Abstract
Most diagnostic tests for tuberculosis (TB) rely on sputum samples, which are difficult to obtain and have low sensitivity in immunocompromised patients, patients with disseminated TB, and children, delaying treatment initiation. The World Health Organization (WHO) calls for the development of a rapid, biomarker-based, non-sputum test capable of detecting all forms of TB at the point-of-care to enable immediate treatment initiation. Lipoarabinomannan (LAM) is the only WHO-endorsed TB biomarker that can be detected in urine, an easily collected sample. This status update discusses the characteristics of LAM as a biomarker, describes the performance of first-generation urine LAM tests and reasons for slow uptake, and presents considerations for developing the next generation of more sensitive and impactful tests. Next-generation urine LAM tests have the potential to reach adult and pediatric patients regardless of HIV status or site of infection and facilitate global TB control. Implementation and scale-up of existing LAM tests and development of next-generation assays should be prioritized.
1. Introduction
Tuberculosis (TB) has infected one-quarter of the world’s population and is the leading infectious cause of mortality worldwide [1]. Ten million people develop active TB each year, one million of whom are children [1]. An estimated 36% of new TB cases remain undiagnosed or unreported, partly due to the major limitations of current diagnostic tools [1]. Most conventional diagnostic tests for microbiological confirmation rely on sputum samples, which can be difficult to obtain and have low diagnostic sensitivity in children, patients with extrapulmonary TB (EPTB), and people living with HIV (PLHIV) [2]. EPTB occurs in one fifth of all incident TB cases, and the majority (60%) of EPTB patients do not have traceable TB in the lungs and sputum [3]. PLHIV experience higher rates of EPTB [1].
In 2014, the World Health Organization (WHO) called for the development of a “rapid biomarker-based non-sputum test capable of detecting all forms of TB by identifying characteristic biomarkers” (Table 1) [4]. The target product profile (TPP) specified that the test must be able to diagnose active TB and have a high specificity to allow initiation of treatment at the same clinical encounter or on the same day [4]. A point of care (POC) test that readily detects active TB would reduce diagnostic delays, interrupt transmission with appropriate therapy, and address many of the current gaps in global TB control.

Table 1.
Status of the available AlereLAM, FujiLAM, and sputum-based tuberculosis (TB) diagnosis vs. biomarker target product profile (TPP) [4]. WHO: World Health Organization; PLHIV: people living with HIV; TPP: Target product profile; CRS: Composite reference standard; IVD: in vitro diagnostic; “✓” indicates that the TPP minimal criteria is met; “x” indicates that the TPP minimal criteria is not met; “?” indicates that it is currently unclear whether the TPP criteria is met.
2. Lipoarabinomannan in Active TB Disease
Active Mycobacterium tuberculosis (Mtb) infection begins when Mtb enters the lungs via inhalation and invades the lung interstitial tissue where the process of infection evolves (Figure 1). This leads to the recruitment of an increasing number of immune cells to the lung parenchyma to form a granuloma (Figure 1). On the one hand, macrophages in the granuloma are capable of killing or at least controlling the growth of Mtb with the potential to ‘ward off’ infection from the rest of the body. On the other hand, granulomas are a growing collection of phagocytic cells that Mtb can infect and replicate within [5]. If the bacterial load becomes too great, the granuloma will fail to contain the infection, allowing Mtb to enter the bloodstream or the lymphatic system, disseminate to other extrapulmonary sites (Figure 1), or re-enter the respiratory tract to be released. The patient is now infectious and is said to have active TB disease.
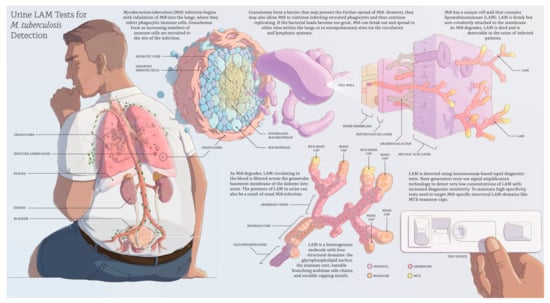
Figure 1.
Overview of lipoarabinomannan (LAM) detection in urine for the diagnosis of active tuberculosis (developed by Digizyme, Inc., Boston, MA, USA and FIND, Geneva, Switzerland).
Mtb has a unique cell wall with multiple lipid-based molecules that create a thick ‘waxy’ surface [6]. A major component of this cell envelope is lipoarabinomannan (LAM), which represents up to 15% of the bacterial mass [7]. LAM is firmly but non-covalently attached to the inner membrane and extends to the exterior of the cell wall (Figure 1) [8] where it interacts as a potent virulence factor that modulates the host immune response and plays an important role in the pathogenesis of Mtb infection [9]. The exact molecular structure and size of LAM in vivo is unknown and might differ in different parts of the body. If produced in vitro, LAM’s average molecular weight is 17.4 kilodaltons, but the molecule is heterogeneous in size, branching pattern, acylation, and phosphorylation on the arabinan and mannan portions [10,11]. LAM has four structural domains (Figure 1): (I) the glycophospholipid anchor, which attaches the molecule non-covalently to the inner membrane, (II) the attached mannan core, which is highly conserved across mycobacterial species, and (III) the variable branching arabinan side chains with (IV) variable capping motifs that give rise to the intra- and inter-species diversity of LAM molecules [12]. According to the capping motifs, LAM can be classified into three structural families: LAM from fast-growing, non-pathogenic species, such as M. smegmatis, have uncapped ends (AraLAM) or inositol phosphate caps (phosphatidyl-myo-inositol capped LAM, or PILAM) whereas LAM from slow-growing mycobacteria (Mtb, M. leprae, M. avium, and M. kansasii) is modified with one to three α (1→2)-linked mannopyranose (Manp) units (ManLAM). Mtb ManLAM can contain an additional cap modification, 5-deoxy-5-methylthio-xylofuranose (MTX), attached to the terminal Manp [13,14,15].
As replicating Mtb degrades, LAM circulating in the blood is filtered across the glomerular basement membrane of the kidneys into urine (Figure 1). The presence of LAM in urine can also be a result of renal Mtb infection, as has been shown in autopsy studies [16].
There are few studies reporting LAM concentrations in clinical specimens and direct comparisons between different sample types and assays are complicated by the absence of standardized LAM control materials, sample panels, and reference assays. Four recent studies used the same purified LAM material for calibration and similar antibody reagents for immunoassay-based LAM detection (though different detection platforms) and reported LAM concentrations in sputum [33], blood [34,35], and urine [36] in subjects with active pulmonary TB, allowing for a rough comparison of LAM concentration ranges. For sputum, Kawasaki and colleagues showed that an immunoassay with a cut-off of 15 pg/mL detected all smear-positive and 50% of smear-negative TB patients [33] and sputum LAM concentrations ranged from 15.4 pg/mL to 1,869,000 pg/mL (median 5512 pg/mL). LAM concentration in sputum was linearly correlated to colony forming units (CFU) with 1 pg/mL of LAM correlating to 8 CFU/mL, suggesting that one Mtb bacterium contains approximately 125 fg of LAM [33]. Brock et al. [35] developed a sensitive serum assay using a Single Molecule Array (SIMOA); using a cutoff of 2.3 pg/mL, the assay sensitivity in smear-positive TB patients was 40%, and 60% in HIV-positive/smear-positive TB patients at 100% specificity. Serum LAM concentrations in this study were highest in HIV-positive/smear-positive patients (median 3.97 pg/mL). Broger and colleagues [34,36] developed sensitive immunoassays and compared serum LAM to urine LAM concentrations in matched samples from smear-positive TB patients. The serum assay detected LAM in 55% of smear-positive patients with concentrations of 6 pg/mL to 70,000 pg/mL but 45% of patients were below the cut-off of 6 pg/mL, suggesting a median concentration in serum of roughly 10 pg/mL. In the same sample set, the urinary LAM assay with a cut-off of 11 pg/mL showed that nearly all patients (93%) had concentrations in the range of 12 pg/mL to 90,000 pg/mL (median 111 pg/mL). In summary, data suggest roughly 50-times higher median LAM concentrations in sputum compared to urine, roughly 500-times higher median LAM concentrations in sputum compared to serum, and roughly 10-times higher median LAM concentrations in urine compared to serum. However, further confirmation is required as detection might have been influenced by reagents (e.g., cross-reactivity of antibodies with LAM from non-Mtb bacteria), structural differences of LAM in different sample types, assay designs, and detection platforms.
LAM concentrations in the different body fluids can be drastically influenced by factors such as bacterial burden, infection site (i.e., pulmonary vs. extrapulmonary TB, including urinary tract disease), and co-morbidities such as HIV. There is evidence that HIV/TB co-infected patients with immunosuppression and disseminated TB have higher LAM concentrations in their urine [37]. Biological mechanisms to explain this are not fully understood; whether this is due to an overall higher bacterial body burden or renal TB infection is unclear [16,38]. While HIV co-infection or urinary tract disease are not necessary for LAM antigenuria, they both can lead to higher urine LAM concentrations.
3. First-Generation LAM Tests
Urine LAM has been evaluated as a diagnostic biomarker for TB testing since 1997 [39]. In 2003, Chemogen Inc. (South Portland, OR, USA) developed a lab-based urine ELISA for LAM detection, which was later commercialized by Inverness Inc. as the Clearview® TB ELISA assay. In 2010, Inverness changed its name to Alere and developed the first rapid POC LAM test, the Determine TB LAM Ag lateral flow assay (“AlereLAM”). In 2017, Alere was acquired by Abbott Diagnostics (Lake Bluff, USA). The AlereLAM test likely uses the same polyclonal antibodies as the Clearview ELISA, and there is high observed agreement between the two assays [40]. The AlereLAM test meets the operational characteristics of the WHO TPP for a biomarker-based, non-sputum TB test, but falls short on test accuracy (Table 1). In 2019, a meta-analysis by Bjerrum et al. [21] compared pooled sensitivity and specificity against a microbiological reference standard (MRS), defined as a positive TB culture or TB nucleic acid amplification test (NAAT). When compared against an MRS, pooled sensitivity and specificity in HIV-positive adults were 42% (95% CI: 31–55%) and 91% (95% CI: 85–95%), respectively [21]. It is likely that the MRS from Bjerrum and colleagues’ meta-analysis underestimates AlereLAM specificity; an earlier meta-analysis reported 96–98% specificity against a composite reference standard (CRS) [26].
Among symptomatic participants with CD4 counts >200 cells/µL, pooled sensitivity and specificity were 16% (95% CI: 8–31%) and 94% (95% CI: 81–97%) [21]. Among symptomatic participants with CD4 counts ≤200 cells/µL, pooled sensitivity and specificity were 45% (95% CI: 31–61%) and 89% (95% CI: 77–94%) [21]. Among children, data were limited, but five prospective pediatric cohort studies found a pooled sensitivity of 47% (95% CI: 27–69%) and specificity of 82% (95% CI: 71–89%) [24]. A possible reason for the lower specificity in children could be cross-reactivity of AlereLAM with bacteria from perineal skin or stool that contaminate the urine sample during collection, as urine bags may remain on the skin for several hours until the child produces urine [41], or limitations of the reference standard. Based on these meta-analysis results [21], the WHO announced updated guidelines for use of AlereLAM in 2019, which are summarized in Table 2 [42].

Table 2.
Updated 2019 WHO guidelines for the use of AlereLAM [42].
To date, three randomized controlled trials (RCT) of test implementation have assessed the effect of urine AlereLAM testing on mortality outcomes. Two studies randomly assigned patients 1:1 to either the standard of care (SOC) arm (a combination of Xpert-MTB/RIF, smear microscopy, and culture in the first RCT, and Xpert-MTB/RIF alone in the second RCT) or the interventional arm (SOC plus LAM testing). The first RCT (LAMRCT, [28]) enrolled HIV-positive adults with at least one TB symptom in ten hospitals across South Africa, Tanzania, Zambia, and Zimbabwe. Peter et al. [28] found overall 8-week mortality occurred in 25% of the SOC arm and 21% in the LAM group, and concluded that “LAM-guided initiation of anti-TB treatment” reduced absolute risk of mortality by 4% (95% CI: 1–7%) and relative risk by 17% (95% CI: 4–28%). The second RCT (STAMP trial, [29]) enrolled HIV-positive adults from two hospitals in Malawi and South Africa and found overall 8-week mortality occurred in 21% of the SOC arm and 18% in the LAM group; an adjusted risk reduction of −2.8% (95% CI: −5.8–0.3%; p = 0.074) [29]. This study found that urine LAM testing significantly reduced the risk of mortality in three pre-specified clinical subgroups: patients with CD4 counts <100 cells/µL, by −7.1% (95% CI: −13.7% to −0.4%; p = 0.036), severely anemic patients, by −9.0% (95% CI: −16.6% to −1.3%; p = 0.021), and those with clinically suspected TB, by −5.7% (95% CI: −10.9% to −0.5%; p = 0.033) [29]. The authors concluded that urine LAM could reduce mortality in these high-risk groups and argued for a use for screening in all HIV-positive inpatients. A third RCT (TB Fast Track, [30]) randomly assigned HIV-positive adults (aged ≥18 years) with CD4 counts of ≤150 cells/μL, who had not had antiretroviral therapy (ART) in the past 6 months 1:1 to either routine care or the interventional arm [30]. In the interventional arm, nurses assessed participants on the basis of tuberculosis symptoms, body-mass index, point-of-care hemoglobin concentrations, and urine AlereLAM results. Participants in the interventional arm classified as having high probability of tuberculosis were recommended to start tuberculosis treatment immediately, followed by ART two weeks later; participants classified as medium probability were recommended to have symptom-guided investigation; and participants classified as low probability were recommended to start ART immediately. Although the intervention substantially increased coverage of tuberculosis treatment in this high-risk population, it did not reduce mortality, and the authors proposed to prioritize the development of more sensitive TB diagnostic tests suitable for use by nurses in primary health-care clinics.
Despite the initial WHO recommendation in 2015 [24] and the availability of Global Fund and The President’s Emergency Plan for AIDS Relief (PEPFAR) funding for test procurement, the rollout and uptake of the AlereLAM test has been slow [43]. Only approximately 400,000 AlereLAM tests were sold in 2018 [44]. The majority of uptake has come from three high-burden countries: South Africa, Uganda, and Kenya. According to the Treatment Action Group’s (TAG’s) March 2019 report [17], an in-depth review of the Global Fund’s grant documents included mention of LAM procurement in six additional countries (Burundi, Cameroon, Eswatini, Guatemala, Ukraine, and Vietnam), and PEPFAR country operation plans included mention of LAM procurement in six other countries (Côte d’Ivoire, Democratic Republic of the Congo, Eswatini, Kenya, Malawi, and Zambia). The lack of widespread POC LAM implementation is related to multiple factors, including the conditional WHO recommendation and the lack of coordination between National TB Programs (NTPs) and HIV programs in low and middle-income countries [45]. Some NTP representatives consider LAM to be a “niche test” with clinical utility limited to a small population. LAM testing has increased as a result of the 2017 WHO HIV Advanced Care guidance [46] inclusion of AlereLAM test usage within its algorithms. The uptake has been further boosted by the involvement of patient advocacy groups such as TAG and others [17,47]. The addition of AlereLAM to the WHO Essential Diagnostic List (EDL) [48] and to the Global Drug Facility TB Diagnostics Catalog in August 2018 may further boost procurement [49]. These developments, together with the recently extended WHO recommendation for 2019 (Table 2, [42]), and the consequent policy and stakeholder support globally, provide support for both increased use of AlereLAM and development of a better-performing LAM test in the near future [50].
4. The Need for Next-Generation, Highly Sensitive, and Specific LAM Tests
The fundamental goal of next-generation LAM tests is to achieve a high diagnostic accuracy (sensitivity and specificity) while maintaining the operational characteristics of an easy-to-use, rapid, biomarker-based, non-sputum POC test in order to fulfill the WHO high priority TPP requirements (Table 1).
Several recent research reports indicate that lower detection limits will translate into higher diagnostic sensitivity and demonstrate necessary measures to improve the analytical sensitivity. First, Paris et al. developed a sample preparation device that concentrates antigens and detects LAM down to 14 pg/mL, resulting in 95% sensitivity at 80% specificity in a study of 48 HIV-negative TB-positive subjects and 58 TB-negatives with other respiratory diseases and healthy controls [51]. Shapiro et al. developed a device to concentrate and detect LAM in urine and reported 52% sensitivity in HIV-negative TB-positive persons at 67% specificity in a study with 93 patients [52]. Connelly et al. developed a prototype POC urine assay consisting of a concentration step and lateral flow assay and reported 60% sensitivity at 80% specificity in a cross-sectional study with 86 HIV-positive and 206 HIV-negative patients [53]. Hamasur et al. used magnetic particles to remove undisclosed ‘urine inhibitors’ in combination with LAM enrichment methods and reached 65.5% sensitivity at 84% specificity in a study of 45 HIV-positive and 74 HIV-negative patients [54]. While all these assays showed promising sensitivities (65–95%) they all have specificities below 85% and would not meet the minimal target of ≥98% of the biomarker TPP (Table 1).
In contrast, Sigal et al. developed a sensitive research assay that approaches the performance targets of the biomarker TPP. The assay targets the MTX-LAM epitope (Figure 1) in urine and reached 93% sensitivity and 97% specificity in a case-control study with 40 TB-positive and 35 HIV-negative patients with a cut-off of 11 pg/mL [36]. The study underlines the importance of using reagents that target Mtb-characteristic LAM epitopes to maintain a high diagnostic specificity at very low assay cut-offs.
This work by Sigal et al. informed the development of the Fujifilm SILVAMP TB LAM test (“FujiLAM”; Fujifilm, Tokyo, Japan, Figure 1), which is a well-advanced next-generation LAM test (CE-marked) [20,27,55] (Table 1). The assay combines a pair of high-affinity monoclonal antibodies directed towards the largely Mtb-specific MTX-LAM epitopes and a silver-based amplification step that increases the visibility of test and control lines of a lateral-flow assay to reach a cut-off around 30 pg/mL [27]. This enables the detection of approximately 30-fold lower concentrations of LAM in urine compared to the AlereLAM test. FujiLAM showed approximately 30% improved diagnostic sensitivity in HIV-positive inpatients compared to AlereLAM at 95.7% specificity [27]. Additional studies in HIV-infected outpatients [31] and a recent meta-analysis of 1595 HIV-positive inpatients and outpatients [22] have confirmed FujiLAM’s superiority over AlereLAM. The meta-analysis reported 70.7% (95% CI: 59.0–80.8%) sensitivity for FujiLAM, a sensitivity increase of 35.8% compared to AlereLAM [22]. FujiLAM also showed high performance for the detection of EPTB in HIV-infected patients [56]. Two recent studies [31,32] showed that FujiLAM could have rapidly diagnosed TB in up to 90% of HIV-positive patients that died within 3 to 6 months, whereas the probability of survival in the case of a FujiLAM-negative result was 86–97%. A limitation of published FujiLAM studies is their use of biobanked samples and, accordingly, testing in a laboratory setting. Notably, a recent study with a cohort of 182 patients from Zambia demonstrated that the use of biobanked specimens delivers nearly equivalent results compared with fresh samples [57]. Prospective studies now need to confirm the initial findings, quantify FujiLAM’s effect on clinical outcomes, establish performance in HIV-negative patients and children, and assess the feasibility for POC implementation in a variety of clinical settings. Furthermore, cost-effectiveness analyses and impact modeling are needed. WHO evaluation of FujiLAM is expected in Q4/2020 and market entry in Q1/2021 [20]. Interestingly, Abbott, the manufacturer of AlereLAM, announced the development of a next-generation LAM test to extend the indication to the HIV-negative population but have not communicated a launch date [20].
5. Considerations for Evaluating LAM Assays
There are several common challenges and limitations faced in diagnostic accuracy studies of LAM tests. One major difficulty in evaluating non-sputum-based tests is an imperfect reference standard, particularly for patients with EPTB and paucibacillary disease including PLHIV and children. Studies assessing AlereLAM and other non-sputum-based tests have discussed this limitation being responsible for at least some of the false-positive results as defined by the index test [58]. In order to improve upon this, a combination of a microbiological reference standard (MRS) and a composite reference standard (CRS) or latent class analysis should be considered [59,60]. The MRS should combine results from several pulmonary samples (at least two, and including sputum induction if needed) as well as extrapulmonary samples (such as urine, blood, or others as indicated by the patient’s presentation) to perform multiple Xpert tests and mycobacterial cultures to define ‘Definite TB’. The CRS combines this with chest X-ray, clinical suspicion, and treatment initiation to define ‘Possible TB’ [60]. A patient follow-up period of 8 to 12 weeks or longer, including observation of clinical improvement in the absence of treatment, is critical to establish a ‘Non-TB’ diagnosis [60]. Cross-reactivity to other pathogens and colonizing organisms also needs to be critically evaluated in both analytical and clinical studies. Importantly, LAM assays can diagnose TB in patients unable to produce sputum, therefore, the analysis of diagnostic yield is important and patients unable to produce sputum should not be excluded as this group might benefit most from urinary LAM testing. Also, evaluation of incremental diagnostic yield of urinary LAM diagnostics in combination with sputum-based diagnostics should be considered. Other common considerations are user subjectivity in test interpretation if the readout is visual, as is the case for AlereLAM and FujiLAM, and ensuring generalizability across settings of implementation.
6. Future Directions and Potential Impact of LAM Tests
The impact of any large-scale rollout of a next-generation LAM test will ultimately be measured by reduction in disease mortality and incidence at the population level. In high-burden settings, this will depend significantly on achieving reductions in TB transmission through a combination of earlier case identification and reduction in loss-to-follow-up from the point of diagnosis to the initiation of treatment. A POC diagnostic test could significantly reduce initial loss-to-follow-up in the care cascade. Analyses of country-level data in South Africa have shown losses of approximately 12% between diagnosis and treatment initiation, which could potentially be diminished by POC testing [61,62]. Importantly, the ability to reduce these losses will also depend on other aspects of the overall care cascade such as the ability to perform drug susceptibility testing in LAM-positive patients. Modelling of intensified case-finding among HIV clinic attendees in South Africa has suggested reductions in mortality of up to 30% over 10 years if LAM test sensitivity is near the biomarker TPP of 65% [63]. Analyses of the potential impact of introducing LAM into the diagnostic and care cascades will need to view LAM testing in the context of its placement in specific health care systems and its relationship to other available diagnostics.
7. Conclusions
Urine LAM is a promising TB diagnostic biomarker for use in a POC TB test, with the potential to reach adult and pediatric patients regardless of HIV status or site of infection. The ultimate goal of a urine LAM test is to achieve sensitivity high enough to reach and benefit all TB patients while remaining specific, fast, simple, and affordable to use. FujiLAM offers greater sensitivity than AlereLAM and is therefore likely to extend the indication of TB LAM testing beyond seriously ill HIV-positive patients. However, several research questions remain to be addressed (Table 3). With dedicated research driving the next generation of urine LAM diagnostics for clinic-based TB detection, there is a clear path forward to making a significant public health impact and facilitating global TB control. Implementation and scale-up of existing LAM tests and development of next-generation assays should be prioritized.

Table 3.
Research, development, and implementation questions for future urine Lipoarabinomannan (LAM) Tests. POC: point of care.
Author Contributions
Conceptualization, M.A.B., C.M.D., P.K.D. and T.B.; Methodology, M.A.B and T.B.; Writing-Original Draft Preparation, M.A.B., B.W., M.R.-J., A.S., N.R.P., E.M., C.M.D., P.K.D. and T.B.; Writing-Review & Editing, M.A.B., B.W., M.R.-J., A.S., N.R.P., E.M., C.M.D., P.K.D. and T.B.; Visualization, M.A.B. and T.B.; Supervision, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
T.B. and C.M.D. were previously employed by FIND, and E.M., A.S. and M.R. are currently employed by FIND. T.B. reports a patent application in the field of LAM detection. All other authors declare no conflict of interest.
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Huerga, H.; Ferlazzo, G.; Bevilacqua, P.; Kirubi, B.; Ardizzoni, E.; Wanjala, S.; Sitienei, J.; Bonnet, M. Incremental Yield of Including Determine-TB LAM Assay in Diagnostic Algorithms for Hospitalized and Ambulatory HIV-Positive Patients in Kenya. PLoS ONE 2017, 12, e0170976. [Google Scholar] [CrossRef] [PubMed]
- Herchline, T.; Amorosa, J. Tuberculosis (TB). Medscape, 15 August 2019. Available online: https://emedicine.medscape.com/article/230802-overview (accessed on 29 December 2019).
- World Health Organization (WHO). High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Robert Wood Johnson Foundation (RWJF) Microbiology Immunology & Infectious Diseases; Stanford Medicine; Duke University; UW Medicine; UCSF. Tuberculosis: Mycobacterial Cell Envelope. Available online: https://www.youtube.com/watch?v=yuHUikQy2vk (accessed on 29 December 2019).
- Correia-Neves, M.; Fröberg, G.; Korshun, L.; Viegas, S.; Vaz, P.; Ramanlal, N.; Bruchfeld, J.; Hamasur, B.; Brennan, P.; Källenius, G. Biomarkers for tuberculosis: the case for lipoarabinomannan. ERJ Open Res. 2019, 5, 115–2018. [Google Scholar] [CrossRef] [PubMed]
- Besra, G.S.; Brennan, P.J. The Mycobacterial Cell Envelope. J. Pharm. Pharmacol. 1997, 49, 25–30. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Chatterjee, D.; Khoo, K.H. Mycobacterial lipoarabinomannan: An extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 1998, 8, 113–120. [Google Scholar] [CrossRef]
- Venisse, A.; Berjeaud, J.M.; Chaurand, P.; Gilleron, M.; Puzo, G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J. Biol. Chem. 1993, 268, 12401–12411. [Google Scholar]
- Lawn, S.D. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: A state of the art review. BMC Infect. Dis. 2012, 12, 103. [Google Scholar] [CrossRef]
- Treumann, A.; Xidong, F.; McDonnell, L.; Derrick, P.J.; Ashcroft, A.E.; Chatterjee, D.; Homans, S.W. 5-methylthiopentose: A new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J. Mol. Biol. 2002, 316, 89–100. [Google Scholar] [CrossRef]
- Joe, M.; Sun, D.; Taha, H.; Completo, G.C.; Croudace, J.E.; Lammas, D.A.; Besra, G.S.; Lowary, T.L. The 5-Deoxy-5-methylthio-xylofuranose Residue in Mycobacterial Lipoarabinomannan. Absolute Stereochemistry, Linkage Position, Conformation, and Immunomodulatory Activity. J. Am. Chem. Soc. 2006, 128, 5059–5072. [Google Scholar] [CrossRef]
- De, P.; Shi, L.; Boot, C.; Ordway, D.; McNeil, M.; Chatterjee, D. Comparative Structural Study of Terminal Ends of Lipoarabinomannan from Mice Infected Lung Tissues and Urine of a Tuberculosis Positive Person. ACS Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Lukande, R.L.; Kalungi, S.; Van Marck, E.; Van de Vijver, K.; Kambugu, A.; Nelson, A.M.; Colebunders, R.; Manabe, Y.C. Is Urinary Lipoarabinomannan the Result of Renal Tuberculosis? Assessment of the Renal Histology in an Autopsy Cohort of Ugandan HIV-Infected Adults. PLoS ONE 2015, 10, e0123323. [Google Scholar] [CrossRef] [PubMed]
- Treatment Action Group (TAG). The LAM Test: Vital for Diagnosing TB in People with Advanced HIV. Available online: https://www.treatmentactiongroup.org/wp-content/uploads/2017/09/LAM-Guide-V3-1.pdf (accessed on 29 December 2019).
- Reddy, K.; Denkinger, C.M.; Broger, T.; McCann, N.; Gupta-Wright, A.; Shebl, F.; Fielding, K.; Nicol, M.P.; Wood, R.; Walensky, R. A higher-sensitivity urine LAM assay for TB testing in hospitalized patients with HIV: Cost-effectiveness analysis. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- FIND. Accessible Pricing. Available online: https://www.finddx.org/find-negotiated-product-pricing (accessed on 29 December 2019).
- Treatment Action Group (TAG). Pipeline Report: Tuberculosis Diagnostics. Available online: http://www.treatmentactiongroup.org/sites/default/files/pipeline_tb_diagnotics_2019_db_final.pdf (accessed on 29 December 2019).
- Bjerrum, S.; Schiller, I.; Dendukuri, N.; Kohli, M.; Nathavitharana, R.R.; Zwerling, A.A.; Denkinger, C.M.; Steingart, K.R.; Shah, M. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV (Review). Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Nicol, M.P.; Szekely, R.; Bjerrum, S.; Sossen, B.; Schutz, C.; Opintan, J.A.; Johansen, I.S.; Mitarai, S.; Chikamatsu, K.; et al. Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for people living with HIV–a meta-analysis of in-and outpatient data. PLoS Med. Submitted.
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV: Policy Guidance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Minion, J.; Leung, E.; Talbot, E.; Dheda, K.; Pai, M.; Menzies, D. Diagnosing tuberculosis with urine lipoarabinomannan: Systematic review and meta-analysis. Eur. Respir. J. 2011, 38, 1398–1405. [Google Scholar] [CrossRef]
- Shah, M.; Hanrahan, C.; Wang, Z.Y.; Dendukuri, N.; Lawn, S.D.; Denkinger, C.M.; Steingart, K.R. The lateral flow urine lipoarabinomannan (LF-LAM) test for diagnosis of tuberculosis in people living with human immunodeficiency virus (HIV). Cochrane Database Syst. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Ivanova Reipold, E.; Ward, A.; Barr, D.A.; Macé, A.; Trollip, A.; et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: A diagnostic accuracy study. Lancet Infect. Dis. 2019, 19, 852–861. [Google Scholar] [CrossRef]
- Peter, J.G.; Zijenah, L.S.; Chanda, D.; Clowes, P.; Lesosky, M.; Gina, P.; Mehta, N.; Calligaro, G.; Lombard, C.J.; Kadzirange, G.; et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: A pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016, 387, 1187–1197. [Google Scholar] [CrossRef]
- Gupta-Wright, A.; Corbett, E.L.; van Oosterhout, J.J.; Wilson, D.; Grint, D.; Alufandika-Moyo, M.; Peters, J.A.; Chiume, L.; Flach, C.; Lawn, S.D.; et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): A pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018, 392, 292–301. [Google Scholar] [CrossRef]
- Grant, A.D.; Charalambous, S.; Tlali, M.; Karat, A.S.; Dorman, S.E.; Hoffmann, C.J.; Johnson, S.; Vassall, A.; Churchyard, G.J.; Fielding, K.L. Algorithm-guided empirical tuberculosis treatment for people with advanced HIV (TB Fast Track): An open-label, cluster-randomised trial. Lancet HIV 2019. [Google Scholar] [CrossRef]
- Bjerrum, S.; Broger, T.; Szekely, R.; Mitarai, S.; Opintan, J.A.; Kenu, E.; Lartey, M.; Addo, K.K.; Chikamatsu, K.; Macé, A.; et al. Diagnostic accuracy of a novel and rapid lipoarabinomannan test for diagnosing tuberculosis among people living with HIV. OFID. in press. [CrossRef]
- Sossen, B.; Broger, T.; Kerkhoff, A.D.; Schutz, C.; Trollip, A.; Moreau, E.; Schumacher, S.G.; Burton, R.; Ward, A.; Wilkinson, R.J.; et al. ‘SILVAMP TB LAM’ rapid urine tuberculosis test predicts mortality in hospitalized HIV patients in South Africa. Clin. Infect. Dis. Submitted.
- Kawasaki, M.; Echiverri, C.; Raymond, L.; Cadena, E.; Reside, E.; Gler, M.T.; Oda, T.; Ito, R.; Higashiyama, R.; Katsuragi, K.; et al. Lipoarabinomannan in sputum to detect bacterial load and treatment response in patients with pulmonary tuberculosis: Analytic validation and evaluation in two cohorts. PLoS Med. 2019, 16, e1002780. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Tsionksy, M.; Mathew, A.; Lowary, T.L.; Pinter, A.; Plisova, T.; Bartlett, D.; Barbero, S.; Denkinger, C.M.; Moreau, E.; et al. Sensitive electrochemiluminescence (ECL) immunoassays for detecting lipoarabinomannan (LAM) and ESAT-6 in urine and serum from tuberculosis patients. PLoS ONE 2019, 14, e0215443. [Google Scholar] [CrossRef]
- Brock, M.; Hanlon, D.; Zhao, M.; Polluck, N.R. Detection of mycobacterial lipoarabinomannan in serum for diagnosis of active tuberculosis. Diagn. Microbiol. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Sigal, G.B.; Pinter, A.; Lowary, T.L.; Kawasaki, M.; Li, A.; Mathew, A.; Tsionsky, M.; Zheng, R.B.; Plisova, T.; Shen, K.; et al. A Novel Sensitive Immunoassay Targeting the 5-Methylthio-d-Xylofuranose–Lipoarabinomannan Epitope Meets the WHO’s Performance Target for Tuberculosis Diagnosis. J. Clin. Microbiol. 2018, 56, e01338-18. [Google Scholar] [CrossRef]
- Gupta-Wright, A.; Peters, J.A.; Flach, C.; Lawn, S.D. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: A systematic review and meta-analysis. BMC Med. 2016, 14, 53. [Google Scholar] [CrossRef]
- Wood, R.; Racow, K.; Bekker, L.-G.; Middelkoop, K.; Vogt, M.; Kreiswirth, B.N.; Lawn, S.D. Lipoarabinomannan in urine during tuberculosis treatment: Association with host and pathogen factors and mycobacteriuria. BMC Infect. Dis. 2012, 12, 47. [Google Scholar] [CrossRef]
- Hamasur, B.; Bruchfeld, J.; Haile, M.; Pawlowski, A.; Bjorvatn, B.; Källenius, G.; Svenson, S.B. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J. Microbiol. Methods 2001, 45, 41–52. [Google Scholar] [CrossRef]
- Lawn, S.D.; Kerkhoff, A.D.; Vogt, M.; Wood, R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: A descriptive study. Lancet Infect. Dis. 2012, 12, 201–209. [Google Scholar] [CrossRef]
- Nicol, M.P.; Allen, V.; Workman, L.; Isaacs, W.; Munro, J.; Pienaar, S.; Black, F.; Adonis, L.; Zemanay, W.; Ghebrekristos, Y. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: A prospective study. Lancet Glob. Health 2014, 2, e278–e284. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis of Active Tuberculosis in People Living with HIV, 2019 Update; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Medecins Sans Frontieres; Stop TB Partnership. Out of Step 2017: TB Policies in 29 Countries. A Survey of Prevention, Testing and Treatment Policies and Practices; Medecins Sans Frontieres: Geneva, Switzerland, 2017. [Google Scholar]
- Mandavilli, A. Global Health: The World Needs a Urine Test for TB. But It’s Already Here. The New York Times, 17 December 2018. [Google Scholar]
- Nathavitharana, R.R.; Pai, M. New strategies for inpatients with HIV and tuberculosis. Lancet 2018, 392, 256–258. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Médecins Sans Frontiéres. Activists Call on Countries and Donors to Immediately Scale up Use of Life-Saving TB LAM Test. Available online: https://www.msfaccess.org/activists-call-countries-and-donors-immediately-scale-use-life-saving-tb-lam-test (accessed on 29 December 2019).
- World Health Organization (WHO). World Health Organization Model List of Essential In Vitro Diagnostics, 1st ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Stop TB Partnership. Global Drug Facility: Diagnostics Catalog August 2018. Available online: http://www.stoptb.org/assets/documents/gdf/Diagnostics_Catalog_2018_WEB.pdf (accessed on 29 December 2019).
- Maclean, E.; Pai, M. Urine Lipoarabinomannan for Tuberculosis Diagnosis: Evolution and Prospects. Clin. Chem. 2018, 64. [Google Scholar] [CrossRef]
- Paris, L.; Magni, R.; Zaidi, F.; Araujo, R.; Saini, N.; Harpole, M.; Coronel, J.; Kirwan, D.E.; Steinberg, H.; Gilman, R.H.; et al. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Sci. Transl. Med. 2017, 9, eaal2807. [Google Scholar] [CrossRef]
- Shapiro, A.E.; Wittwer, T.; Ngwane, N.W.; Magcaba, Z.P.; Mullins, B.; Aamotsbakken, R.; Berry, S.; Beebe, D.J.; Wilson, D.P.K.; Drain, P.K. A point-of-care test to concentrate and detect urine LAM for TB diagnosis: results from the first-in-human study of FLOW-TB. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- Connelly, J.T.; Andama, A.; Grant, B.; Ball, L.; Lopez, B.B.; Hunt, V.; Ignatowicz, L.; Hamasur, B.; Cattamanchi, A.; Bell, D.; et al. Clinical Performance of Prototype Point-of-Care TB Lipoarabinomannan (LAM) Test in Uganda. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- Hamasur, B.; Ignatowicz, L.; Ramachandraiah, H. SpeClean: Urine sample treatment method for ultra-sensitive LAM diagnostics. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Ivanova Reipold, E.; Ward, A.; Barr, D.A.; Macé, A.; Trollip, A.; et al. YouTube Video. Fujifilm SILVAMP TB LAM Test Procedure. Available online: https://www.youtube.com/watch?v=aK-QtzkLBug (accessed on 24 April 2019).
- Kerkhoff, A.D.; Sossen, B.; Schutz, C.; Reipold, E.I.; Trollip, A.; Moreau, E.; Schumacher, S.G.; Burton, R.; Ward, A.; Nicol, M.P.; et al. Diagnostic sensitivity of SILVAMP TB-LAM (FujiLAM) point-of-care urine assay for extra-pulmonary tuberculosis in people living with HIV. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Broger, T.; Muyoyeta, M.; Kerkhoff, A.D.; Denkinger, C.M.; Moreau, E. Tuberculosis test results using fresh versus biobanked urine samples with FujiLAM. Lancet Infect. Dis. 2020, 20, 22–23. [Google Scholar] [CrossRef]
- Lawn, S.D.; Kerkhoff, A.D.; Nicol, M.P.; Meintjes, G. Underestimation of the True Specificity of the Urine Lipoarabinomannan Point-of-Care Diagnostic Assay for HIV-Associated Tuberculosis. J. Acquir. Immune Defic. Syndr. 2015, 69, 144–146. [Google Scholar] [CrossRef]
- Denkinger, C.M.; Schumacher, S.G.; Gilpin, C.; Korobitsyn, A.; Wells, W.A.; Pai, M.; Leeflang, M.; Steingart, K.R.; Bulterys, M.; Schünemann, H.; et al. Guidance for the Evaluation of Tuberculosis Diagnostics That Meet the World Health Organization (WHO) Target Product Profiles: An Introduction to WHO Process and Study Design Principles. J. Infect. Dis. 2019, 220, S91–S98. [Google Scholar] [CrossRef]
- Drain, P.K.; Gardiner, J.L.; Hannah, H.; Broger, T.; Dheda, K.; Fielding, K.; Walzl, G.; Kaforou, M.; Kranzer, K.; Joosten, S.A.; et al. Guidance for Studies Evaluating the Accuracy of Biomarker-Based Nonsputum Tests to Diagnose Tuberculosis. J. Infect. Dis. 2019, 220, S108–S115. [Google Scholar] [CrossRef]
- Naidoo, P.; Theron, G.; Rangaka, M.X.; Chihota, V.N.; Vaughan, L.; Brey, Z.O.; Pillay, Y. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J. Infect. Dis. 2017, 216, S702–S713. [Google Scholar] [CrossRef] [PubMed]
- Padayatchi, N.; Daftary, A.; Naidu, N.; Naidoo, K.; Pai, M. Tuberculosis: Treatment failure, or failure to treat? Lessons from India and South Africa. BMJ Glob. Health 2019, 4, e001097. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.G.; Chang, S.T.; Hannah, H. Estimating the impact of a combined C-reactive protein and LAM-based diagnostic algorithm for TB disease in HIV clinics in South Africa: A mathematical modeling-based analysis. In Proceedings of the 49th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (the Union), The Hague, The Netherlands, 24–27 October 2018. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).