Metoclopramide and Levosulpiride Use and Subsequent Levodopa Prescription in the Korean Elderly: The Prescribing Cascade
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
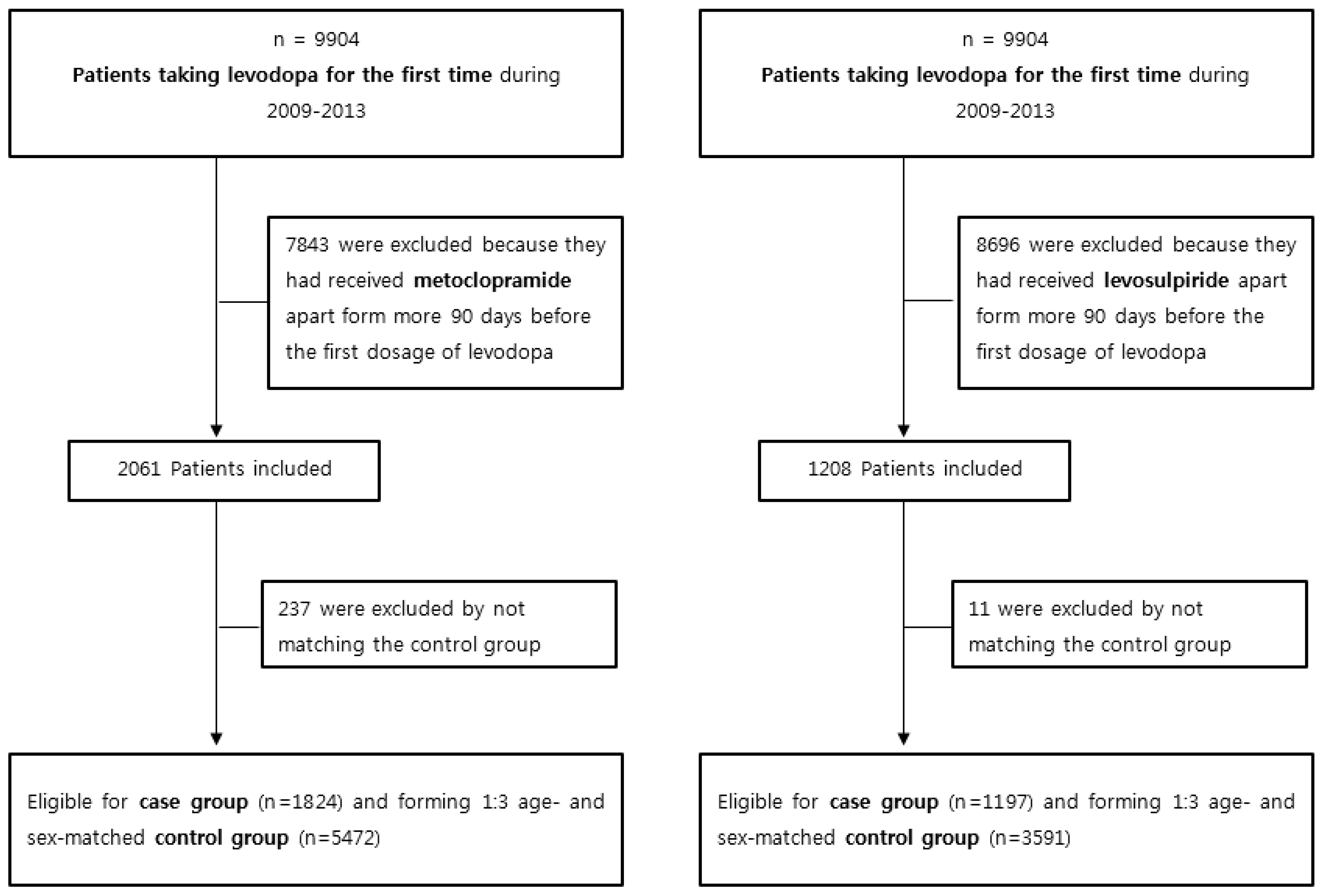
2.2. Patient Selection and Criteria
2.3. Medication Exposure
2.4. Statistical Analysis
3. Results
3.1. Metoclopramide Use and Subsequent Levodopa Prescription
3.2. Levosulpiride Use and Subsequent Levodopa Prescription
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rochon, P.A.; Gurwitz, J.H. Optimising drug treatment for elderly people: The prescribing cascade. BMJ Br. Med. J. 1997, 315, 1096. [Google Scholar] [CrossRef]
- Vouri, S.M.; van Tuyl, J.S.; Olsen, M.A.; Xian, H.; Schootman, M. An evaluation of a potential calcium channel blocker-lower-extremity edema-loop diuretic prescribing cascade. J. Am. Pharm. Assoc. 2018, 58, 534–539. [Google Scholar] [CrossRef]
- Petrone, K.; Katz, P. Approaches to appropriate drug prescribing for the older adult. Prim. Care 2005, 32, 755–775. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Goldman, D. Treatment of psychotic states with chlorpromazine. J. Am. Med. Assoc. 1955, 157, 1274–1278. [Google Scholar] [CrossRef]
- Ayd, F.J. A survey of drug-induced extrapyramidal reactions. JAMA 1961, 175, 1054–1060. [Google Scholar] [CrossRef]
- Jabs, B.E.; Bartsch, A.J.; Pfuhlmann, B. Susceptibility to neuroleptic-induced parkinsonism—Age and increased substantia nigra echogenicity as putative risk factors. Eur. Psychiatry 2003, 18, 177–181. [Google Scholar] [CrossRef]
- Richardson, M.A.; Haugland, G.; Craig, T.J. Neuroleptic use, parkinsonian symptoms, tardive dyskinesia, and associated factors in child and adolescent psychiatric patients. Am. J. Psychiatry 1991, 148, 1322. [Google Scholar]
- Avorn, J.; Bohn, R.L.; Mogun, H.; Gurwitz, J.H.; Monane, M.; Everitt, D.; Walker, A. Neuroleptic drug exposure and treatment of parkinsonism in the elderly: A case-control study. Am. J. Med. 1995, 99, 48–54. [Google Scholar] [CrossRef]
- Susatia, F.; Fernandez, H.H. Drug-induced parkinsonism. Curr. Treat. Options Neurol. 2009, 11, 162–169. [Google Scholar] [CrossRef]
- Thanvi, B.; Treadwell, S. Drug induced parkinsonism: A common cause of parkinsonism in older people. Postgrad. Med J. 2009, 85, 322–326. [Google Scholar] [CrossRef]
- Volkow, N.D.; Yu-Shin, D.; Fowler, J.S.; Gene-Jack, W. Dopamine transporters decrease with age. J. Nucl. Med. 1996, 37, 554. [Google Scholar]
- Shin, H.W.; Chung, S.J. Drug-induced parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef]
- Ma, H.I.; Kim, J.H.; Chu, M.K.; Oh, M.S.; Yu, K.H.; Kim, J.; Hahm, W.; Kim, Y.J.; Lee, B.C. Diabetes mellitus and drug-induced Parkinsonism: A case-control study. J. Neurol. Sci. 2009, 284, 140–143. [Google Scholar] [CrossRef]
- Lopez-Sendon, J.L.; Mena, M.A.; de Yebenes, J.G. Drug-induced parkinsonism in the elderly: Incidence, management and prevention. Drugs Aging 2012, 29, 105–118. [Google Scholar] [CrossRef]
- Shin, H.W.; Kim, M.J.; Kim, J.S.; Lee, M.C.; Chung, S.J. Levosulpiride-induced movement disorders. Mov. Disord. 2009, 24, 2249–2253. [Google Scholar] [CrossRef]
- Madjunkova, S.; Maltepe, C.; Farine, D.; Koren, G. Patterns of antiemetic use among American women with nausea and vomiting of pregnancy. Obstet. Gynecol. 2014, 123, 155S. [Google Scholar] [CrossRef]
- Wallenborn, J.; Gelbrich, G.; Bulst, D.; Behrends, K.; Wallenborn, H.; Rohrbach, A.; Krause, U.; Kühnast, T.; Wiegel, M.; Olthoff, D. Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: Randomised double blind multicentre trial. BMJ 2006, 333, 324. [Google Scholar] [CrossRef]
- Schriever, J.; Bühlen, M.; Broich, K. Current state of knowledge and developments in the prophylaxis and acute treatment of migraine. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014, 57, 974–982. [Google Scholar] [CrossRef]
- Sayana, A.; Barshiliya, Y. Comparative Study of Metoclopramide, Ondansetron, and Granisetron in Prophylaxis of Post operative Nausea and Vomiting in Patient Undergoing Laparoscopic Cholecystectomy Under General Anaesthesia. Asian J. Pharm. Life Sci. 2012, 2231, 4423. [Google Scholar]
- Tonini, M.; Cipollina, L.; Poluzzi, E.; Crema, F.; Corazza, G.; De Ponti, F. Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment. Pharmacol. Ther. 2004, 19, 379–390. [Google Scholar] [CrossRef]
- Llau, M.; Nguyen, L.; Senard, J.; Rascol, O.; Montastruc, J. Drug-induced parkinsonian syndromes: A 10-year experience at a regional center of pharmaco-vigilance. Rev. Neurol. 1994, 150, 757–762. [Google Scholar]
- Martí, J.M.; Poza, J. Drug-induced or aggravated parkinsonism: Clinical signs and the changing pattern of implicated drugs. Neurologia 1996, 11, 10–15. [Google Scholar]
- Kim, S.; Cheon, S.M.; Suh, H.S. Association Between Drug Exposure and Occurrence of Parkinsonism in Korea: A Population-Based Case-Control Study. Ann. Pharm. 2019. [Google Scholar] [CrossRef]
- Avorn, J.; Gurwitz, J.H.; Bohn, R.L.; Mogun, H.; Monane, M.; Walker, A. Increased incidence of levodopa therapy following metoclopramide use. JAMA 1995, 274, 1780–1782. [Google Scholar] [CrossRef]
| Cases N = 1824 | Controls N = 5472 | ||
|---|---|---|---|
| Sex | Male | 960 (52.63) | 2880 (52.63) |
| Female | 86 (47.37) | 2592 (47.37) | |
| Age (Years) | 65–74 | 694 (38.05) | 2082 (38.05) |
| 75–84 | 918 (50.33) | 2754 (50.33) | |
| ≥85 | 212 (11.62) | 636 (11.62) | |
| Exposure to Metoclopramide | No | 1645 (90.19) | 5280 (96.49) |
| Yes | 179 (9.81) | 192 (3.51) | |
| Duration of Metoclopramide prescription | None | 1645 (90.19) | 5280 (96.49) |
| 1–19 days | 157 (8.61) | 175 (3.2) | |
| ≥20 days | 22 (1.21) | 17 (0.31) | |
| Crude OR (95% CI) | Adjusted OR (95% CI) * | ||
|---|---|---|---|
| Exposure to metoclopramide | No | 1 ** | 1 ** |
| Yes | 3.04 (2.46,3.77) | 2.94 (2.35,3.67) | |
| Duration | None | 1 ** | 1 ** |
| 1–19 days | 2.93 (2.34,3.68) | 2.82 (2.23,3.56) | |
| ≥20 days | 4.18 (2.21,7.89) | 4.14 (2.15,7.98) | |
| Cases N = 1197 | Controls N = 3591 | ||
|---|---|---|---|
| Sex | Male | 609 (50.88) | 1827 (50.88) |
| Female | 588 (49.12) | 1764 (49.12) | |
| Age (Years) | 65–74 | 472 (39.43) | 1416 (39.43) |
| 75–84 | 584 (48.79) | 1752 (48.79) | |
| ≥85 | 141 (11.78) | 423 (11.78) | |
| Exposure to levosulpiride | No | 1070 (89.39) | 3465 (96.49) |
| Yes | 127 (10.61) | 126 (3.51) | |
| Duration of levosulpiride prescription | None | 1070 (89.39) | 3465 (96.49) |
| 1–19 days | 75 (6.27) | 109 (3.04) | |
| ≥20 days | 52 (4.34) | 17 (0.47) | |
| Crude OR (95% CI) | Adjusted OR (95% CI) * | ||
|---|---|---|---|
| Exposure to Levosulpiride | No | 1 ** | 1 ** |
| Yes | 3.32 (2.56,4.3) | 3.3 (2.52,4.32) | |
| Duration | Non | 1 ** | 1 ** |
| 1–19 days | 2.29 (1.69,3.11) | 2.24 (1.64,3.08) | |
| ≥20 days | 9.79 (5.65,16.97) | 10.24 (5.82,17.99) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, Y.; Kim, D.-H.; Choi, M.; Park, J.-H.; Kwon, D.-Y.; Jung, J.-H.; Han, K.; Park, Y.-G. Metoclopramide and Levosulpiride Use and Subsequent Levodopa Prescription in the Korean Elderly: The Prescribing Cascade. J. Clin. Med. 2019, 8, 1496. https://doi.org/10.3390/jcm8091496
Huh Y, Kim D-H, Choi M, Park J-H, Kwon D-Y, Jung J-H, Han K, Park Y-G. Metoclopramide and Levosulpiride Use and Subsequent Levodopa Prescription in the Korean Elderly: The Prescribing Cascade. Journal of Clinical Medicine. 2019; 8(9):1496. https://doi.org/10.3390/jcm8091496
Chicago/Turabian StyleHuh, Youn, Do-Hoon Kim, Moonyoung Choi, Joo-Hyun Park, Do-Young Kwon, Jin-Hyung Jung, Kyungdo Han, and Yong-Gyu Park. 2019. "Metoclopramide and Levosulpiride Use and Subsequent Levodopa Prescription in the Korean Elderly: The Prescribing Cascade" Journal of Clinical Medicine 8, no. 9: 1496. https://doi.org/10.3390/jcm8091496
APA StyleHuh, Y., Kim, D.-H., Choi, M., Park, J.-H., Kwon, D.-Y., Jung, J.-H., Han, K., & Park, Y.-G. (2019). Metoclopramide and Levosulpiride Use and Subsequent Levodopa Prescription in the Korean Elderly: The Prescribing Cascade. Journal of Clinical Medicine, 8(9), 1496. https://doi.org/10.3390/jcm8091496





