48.6 ± 17.2%pred) Arterial hypertension: n = 12 Coronary heart disease: n = 7 Pulmonary hypertension: n = 5 | Aerobic and strength training 50%–70% of WRpeak 70%–90% of average walking speed in the 6MWT 3–6 in the mBorg | 12 weeks 2 sessions/week 60 min/session | HR at rest (bpm) | Mean Pre/Post difference −2.4 ± 9.1 ES = −0.26 |
| Systolic blood pressure at rest (mmHg) | Mean Pre/Post difference −2.9 ± 13.6 ES = −0.21 | |||||
| Diastolic blood pressure at rest (mmHg) | Mean Pre/Post difference 1.5 ± 7.1 ES = 0.21 | |||||
| Oxygen pulse (ml/beat) | Mean Pre/Post difference 0.9 ± 1.5 ES = 0.62 | |||||
| Marcellis et al., 2015 The Netherlands | Pre-Post study | 18 patients with Sarcoidosis (14♂; 50.3 ± 10.4 years; FEV1 93.6 ± 17.0%pred; FVC 102.2 ± 18.1%pred; DLCO 91.2 ± 18.4%pred) | Aerobic and strength training 40% of 1RM 60% of maximal walking speed in the 6MWT 50% of WRmax | 13 weeks 3 sessions/week 1 h/session | HR at rest (bpm) | Pre 82.7 ± 13.1 vs. Post 77.1 ± 12.8, p = 0.11 ES = −0.43 |
| Vainshelboim at al., 2015 Israel | Randomized controlled trial | 15 patients with IPF (10♂; 68.8 ± 6 years; FVC 66.1 ± 14.8%pred; DLCO 48.6 ± 17.2%pred) Arterial hypertension: n = 12 Coronary heart disease: n = 7 Pulmonary hypertension: n = 5 | Aerobic and strength training 50%–70% of WRpeak 70%–90% of average walking speed in the 6MWT Perceived exertion 3–6 in the mBorg | 12 weeks 2 sessions/week 60 min/session | ||
| Boström et al., 2016 Sweden | Randomized controlled trial | 18 patients with Systemic lupus erythematosus (0♂; 52.0 ± 10.0 years) Arterial hypertension: n = 6 | Pulmonary rehabilitation 65%–80% of HRmax Perceived exertion 13–16 in the Borg | 12 weeks 2 sessions/week 60 min/session | HR at rest Blood pressure at rest | Pre vs. Post, p = 0.04 Pre vs. Post, p > 0.05 |
| Vainshelboim et al., 2017 Israel | Randomized controlled trial | 15 patients with IPF (10♂; 68.8 ± 6.0 years; FVC 66.1 ± 14.8%pred; DLCO 48.6 ± 17.2%pred) Arterial hypertension: n = 12 Coronary heart disease: n = 7 Pulmonary hypertension: n = 5 | Aerobic and strength training 50%–70% of WRpeak 70%–90% of average walking speed in the 6MWT | 12 weeks 2 sessions/week 60 min/session | HR (bpm) | Mean Pre/Post difference −2.4 ± 9.1 ES = −0.26 |
| HR reserve (bpm) | Mean Pre/Post difference 6.7 ± 11.0 ES = 0.61 | |||||
| Systolic blood pressure (mmHg) | Mean Pre/Post difference −2.9 ± 13.6 ES = −0.21 | |||||
| Diastolic blood pressure (mmHg) | Mean Pre/Post difference 1.5 ± 7.1 ES = 0.21 | |||||
| Rate pressure product (bpm/mmHg) | Mean Pre/Post difference 1685.0 ± 3338.0 ES = 0.50 | |||||
| Left atrium diameter (cm) | Mean Pre/Post difference 0.0 ± 0.5 ES = 0.04 | |||||
| Left atrium area (cm2) | Mean Pre/Post difference 0.2 ± 2.7 ES = 0.07 | |||||
| Left ventricle posterior wall thickness (cm) | Mean Pre/Post difference 0.0 ± 0.1 ES = 0.30 | |||||
| Intra-ventricular septum thickness (cm) | Mean Pre/Post difference 0.1 ± 0.1 ES = 0.60 | |||||
| Left ventricle end systolic diameter index (cm/m2) | Mean Pre/Post difference −0.1 ± 0.3 ES = −0.40 | |||||
| Left ventricle end diastolic diameter index (cm/m2) | Mean Pre/Post difference −0.1 ± 0.3 ES = −0.47 | |||||
| Stroke volume (mL/beat) | Mean Pre/Post difference −4.5 ± 13.4 ES = −0.34 | |||||
| Cardiac output (L/min) | Mean Pre/Post difference −0.4 ± 0.8 ES = −0.50 | |||||
| Cardiac index (L/min/m2) | Mean Pre/Post difference −0.2 ± 0.4 ES = −0.50 | |||||
| Ejection fraction (%) | Mean Pre/Post difference 0.8 ± 3.0 ES = 0.27 | |||||
| Fractioning shortening (%) | Mean Pre/Post difference 0.9 ± 6.2 ES = 0.15 | |||||
| Earlier transmitral velocity (E) (ms) | Mean Pre/Post difference 0.8 ± 16.9 ES = 0.05 | |||||
| Late trasmitral velocity (A) (ms) | Mean Pre/Post difference 5.1 ± 20.7 ES = 0.25 | |||||
| E/A ratio | Mean Pre/Post difference 0.0 ± 0.4 ES = 0.00 | |||||
| Isovolumic relaxation time (ms) | Mean Pre/Post difference 9.1 ± 32.1 ES = 0.28 | |||||
| Deceleration time (ms) | Mean Pre/Post difference 11.0 ± 52.7 ES = 0.21 | |||||
| Systolic pulmonary arterial pressure (mmHg) | Mean Pre/Post difference −0.5 ± 6.8 ES = −0.07 | |||||
| Peak circulatory power (mLO2/kg/min/mmHg) | Mean Pre/Post difference 490.0 ± 637.0 ES = 0.77 | |||||
| Peak cardiac power output (W) | Mean Pre/Post difference 0.3 ± 0.3 ES = 0.94 | |||||
| Peak stroke work (mLO2/beat/mmHg) | Mean Pre/Post difference 221.0 ± 343.0 ES = 0.64 | |||||
| Naz et al., 2018a Turkey | Pre-Post study | 14 patients with ILD (5♂; 63.0 (53.0; 70.0) years; FEV1 78.0 (69.0; 83.0)%pred; FVC 74.0 (67.0; 78.0)%pred; DLCO 40.0 (19.0; 45.0)%pred) Arterial hypertension: n = 7 Congestive heart failure: n = 2 | Aerobic and strength training 80% of peak walking speed in the 6MWT 70% of WRmax Dyspnoea and perceived exertion 4–6 in the mBorg | 12 weeks 2 sessions/week 60–90 min/session | ||
| Naz et al., 2018b Turkey | Randomized controlled trial | 9 patients with Sarcoidosis (33.3%♂; 59.0 (52.0; 64.0) years; FEV1 73.0 (65.0; 85.0)%pred; FVC 76.0 (66.0; 90.0)%pred; DLCO 45.0 (36.0; 54.0)%pred) | Aerobic and strength training 80% of the peak speed in the 6MWT Fatigue 4–6 in the mBorg | 12 weeks 2 sessions/week | HR (bpm) | Median Pre/Post difference 0.0 [−6.0; 5.0], p > 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, A.; Quadflieg, K.; Oliveira, A.; Keytsman, C.; Marques, A.; Hansen, D.; Burtin, C. Exercise Training in Patients with Chronic Respiratory Diseases: Are Cardiovascular Comorbidities and Outcomes Taken into Account?—A Systematic Review. J. Clin. Med. 2019, 8, 1458. https://doi.org/10.3390/jcm8091458
Machado A, Quadflieg K, Oliveira A, Keytsman C, Marques A, Hansen D, Burtin C. Exercise Training in Patients with Chronic Respiratory Diseases: Are Cardiovascular Comorbidities and Outcomes Taken into Account?—A Systematic Review. Journal of Clinical Medicine. 2019; 8(9):1458. https://doi.org/10.3390/jcm8091458
Chicago/Turabian StyleMachado, Ana, Kirsten Quadflieg, Ana Oliveira, Charly Keytsman, Alda Marques, Dominique Hansen, and Chris Burtin. 2019. "Exercise Training in Patients with Chronic Respiratory Diseases: Are Cardiovascular Comorbidities and Outcomes Taken into Account?—A Systematic Review" Journal of Clinical Medicine 8, no. 9: 1458. https://doi.org/10.3390/jcm8091458
APA StyleMachado, A., Quadflieg, K., Oliveira, A., Keytsman, C., Marques, A., Hansen, D., & Burtin, C. (2019). Exercise Training in Patients with Chronic Respiratory Diseases: Are Cardiovascular Comorbidities and Outcomes Taken into Account?—A Systematic Review. Journal of Clinical Medicine, 8(9), 1458. https://doi.org/10.3390/jcm8091458





