The Feasibility and Effectiveness of a New Practical Multidisciplinary Treatment for Low-Back Pain: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
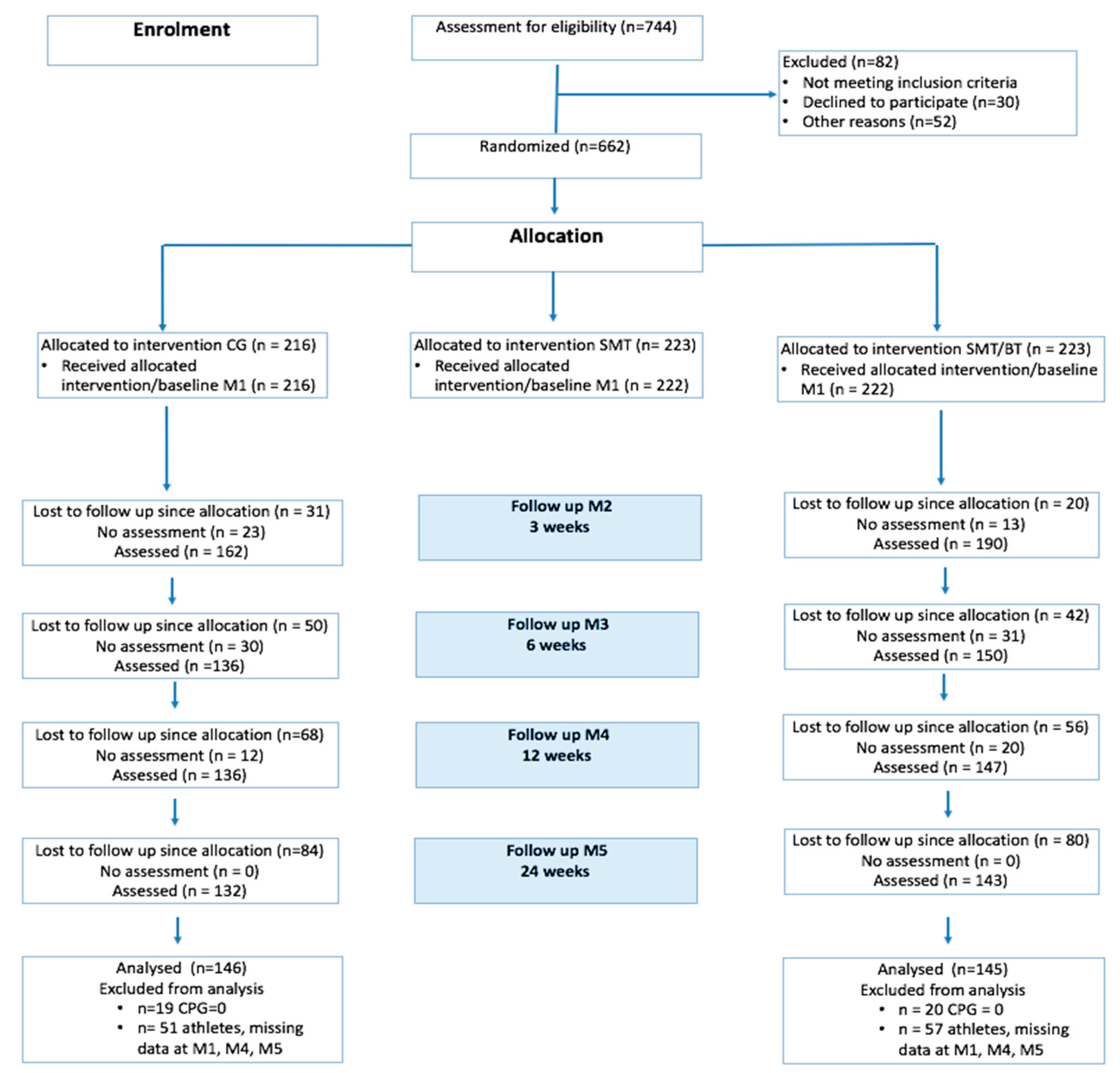
2.2. Design and Procedure
2.3. Intervention
2.3.1. Sensorimotor Training
2.3.2. Multidisciplinary Intervention
2.3.3. Rationale of Selection
2.4. Instruments
2.5. Statistical Analysis
3. Results
3.1. Descriptive for Pain, Pain-Related Cognitions, and Mental Health
3.2. Objective 1: Within Group Comparison
3.3. Objective 2: Differences between the Multidisciplinary Intervention and the Control Group
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CPG | Chronic Pain Grade Questionnaire |
| CPI | Characteristic Pain intensity, scale of Chronic Pain Grade questionnaire |
| DISS | subjective pain disability, scale of Chronic Pain Grade questionnaire |
| DRKS | German Clinical Trials Register |
| FABQ-D | Fear-Avoidance Questionnaire |
| HADS | Hamilton Anxiety and Depression Scale |
| LBP | Low Back Pain |
| M1, M2, M3, M4, M5 | visits 1–5 |
| MiSpEx | the German Research Network of Medicine in Spine Exercise |
| MBSR | Mindfulness-Based Stress Reduction Program |
| N | number of participants |
| p-values | significance level: p < 0.01, p < 0.05 or p < 0.10 |
| PSS | Perceived Stress Scale |
| PVAQ | Pain Vigilance Avoidance Questionnaire |
| SMT | sensorimotor training |
| SMT-BT | sensorimotor training with behavioural therapy elements |
| VE | Vital Exhaustion |
| MBSR | Mindfulness-Based Stress Reduction Program |
References
- Institute for Health Metrics and Evaluation. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Techniker Krankenkasse. Gesundheitsreport 2014—Veröffentlichungen zum Betrieblichen Gesundheitsmanagement der Techniker Krankenkasse; Techniker Krankenkasse: Hamburg, Germany, 2014. [Google Scholar]
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P.; Hartvigsen, J.; Cherkin, D.; Foster, N.E.; et al. Low back pain: A call for action. Lancet 2018, 391, 9–15. [Google Scholar] [CrossRef]
- National Association of Statutory Health Insurance Physicians; Association of the Scientific Medical Societies in Germany. Nationale Versorgungsleitlinie Nicht-Spezifischer Kreuzschmerz—Langfassung. National Therapy Guideline for Non-Specific Low Back Pain; National Association of Statutory Health Insurance Physicians; Association of the Scientific Medical Societies in Germany: Berlin, Germany, 2016. [Google Scholar]
- Nicholas, M.K.; Linton, S.J.; Watson, P.J.; Main, C.J.; “Decade of the Flags” Working Group. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: A reappraisal. Phys. Ther. 2011, 96, 1–17. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Engers, A.J.; Jellema, P.; Wensing, M.; van der Windt, D.A.; Grol, R.; van Tulder, M.W. Individual patient education for low back pain. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Heymans, M.W.; van Tulder, M.W.; Esmail, R.; Bombardier, C.; Koes, B.W. Back schools for non-specific low-back pain. Cochrane Database Syst. Rev. 2004, 4. [Google Scholar] [CrossRef]
- Henschke, N.; Ostelo, R.W.; van Tulder, M.W.; Vlaeyen, J.W.; Morley, S.; Assendelft, W.J.; Main, C.J. Behavioural treatment for chronic low-back pain. Cochrane Database Syst. Rev. 2010, CD002014. [Google Scholar] [CrossRef]
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.; Costa, L.C.M.; Ostelo, R.W.; Macedo, L.G. Motor control exercise for chronic non-specific low back pain: Cochrane review. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.; Ostelo, R.W.; Guzman, J.; van Tulder, M.W. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Hancock, M.J.; Maher, C.G.; Laslett, M.; Hay, E.; Koes, B. Discussion paper: What happened to the ‘bio’ in the bio-psycho-social model of low back pain? Eur. Spine J. 2011, 20, 2105–2110. [Google Scholar] [CrossRef]
- Wippert, P.-M.; Wiebking, C. Stress and Alterations in the Pain Matrix: A Biopsychosocial Perspective on Back Pain and Its Prevention and Treatment. Int. J. Environ. Res. Public Health 2018, 15, 785. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Wippert, P.-M.; Wippert, P.-M.; Niederer, D.; Drießlein, D.; Beck, H.; Banzer, W.; Schneider, C.; Schiltenwolf, M.; Mayer, F. The Chicken-Egg-Affair: Moderators and Mediators of the responsiveness to exercise in low back pain: A Randomized Controlled Trial. Br. J. Sports Med. 2019. under review. [Google Scholar]
- Mayer, F.; Arampatzis, A.; Banzer, W.; Beck, H.; Brüggemann, G.P.; Hasenbring, M.; Kellmann, M.; Kleinert, J.; Schiltenwolf, M.; Schmidt, H.; et al. Medicine in Spine Exercise [MiSpEx]—A National Research Network to evaluate Back Pain in High-performance Sports as well as the General Population. Ger. J. Sports Med. 2018, 69, 229–235. [Google Scholar]
- Hönning, A.; Stengel, D.; Güthoff, C. Statistical strategies to address main research questions of the MiSpEx network and meta-analytical approaches. Ger. J. Sports Med. 2018, 69, 236–239. [Google Scholar] [CrossRef]
- Wippert, P.M.; de Witt Huberts, J.; Klipker, K.; Gantz, S.; Schiltenwolf, M.; Mayer, F. Beschreibung und empirische Fundierung des verhaltenstherapeutischen Moduls der MiSpEx-Intervention. Schmerz 2015, 29, 658–663. [Google Scholar] [CrossRef]
- Hayden, J.A.; Cartwright, J.L.; Riley, R.D. Exercise therapy for chronic low back pain: Protocol for an individual participant data meta-analysis. Syst. Rev. 2012, 1, 64. [Google Scholar] [CrossRef]
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.; Costa, L.C.M.; Ostelo, R.W.; Macedo, L.G. Motor control exercise for chronic non-specific low back pain: A Cochrane review. Spine 2016, 41, 1284–1295. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database of Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Standaert, C.J.; Weinstein, S.M.; Rumpeltes, J. Evidence-informed management of chronic low back pain with lumbar stabilization exercises. Spine J. 2008, 8, 114–120. [Google Scholar] [CrossRef]
- Burciu, R.G.; Fritsche, N.; Granert, O.; Schmitz, L.; Spönemann, N.; Konczak, J.; Theysohn, N.; Gerwig, M.; van Eimeren, T.; Timmann, D. Brain changes associated with postural training in patients with cerebellar degeneration: A voxel-based morphometry study. J. Neurosci. 2013, 33, 4594–4604. [Google Scholar] [CrossRef]
- Vivar, C.; Potter, M.C.; van Praag, H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Topics Behav. Neurosci. 2013, 15, 189–210. [Google Scholar]
- Erickson, K.I.; Gildengers, A.G.; Butters, M.A. Physical activity and brain plasticity in late adulthood. Dialogues Clin. Neurosci. 2013, 15, 99–108. [Google Scholar]
- Naugle, K.M.; Fillingim, R.B.; Riley, J.L. A Meta-Analytic Review of the Hypoalgesic Effects of Exercise. J. Pain 2012, 13, 1139–1150. [Google Scholar] [CrossRef]
- Hoeger-Bement, M.K. Exercise-induced hypoalgesia: An evidenced-based review. In Management and Mechanisms of Pain for the Physical Therapist; Sluka, K.A., Ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2009. [Google Scholar]
- Dapunt, U.; Gantz, S.; Zhuk, A.; Gather, K.; Wang, H.; Schiltenwolf, M. Quantitative sensory testing in physically active individuals and patients who underwent multidisciplinary pain therapy in the longitudinal course. J. Pain Res. 2018, 11, 2323–2330. [Google Scholar] [CrossRef]
- Niederer, D.; Vogt, L.; Wippert, P.M.; Puschmann, A.K.; Pfeifer, A.C.; Schiltenwolf, M.; Banzer, W.; Mayer, F. Medicine in spine exercise (MiSpEx) for nonspecific low back pain patients: Study protocol of a multicentre, single-blind randomised controlled trial. Trials 2016, 17, 507. [Google Scholar] [CrossRef]
- Mueller, J.; Stoll, J.; Mueller, S.; Mayer, F. Dose-response relationship of core-specific sensorimotor interventions in healthy, well-trained participants: Study protocol for a (MiSpEx) randomized controlled trial. Trials 2018, 19, 424. [Google Scholar] [CrossRef]
- Arampatzis, A.; Schroll, A.; Catalá, M.M.; Laube, G.; Schüler, S.; Dreinhofer, K. A random-perturbation therapy in chronic non-specific low-back pain patients: A randomised controlled trial. Eur. J. Appl. Physiol. 2017, 117, 2547–2560. [Google Scholar] [CrossRef]
- Doumas, M.; Rapp, M.A.; Krampe, R.T. Working memory and postural control: Adult age differences in potential for improvement, task priority, and dual tasking. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2009, 64, 193–201. [Google Scholar] [CrossRef]
- Resch, J.E.; May, B.; Tomporowski, P.D.; Ferrara, M.S. Balance performance with a cognitive task: A continuation of the dual-task testing paradigm. J. Athlethic Train. 2011, 46, 170–175. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Huber, C.; Schöfer, D.; Kunz, M.; Parthum, A.; Weber, P.G.; Roman, C.; Griessinger, N.; Sittl, R. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: A comparison with other psychological and physiological predictors. Pain 2010, 151, 722–731. [Google Scholar] [CrossRef]
- Verhoeven, K.; Crombez, G.; Eccleston, C.; Van Ryckeghem, D.M.; Morley, S.; Van Damme, S. The role of motivation in distracting attention away from pain: An experimental study. Pain 2010, 149, 229–234. [Google Scholar] [CrossRef]
- Villemure, C.; Bushnell, M.C. Mood influences supraspinal pain processing separately from attention. J. Neurosci. 2009, 29, 705–715. [Google Scholar] [CrossRef]
- Valet, M.; Sprenger, T.; Boecker, H.; Willoch, F.; Rummeny, E.; Conrad, B.; Erhard, P.; Tolle, T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—An fMRI analysis. Pain 2004, 109, 399–408. [Google Scholar] [CrossRef]
- Villarreal, E.A.; Brattico, E.; Vase, L.; Østergaard, L.; Vuust, P. Superior analgesic effect of an active distraction versus pleasant unfamiliar sounds and music: The influence of emotion and cognitive style. PLoS ONE 2012, 7, 1e29397. [Google Scholar] [CrossRef]
- Tracey, I.; Ploghaus, A.; Gati, J.S.; Clare, S.; Smith, S.; Menon, R.S.; Matthews, P.M. Imaging attentional modulation of pain in the periaqueductal gray in humans. J. Neurosci. 2002, 22, 2748–2752. [Google Scholar] [CrossRef]
- Pichierri, G.; Wolf, P.; Murer, K.; de Bruin, E.D. Cognitive and cognitive-motor interventions affecting physical functioning: A systematic review. BMC Geriatr. 2011, 11, 29. [Google Scholar] [CrossRef]
- Niemann, H. California Verbal Learning Test (CVLT)—Deutsche Adaptation; Pearson: London, UK, 2008. [Google Scholar]
- Zeidan, F.; Grant, J.A.; Brown, C.A.; McHaffie, J.G.; Coghill, R.C. Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neurosci. Lett. 2012, 520, 165–173. [Google Scholar] [CrossRef]
- Wiebking, C.; Duncan, N.W.; Tiret, B.; Hayes, D.J.; Marjaǹska, M.; Doyon, J.; Bajbouj, M.; Northoff, G. GABA in the insula—A predictor of the neural response to interoceptive awareness. Neuroimage 2014, 86, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Segal, Z.V.; Teasdale, J.D.; Williams, J.M.G. Mindfulness-Based Cognitive Therapy: Theoretical Rationale and Empirical Status. In Mindfulness and Acceptance: Expanding the Cognitive-Behavioral Tradition; Hayes, S.C., Follette, V.M., Linehan, M.M., Eds.; Guilford Press: New York, NY, USA, 2004; pp. 45–65. [Google Scholar]
- Creswell, J.D.; Lindsay, E.K.; Villalba, D.K.; Chin, B. Mindfulness Training and Physical Health. Mechanisms and Outcomes. Psychosom. Med. 2019, 81, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Grossman, P.; Niemann, L.; Schmidt, S.; Walach, H. Mindfulness-based stress reduction and health benefits-A meta-analysis. J. Psychosom. Res. 2004, 57, 35–44. [Google Scholar] [CrossRef]
- Taren, A.A.; Gianaros, P.J.; Greco, C.M.; Lindsay, E.K.; Fairgrieve, A.; Brown, K.W.; Rosen, R.K.; Ferris, J.L.; Julson, E.; Marsland, A.L.; et al. Mindfulness Meditation Training and Executive Control Network Resting State Functional Connectivity: A Randomized Controlled Trial. Psychosom. Med. 2018, 79, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Ditto, B.; Eclache, M.; Goldman, N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann. Behav. Med. A Publ. Soc. Behav. Med. 2006, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Morlion, B.; Coluzzi, F.; Aldington, D.; Kocot-Kepska, M.; Pergolizzi, J.; Mangas, A.C.; Ahlbeck, K.; Kalso, E. Pain chronification: What should a non-pain medicine specialist know? Curr. Med. Res. Opin. 2019, 34, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B. Postmodern pain education “from being to becoming”. Pain 2018, 159, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Appels, A.; Hoppener, P.; Mulder, A. A questionnaire to assess premonitory symptoms of myocardial infarction. Int. J. Cardiol. 1987, 17, 15–24. [Google Scholar] [CrossRef]
- Herrmann-Lingen, C.; Buss, U.; Snaith, R.P. Hospital Anxiety and Depression Scale—Deutsche Version (HADS-D); Verlag Hans Huber: Bern, Switzerland, 1995. [Google Scholar]
- Pfingsten, M.; Leibing, E.; Franz, C.; Bansemer, D.; Busch, O.; Hildebrandt, J. Fear-avoidance-beliefs in patients with backpain. Schmerz 1997, 11, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Capito, E.S.; Horn-Hofmann, C.; Baum, C.; Scheel, J.; Karmann, A.J.; Priebe, J.A.; Lautenbacher, S. Psychometric properties of the German version of the Pain Vigilance and Awareness Questionnaire (PVAQ) in pain-free samples and samples with acute and chronic pain. Int. J. Behav. Med. 2017, 24, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, R.; Schulz, U. Berliner Social-Support Skalen; Freie Universität: Berlin, Germany, 2000. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2019. Available online: https://www.R-project.org/ (accessed on 30 December 2019).
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.S.; Taylor, B.C.; Greer, N.; Murdoch, M.; MacDonald, R.; McKenzie, L.; Rosebush, C.E.; Wilt, T.J. Focused Evidence Review: Psychometric Properties of Patient-Reported Outcome Measures for Chronic Musculoskeletal Pain. J. Gen. Intern. Med. 2018, 33, 61–70. [Google Scholar] [CrossRef]
- Von Korff, M. Assessment of Chronic Pain in Epidemiological and Health Services Research: Empirical Bases and New Directions. In Handbook of Pain Assessment; The Guilford Press: New York, NY, USA, 2011; pp. 455–473. [Google Scholar]
- Krebs, E.E.; Bair, M.J.; Damush, T.M.; Tu, W.; Wu, J.; Kroenke, K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med. Care 2010, 48, 1007–1014. [Google Scholar] [CrossRef]
- Bernard, P.; Romain, A.J.; Caudroit, J.; Chevance, G.; Carayol, M.; Gourlan, M.; Needham Dancause, K.; Moullec, G. Cognitive behavior therapy combined with exercise for adults with chronic diseases: Systematic review and meta-analysis. Health Psychol. 2018, 37, 433–450. [Google Scholar] [CrossRef]
- Broman-Fulks, J.J.; Abraham, C.M.; Thomas, K.; Canu, W.H.; Nieman, D.C. Anxiety sensitivity mediates the relationship between exercise frequency and anxiety and depression symptomology. Stress Health 2018, 34, 500–508. [Google Scholar] [CrossRef]
- Smith, B.E.; Hendrick, P.; Bateman, M.; Holden, S.; Littlewood, C.; Smith, T.O.; Logan, P. Musculoskeletal pain and exercise—Challenging existing paradigms and introducing new. Br. J. Sports Med. 2019, 53, 907–912. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar]


| Characteristic | % | X | SD | n | X | SD | n | X | SD | n |
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M4 | M5 | ||||||||
| Gender (female) | 61.1 | -- | -- | 291 | ||||||
| Higher Education | 40.53 | -- | -- | 227 | ||||||
| Living in partnership | 55.95 | -- | -- | 227 | ||||||
| Age | -- | 39.7 | 12.7 | 291 | ||||||
| CPG CPI CG a | 32.81 | 19.35 | 146 | 28.43 | 20.07 | 83 | 25.42 | 17.69 | 83 | |
| CPG CPI SMT + BT | 36.34 | 18.77 | 145 | 27.27 | 17.85 | 89 | 23.65 | 16.18 | 80 | |
| CPG DISS CG | 17.05 | 21.93 | 146 | 10.32 | 17.11 | 83 | 8.05 | 12.65 | 83 | |
| CPG DISS SMT + BT | 25.08 | 24.66 | 145 | 12.06 | 17.38 | 89 | 8.71 | 13.29 | 80 | |
| fab_activity CG b | 12.56 | 6.48 | 142 | 10.04 | 6.66 | 82 | 14.58 | 3.89 | 36 | |
| fab_activity SMT+ BT | 12.86 | 5.29 | 139 | 13.01 | 5.85 | 88 | 12.26 | 5.71 | 53 | |
| HADS anxiety CG c | 5.17 | 3.04 | 144 | 5.99 | 3.20 | 80 | 5.80 | 3.25 | 79 | |
| HADS anxiety SMT + BT | 5.23 | 3.04 | 142 | 4.63 | 2.86 | 82 | 4.63 | 3.02 | 70 | |
| HADS depression CG | 3.69 | 2.99 | 144 | 3.77 | 3.33 | 80 | 3.79 | 3.06 | 77 | |
| HADS depression SMT + BT | 3.81 | 2.97 | 143 | 3.13 | 3.08 | 84 | 3.18 | 3.30 | 71 | |
| PSS CG d | 16.09 | 6.47 | 139 | 16.55 | 5.65 | 80 | 14.25 | 5.59 | 79 | |
| PSS SMT + BT | 15.88 | 5.77 | 137 | 14.59 | 5.93 | 86 | 13.57 | 7.37 | 76 | |
| VE CG e | 7.00 | 4.87 | 144 | 7.38 | 4.89 | 82 | 6.83 | 4.76 | 82 | |
| VE SMT + BT | 7.57 | 5.15 | 141 | 6.15 | 4.72 | 85 | 6.18 | 5.08 | 79 | |
| PVAQ CG f | 37.27 | 13.02 | 146 | 34.18 | 13.55 | 83 | -- | -- | -- | |
| PVAQ SMT + BT | 38.10 | 12.53 | 145 | 34.15 | 11.68 | 89 | -- | -- | -- | |
| Characteristics | M1 to M4 | M1 to M5 | ||||
|---|---|---|---|---|---|---|
| Groups, Test Statistic | CG | SMT + BT | F (p) | CG | SMT + BT | F (p) |
| CPG CPI a | −2.34 | −8.57 | 0.67 (0.415) | 0.35 | −2.46 | 0.15 (0.700) |
| CPG DISS | −3.40 | −4.63 | 0.02 (0.878) | 4.30 | 4.56 | 0.00 (0.964) |
| FABQ_activity b | 1.91 | −1.23 | 0.91 (0.344) | 0.59 | −0.10 | 0.07 (0.786) |
| HADS anxiety c | 1.62 | −1.10 | 5.96 (0.017) * | 2.21 | −0.39 | 4.00 (0.048) * |
| HADS depression | 0.90 | 0.39 | 0.17 (0.683) | −0.36 | 0.02 | 0.09 (0.765) |
| PSS Perceived stress d | −0.11 | 1.25 | 0.36 (0.552) | −0.64 | 0.98 | 0. 13 (0.579) |
| VE Vital Exhaustion e | 1.33 | −0.95 | 1.70 (0.194) | 0.42 | −2.20 | 2.17 (0.144) # |
| PVAQ Pain Vigilance Avoidance f | 0.47 | 1.41 | 0.06 (0.800) | -- | -- | -- |
| Characteristics | SMT + BT | F (p) | SMT + BT | F (p) |
|---|---|---|---|---|
| Differences in comparison to CG | M4–M1 | M5–M1 | ||
| CPG CPI a | −7.95 | 0.85 (0.359) | −6.24 | 0.34 (0.559) |
| CPG DISS | −11.81 | 1.55 (0.215) | −17.03 | 2.16 (0.109) # |
| FABQ_activity b | −0.11 | 0.72 (0.401) | −2.88 | 0.02 (0.892) |
| HADS anxiety c | −3.54 | 10.55 (0.001) ** | −2.88 | 7.70 (0.006) ** |
| HADS depression | −1.44 | 0.46 (0.501) | −0.61 | 0.00 (0.993) |
| PSS Perceived stress d | 0.09 | 0.09 (0.768) | 0.68 | 0.11 (0.746) |
| VE Vital Exhaustion e | −3.06 | 1.37 (0.244) | −3.01 | 1.86 (0.195) |
| PVAQ Pain Vigilance Avoidance f | −0.43 | 0.34 (0.560) | -- | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wippert, P.-M.; Drießlein, D.; Beck, H.; Schneider, C.; Puschmann, A.-K.; Banzer, W.; Schiltenwolf, M. The Feasibility and Effectiveness of a New Practical Multidisciplinary Treatment for Low-Back Pain: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 115. https://doi.org/10.3390/jcm9010115
Wippert P-M, Drießlein D, Beck H, Schneider C, Puschmann A-K, Banzer W, Schiltenwolf M. The Feasibility and Effectiveness of a New Practical Multidisciplinary Treatment for Low-Back Pain: A Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(1):115. https://doi.org/10.3390/jcm9010115
Chicago/Turabian StyleWippert, Pia-Maria, David Drießlein, Heidrun Beck, Christian Schneider, Anne-Katrin Puschmann, Winfried Banzer, and Marcus Schiltenwolf. 2020. "The Feasibility and Effectiveness of a New Practical Multidisciplinary Treatment for Low-Back Pain: A Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 1: 115. https://doi.org/10.3390/jcm9010115
APA StyleWippert, P.-M., Drießlein, D., Beck, H., Schneider, C., Puschmann, A.-K., Banzer, W., & Schiltenwolf, M. (2020). The Feasibility and Effectiveness of a New Practical Multidisciplinary Treatment for Low-Back Pain: A Randomized Controlled Trial. Journal of Clinical Medicine, 9(1), 115. https://doi.org/10.3390/jcm9010115







