Efficacy of Different Energy Levels Used in Focused and Radial Extracorporeal Shockwave Therapy in the Treatment of Plantar Fasciitis: A Meta-Analysis of Randomized Placebo-Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Type of Outcomes
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
3.3. Meta-Analysis
3.3.1. Different Energy Levels of ESWT regardless of the Types of Shockwave Generators
Success Rate
VAS Score
3.3.2. Radial and Focused ESWTs
Success Rate
VAS Score
3.3.3. Local Anesthesia
Success Rate
VAS Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 3–4 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 4 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 4 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 3-4 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 4 |
| RESULTS | |||
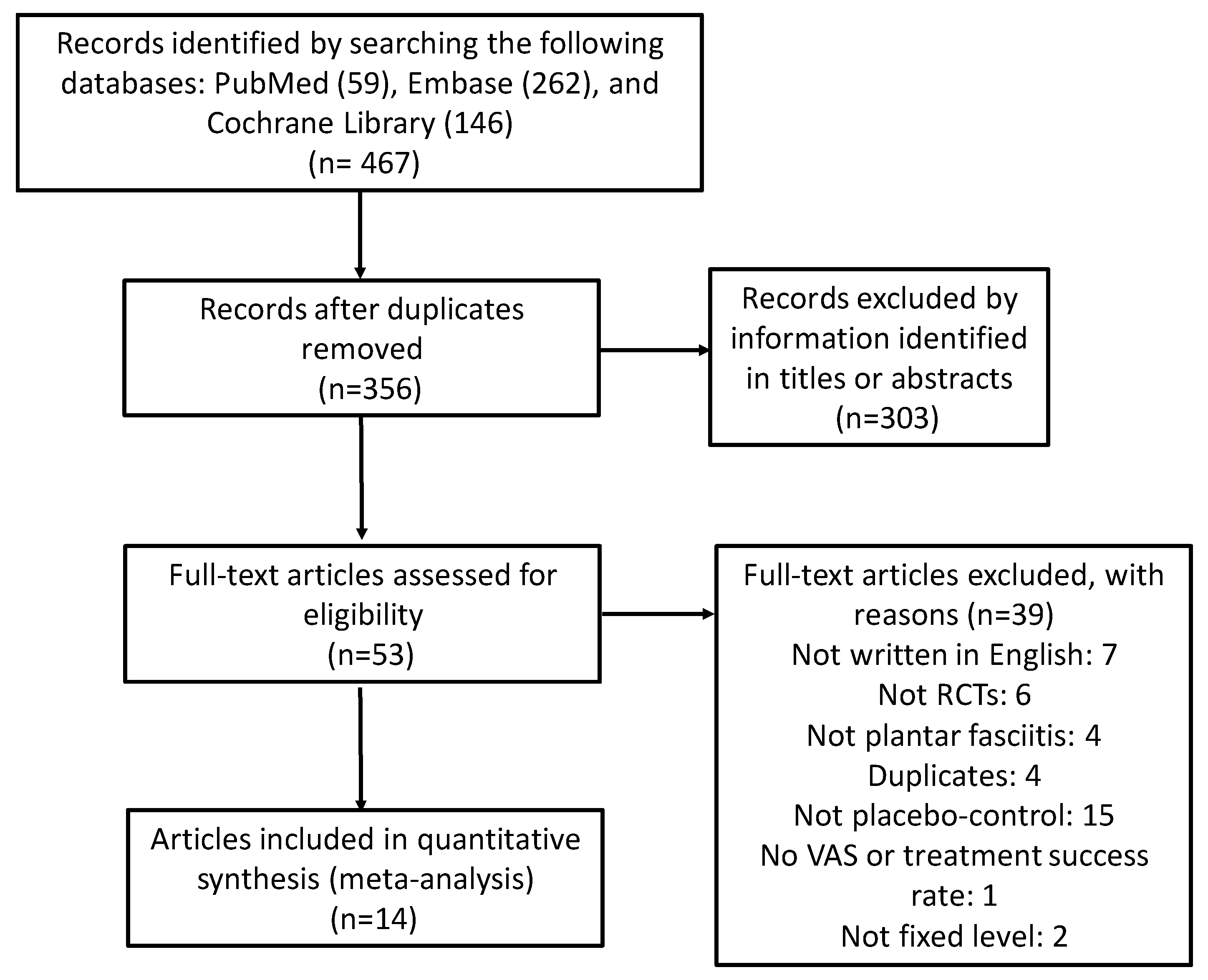
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | Page 4, Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | Page 4, Table 1 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Figure 3 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Pages 9–13, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 9–13 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 8 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see Item [16]). | Table S1 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 13–14 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 15 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 15 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | N/A |
References
- Rompe, J.D. Plantar Fasciopathy. Sports Med. Arthrosc. 2009, 17, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Roxas, M. Plantar fasciitis: Diagnosis and therapeutic considerations. Altern. Med. Rev. 2005, 10, 83–93. [Google Scholar] [PubMed]
- Riddle, D.; Pulisic, M.; Pidcoe, P.; Johnson, R.E. Risk factors for plantar fasciitis: A matched case-control study. J. Bone Jt. Surg. Am. Vol. 2003, 85a, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Demaio, M.; Paine, R.; Mangine, R.E.; Drez, D. Plantar Fasciitis. Orthopedics 1993, 16, 1153–1163. [Google Scholar] [PubMed]
- Palomo-Lopez, P.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodriguez-Sanz, D.; Calvo-Lobo, C.; Lopez-Lopez, D. Impact of plantar fasciitis on the quality of life of male and female patients according to the Foot Health Status Questionnaire. J. Pain Res. 2018, 11, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Irving, D.B.; Cook, J.L.; Young, M.A.; Menz, H.B. Impact of chronic plantar heel pain on health-related quality of life. J. Am. Podiatr. Med. Assoc. 2008, 98, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hyer, C.F.; Vancourt, R.; Block, A. Evaluation of ultrasound-guided extracorporeal shock wave therapy (ESWT) in the treatment of chronic plantar fasciitis. J. Foot Ankle Surg. 2005, 44, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, K.; Sun, H.; Luo, X.; Wang, P.; Fang, S.; Chen, H.; Sun, X. Clinical effects of extracorporeal shock-wave therapy and ultrasound-guided local corticosteroid injections for plantar fasciitis in adults: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e13687. [Google Scholar] [CrossRef] [PubMed]
- Janisse, D.J.; Janisse, E. Shoe modification and the use of orthoses in the treatment of foot and ankle pathology. J. Am. Acad. Orthop. Surg. 2008, 16, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nahin, R.L. Prevalence and Pharmaceutical Treatment of Plantar Fasciitis in United States Adults. J. Pain 2018, 19, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Coombs, R.; Schaden, W.; Zhou, S.S. Musculoskeletal Shockwave Therapy; Greenwich Medical Media, Ltd.: London, UK, 2000. [Google Scholar]
- Eslamian, F.; Shakouri, S.K.; Jahanjoo, F.; Hajialiloo, M.; Notghi, F. Extra Corporeal Shock Wave Therapy Versus Local Corticosteroid Injection in the Treatment of Chronic Plantar Fasciitis, a Single Blinded Randomized Clinical Trial. Pain Med. 2016, 17, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.A.; Alvarez, R.G.; Levitt, R.L.; Johnson, J.E.; Marlow, M.E. Electrohydraulic high-energy shock-wave treatment for chronic plantar fasciitis. J. Bone Jt. Surg. Am. Vol. 2004, 86a, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhou, H.; Jiang, W. Extracorporeal shock wave therapy versus other therapeutic methods for chronic plantar fasciitis. Foot Ankle Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Chen, S.Y.; Chen, W.S.; Tu, Y.K.; Chien, K.L. Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: A systematic review and network meta-analysis. Arch. Phys. Med. Rehabil. 2012, 93, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.A.; Toth-Kischkat, A.; Schultheiss, R. Principles of shock wave therapy. Clin. Orthop. Relat. Res. 2001, 387, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Chow, I.H.; Cheing, G.L. Comparison of different energy densities of extracorporeal shock wave therapy (ESWT) for the management of chronic heel pain. Clin. Rehabil. 2007, 21, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Ye, J.; Yao, M.; Cui, X.J.; Xia, Y.; Shen, Q.X.; Tong, Z.Y.; Wu, X.Q.; Ma, J.M.; Mo, W. Is extracorporeal shock wave therapy clinical efficacy for relief of chronic, recalcitrant plantar fasciitis? A systematic review and meta-analysis of randomized placebo or active-treatment controlled trials. Arch. Phys. Med. Rehabil. 2014, 95, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.N.; Gonzalez-Suarez, C.; Zamora, M.T.; Gambito, E.D. Effectiveness of extracorporeal shock wave therapy in chronic plantar fasciitis: A meta-analysis. Am. J. Phys. Med. Rehabil. 2013, 92, 606–620. [Google Scholar] [CrossRef]
- Li, Z.; Jin, T.; Shao, Z. Meta-analysis of high-energy extracorporeal shock wave therapy in recalcitrant plantar fasciitis. Swiss Med. Wkly 2013, 143, w13825. [Google Scholar]
- Rompe, J.D.; Meurer, A.; Nafe, B.; Hofmann, A.; Gerdesmeyer, L. Repetitive low-energy shock wave application without local anesthesia is more efficient than repetitive low-energy shock wave application with local anesthesia in the treatment of chronic plantar fasciitis. J. Orthop. Res. 2005, 23, 931–941. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.J.M.; Ashton-James, C.E.; Skorpil, N.E.; Heymans, M.W.; Forouzanfar, T. What constitutes a clinically important pain reduction in patients after third molar surgery? Pain Res. Manag. 2013, 18, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of Adult Pain Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthrit. Care Res. 2011, 63, S240–S252. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wang, S.; Liu, S.; Xing, G. Effectiveness of Extracorporeal Shock Wave Therapy without Local Anesthesia in Patients with Recalcitrant Plantar Fasciitis: A Meta-Analysis of Randomized Controlled Trials. Am. J. Phys. Med. Rehabil. 2017, 96, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Kullmer, H.M.; Riehle, B.; Herbsthofer, B.; Eckardt, A.; Burger, R.; Nafe, B.; Eysel, P. Effectiveness of low-energy extracorporal shock waves for chronic plantar fasciitis. Foot Ankle Int. 1996, 2, 215–221. [Google Scholar] [CrossRef]
- Rompe, J.D.; Decking, J.; Schoellner, C.; Nafe, B. Shock wave application for chronic plantar fasciitis in running athletes. A prospective, randomized, placebo-controlled trial. Am. J. Sports Med. 2003, 31, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Speed, C.A.; Nichols, D.; Wies, J.; Humphreys, H.; Richards, C.; Burnet, S.; Hazleman, B.L. Extracorporeal shock wave therapy for plantar fasciitis. A double blind randomised controlled trial. J. Orthop. Res. 2003, 21, 937–940. [Google Scholar] [CrossRef]
- Haake, M.; Buch, M.; Schoellner, C.; Goebel, F.; Vogel, M.; Mueller, I.; Hausdorf, J.; Zamzow, K.; Schade-Brittinger, C.; Mueller, H.H. Extracorporeal shock wave therapy for plantar fasciitis: Randomised controlled multicentre trial. BMJ 2003, 327, 75. [Google Scholar] [CrossRef]
- Theodore, G.H.; Buch, M.; Amendola, A.; Bachmann, C.; Fleming, L.L.; Zingas, C. Extracorporeal shock wave therapy for the treatment of plantar fasciitis. Foot Ankle Int. 2004, 25, 290–297. [Google Scholar] [CrossRef]
- Kudo, P.; Dainty, K.; Clarfield, M.; Coughlin, L.; Lavoie, P.; Lebrun, C. Randomized, placebo-controlled, double-blind clinical trial evaluating the treatment of plantar fasciitis with an extracoporeal shockwave therapy (ESWT) device: A North American confirmatory study. J. Orthop. Res. 2006, 24, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, H.; Diehl, P.; von Korff, A.; Rahlfs, V.W.; Gerdesmeyer, L. Extracorporeal shock wave therapy for chronic painful heel syndrome: A prospective, double blind, randomized trial assessing the efficacy of a new electromagnetic shock wave device. J. Foot Ankle Surg. 2007, 46, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Gerdesmeyer, L.; Frey, C.; Vester, J.; Maier, M.; Lowell, W., Jr.; Weil, L., Sr.; Russlies, M.; Stienstra, J.; Scurran, B.; Fedder, K.; et al. Radial extracorporeal shock wave therapy is safe and effective in the treatment of chronic recalcitrant plantar fasciitis: Results of a confirmatory randomized placebo-controlled multicenter study. Am. J. Sports Med. 2008, 36, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.; Jackiewicz, A.; Witkowski, Z.; Kot, J.; Deja, W.; Lasek, J. Extracorporeal shock-wave therapy (ESWT) with a new-generation pneumatic device in the treatment of heel pain. A double blind randomised controlled trial. Acta Orthop. Belg. 2008, 74, 98–101. [Google Scholar] [PubMed]
- Gollwitzer, H.; Saxena, A.; DiDomenico, L.A.; Galli, L.; Bouche, R.T.; Caminear, D.S.; Fullem, B.; Vester, J.C.; Horn, C.; Banke, I.J.; et al. Clinically relevant effectiveness of focused extracorporeal shock wave therapy in the treatment of chronic plantar fasciitis: A randomized, controlled multicenter study. J. Bone Jt. Surg. Am. Vol. 2015, 97, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hawamdeh, Z.; Alghwiri, A.A.; Nassar, A. The short-term effect of extracorporeal shock wave in treating plantar fasciitis: RCT. J. Med. J. 2016, 50, 1–11. [Google Scholar]
- Ibrahim, M.I.; Donatelli, R.A.; Hellman, M.; Hussein, A.Z.; Furia, J.P.; Schmitz, C. Long-term results of radial extracorporeal shock wave treatment for chronic plantar fasciopathy: A prospective, randomized, placebo-controlled trial with two years follow-up. J. Orthop. Res. 2017, 35, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Takla, M.K.N.; Rezk, S.S.R. Clinical effectiveness of multi-wavelength photobiomodulation therapy as an adjunct to extracorporeal shock wave therapy in the management of plantar fasciitis: A randomized controlled trial. Lasers Med. Sci. 2019, 34, 583–593. [Google Scholar] [CrossRef]
- Sanchez, M.; Collvinent, B.; Miro, O.; Horcajada, J.P.; Moreno, A.; Marco, F.; Mensa, J.; Milla, J. Short-term effectiveness of ceftriaxone single dose in the initial treatment of acute uncomplicated pyelonephritis in women. A randomised controlled trial. Emerg. Med. J. 2002, 19, 19–22. [Google Scholar] [CrossRef][Green Version]
- Akin, C.; Oken, O.; Koseoglu, B.F. Short-Term Effectiveness of Ultrasound Treatment in Patients with Lateral Epicondylitis: Randomized, Single-Blind, Placebo-Controlled, Prospective Study. Turk. J. Rheumatol. 2010, 25, 50–55. [Google Scholar] [CrossRef]
- Ponzani, P. Long-term effectiveness and safety of liraglutide in clinical practice. Minerva Endocrinol. 2013, 38, 103–112. [Google Scholar] [PubMed]
- Riegel, G.; Moreira, L.B.; Fuchs, S.C.; Gus, M.; Nunes, G.; Correa, V.; Wiehe, M.; Goncalves, C.C.; Fernandes, F.S.; Fuchs, F.D. Long-Term Effectiveness of Non-Drug Recommendations to Treat Hypertension in a Clinical Setting. Am. J. Hypertens. 2012, 25, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Labek, G.; Auersperg, V.; Ziernhold, M.; Poulios, N.; Bohler, N. Influence of local anesthesia and energy level on the clinical outcome of extracorporeal shock wave-treatment of chronic plantar fasciitis. Z. Orthop. Ihre Grenzgeb. 2005, 143, 240–246. [Google Scholar] [CrossRef] [PubMed]






| OBS | Study | Study Design | Treatment (Energy) | Use of Local Anesthesia | Number of Patients | Mean Age (Year) | Intensity (mJ/mm2) | Follow-Up Times | Extracted Outcome Data | Definition of Success |
|---|---|---|---|---|---|---|---|---|---|---|
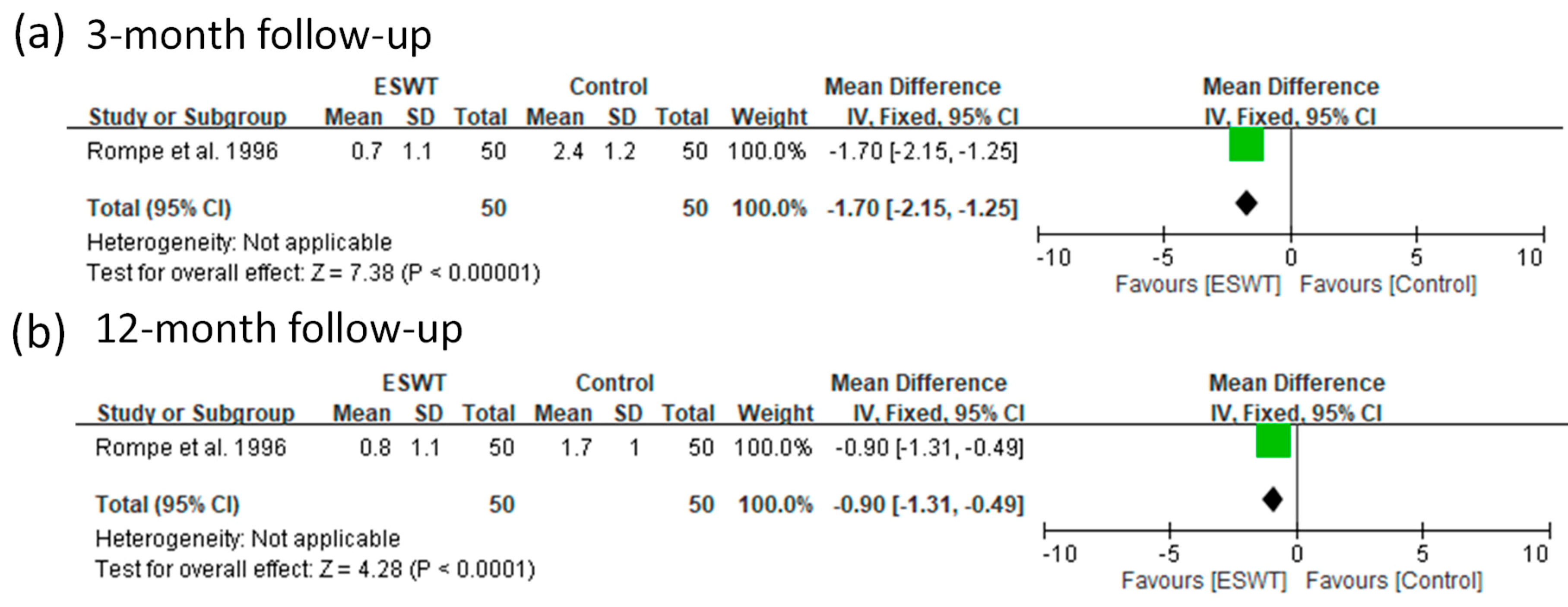
| 1 | Rompe et al. 1996 | NA, RCT | FSW (low) | No | 50 | 44.0 | 0.08 | 3 and 13 months | VAS | |
| Placebo | 50 | 49.0 | ||||||||
| 2 | Rompe et al. 2003 | SB, RCT | FSW (medium) | No | 22 | 43.0 | 0.16 | 6 and 12 months | Success rate and VAS | >50% improvement of pain during the first few minutes of walking scored on VAS |
| Placebo | 23 | 40.0 | ||||||||
| 3 | Speed et al. 2003 | DB, RCT | FSW (medium) | No | 46 | 51.7 | 0.12 | 1, 2, 3, and 6 months | VAS | |
| Placebo | 42 | 52.5 | ||||||||
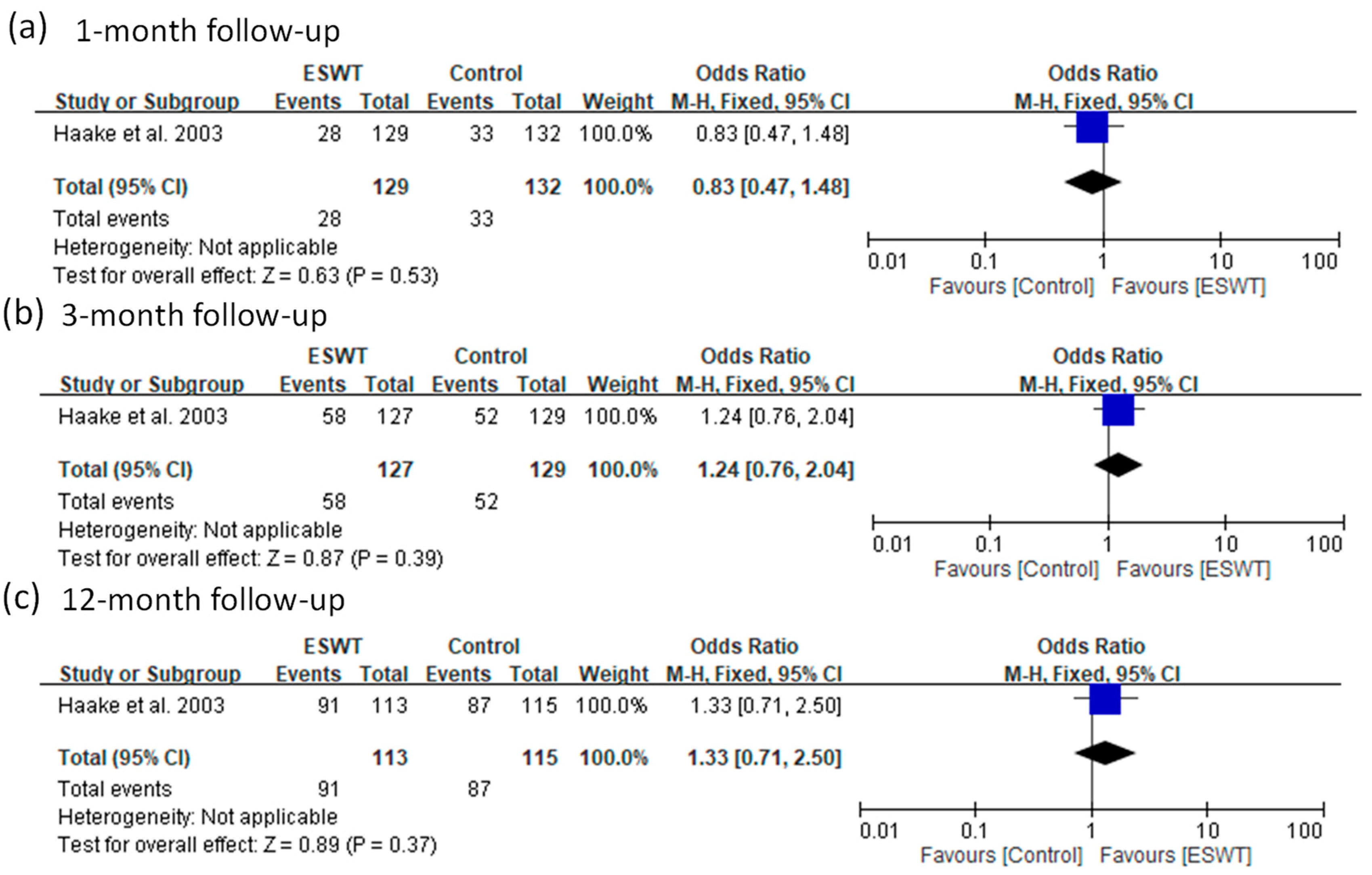
| 4 | Haake et al. 2003 | DB, RCT | SW (low) | Yes | 129 | NA | 0.08 | 6 weeks, 3 months, and 12 months | Success rate | Roles and Maudsley score 1 or 2 |
| Placebo | 132 | NA | ||||||||
| 5 | Ogden et al. 2004 | DB, RCT | FSW (high) | Yes | 144 | NA | 0.22 | 3 months | Success rate | >50% improvement of pain scored on VAS and VAS of <=4 |
| Placebo | 141 | NA | ||||||||
| 6 | Theodore et al. 2004 | DB, RCT | FSW (high) | Yes | 73 | 50.0 | 0.36 | 6 weeks and 3 months | Success rate and VAS | Roles and Maudsley score 1 or 2 |
| Placebo | 73 | 53.0 | ||||||||
| 7 | Kudo et al. 2006 | DB, RCT | FSW (high) | Yes | 53 | 51.1 | 0.64 | 3 months | Success rate and VAS | >60% improvement of pain during the first few minutes of walking scored on VAS |
| Placebo | 52 | 48.8 | ||||||||
| 8 | Gollwitzer et al. 2007 | DB, RCT | FSW (high) | No | 20 | 53.9 | 0.25 | 3 months | Success rate and VAS | Roles and Maudsley score 1 or 2 |
| Placebo | 20 | 58.9 | ||||||||
| 9 | Gerdesmeyer et al. 2008 | DB, RCT | RSW (medium) | No | 123 | 52.4 | 0.16 | 3 and 12 months | Success rate | >60% from baseline at follow-up after treatment for at least 2 of the 3 heel pain (VAS) measurements |
| Placebo | 116 | 52.0 | ||||||||
| 10 | Marks et al. 2008 | DB, RCT | RSW (medium) | No | 16 | 51.9 | 0.16 | 6 months | Success rate | >50% improvement of pain scored on VAS |
| Placebo | 9 | 51.7 | ||||||||
| 11 | Gollwitzer et al. 2015 | DB, RCT | FSW (high) | No | 125 | 50.0 | 0.25 | 3 months | Success rate | >60% from baseline at follow-up after treatment for at least 2 of the 3 heel pain (VAS) measurements. |
| Placebo | 121 | 47.4 | ||||||||
| 12 | Hawamdeh et al. 2016 | SB, RCT | RSW (high) | No | 12 | NA | 0.25 | 3 weeks | VAS | |
| Placebo | 12 | NA | ||||||||
| 13 | Ibrahim et al. 2017 | DB, RCT | RSW (medium) | No | 25 | 56.6 | 0.16 | 1, 3, 6, and 13 months | VAS | |
| Placebo | 25 | 49.1 | ||||||||
| 14 | Takla et al. 2019 | SB, RCT | FSW (high) | No | 30 | NA | 0.22–0.28 | 3 weeks and 3 months | VAS | |
| Placebo | 30 | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Chen, S.-J.; Huang, P.-J.; Huang, H.-T.; Cheng, Y.-M.; Shih, C.-L. Efficacy of Different Energy Levels Used in Focused and Radial Extracorporeal Shockwave Therapy in the Treatment of Plantar Fasciitis: A Meta-Analysis of Randomized Placebo-Controlled Trials. J. Clin. Med. 2019, 8, 1497. https://doi.org/10.3390/jcm8091497
Wang Y-C, Chen S-J, Huang P-J, Huang H-T, Cheng Y-M, Shih C-L. Efficacy of Different Energy Levels Used in Focused and Radial Extracorporeal Shockwave Therapy in the Treatment of Plantar Fasciitis: A Meta-Analysis of Randomized Placebo-Controlled Trials. Journal of Clinical Medicine. 2019; 8(9):1497. https://doi.org/10.3390/jcm8091497
Chicago/Turabian StyleWang, Ying-Chun, Shu-Jung Chen, Peng-Ju Huang, Hsuan-Ti Huang, Yuh-Min Cheng, and Chia-Lung Shih. 2019. "Efficacy of Different Energy Levels Used in Focused and Radial Extracorporeal Shockwave Therapy in the Treatment of Plantar Fasciitis: A Meta-Analysis of Randomized Placebo-Controlled Trials" Journal of Clinical Medicine 8, no. 9: 1497. https://doi.org/10.3390/jcm8091497
APA StyleWang, Y.-C., Chen, S.-J., Huang, P.-J., Huang, H.-T., Cheng, Y.-M., & Shih, C.-L. (2019). Efficacy of Different Energy Levels Used in Focused and Radial Extracorporeal Shockwave Therapy in the Treatment of Plantar Fasciitis: A Meta-Analysis of Randomized Placebo-Controlled Trials. Journal of Clinical Medicine, 8(9), 1497. https://doi.org/10.3390/jcm8091497




