Intraoperative Anesthetic Management of Patients with Chronic Obstructive Pulmonary Disease to Decrease the Risk of Postoperative Pulmonary Complications after Abdominal Surgery
Abstract
1. Introduction
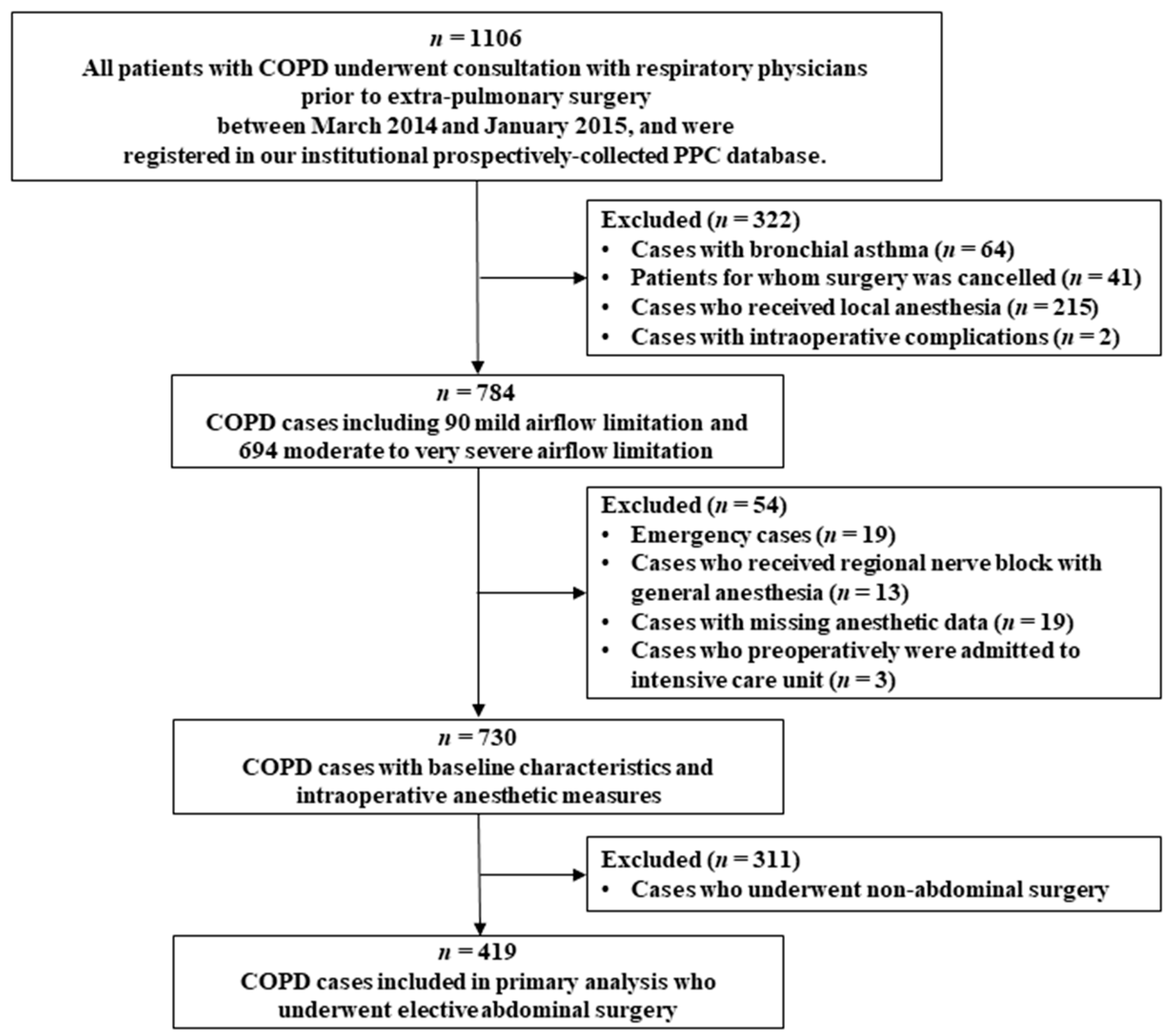
2. Materials and Methods
2.1. Subjects and Data Sources
2.2. Degree of Airflow Limitation
2.3. Statistical Analysis
3. Results
3.1. Multivariable Analysis
3.2. Respective Importance of Each Protective Factor on Each PPC
3.3. Respective Importance of Each Protective Factor on PPC Risk According to the Existing Risk Factors
3.4. Cumulative Effect of Protective Anesthetic Interventions
3.5. Association between PPC and Postoperative Clinical Courses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Gupta, H.; Ramanan, B.; Gupta, P.K.; Fang, X.; Polich, A.; Modrykamien, A.; Schuller, D.; Morrow, L.E. Impact of COPD on postoperative outcomes: Results from a national database. Chest 2013, 143, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Hollmann, M.W.; Jaber, S.; Kozian, A.; Licker, M.; et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: A systematic review and meta-analysis. Lancet Respir. Med. 2014, 2, 1007–1015. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bustamante, A.; Frendl, G.; Sprung, J.; Kor, D.J.; Subramaniam, B.; Martinez Ruiz, R.; Lee, J.W.; Henderson, W.G.; Moss, A.; Mehdiratta, N.; et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017, 152, 157–166. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N. Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef]
- Marseu, K.; Slinger, P. Perioperative lung protection. Korean J. Anesthesiol. 2017, 70, 239–244. [Google Scholar] [CrossRef][Green Version]
- Neto, A.S.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Gajic, O.; El-Tahan, M.R.; Ghamdi, A.A.; Gunay, E.; et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: A meta-analysis of individual patient data. Lancet Respir. Med. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Brandstrup, B.; Tonnesen, H.; Beier-Holgersen, R.; Hjortso, E.; Ording, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648. [Google Scholar] [CrossRef]
- Martinez-Ubieto, J.; Ortega-Lucea, S.; Pascual-Bellosta, A.; Arazo-Iglesias, I.; Gil-Bona, J.; Jimenez-Bernardo, T.; Munoz-Rodriguez, L. Prospective study of residual neuromuscular block and postoperative respiratory complications in patients reversed with neostigmine versus sugammadex. Minerva Anestesiol. 2016, 82, 735–742. [Google Scholar]
- Brueckmann, B.; Sasaki, N.; Grobara, P.; Li, M.K.; Woo, T.; De Bie, J.; Maktabi, M.; Lee, J.; Kwo, J.; Pino, R.; et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: A randomized, controlled study. Br. J. Anaesth. 2015, 115, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Teng, A.; Lee, D.Y.; Rose, K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J. Surg. Res. 2015, 198, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.H.; Shin, B.; Eom, J.S.; Yoo, H.; Song, W.; Han, S.; Lee, K.J.; Jeon, K.; Um, S.W.; Koh, W.J.; et al. Development of a prediction rule for estimating postoperative pulmonary complications. PLoS ONE 2014, 9, e113656. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Lee, H.; Kang, D.; Jeong, B.H.; Kang, H.K.; Chon, H.R.; Koh, W.J.; Chung, M.P.; Guallar, E.; Cho, J.; et al. Airflow limitation severity and post-operative pulmonary complications following extra-pulmonary surgery in COPD patients. Respirology 2017, 22, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, R.D.; Wettstein, R.; Wittnebel, L.; Tracy, M. Incentive spirometry: 2011. Respir. Care 2011, 56, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Valles, J.; Castillo, J.; Sabate, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- McShane, P.J.; Naureckas, E.T.; Tino, G.; Strek, M.E. Non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2013, 188, 647–656. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Choi, J.K.; Paek, D.; Lee, J.O. Normal Predictive Values of Spirometry in Korean Population. Tuberc. Respir. Dis. 2005, 58, 230–242. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.S.; Lee, S.W.; Oh, Y.M. Pulmonary complications after abdominal surgery in patients with mild-to-moderate chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2785–2796. [Google Scholar] [CrossRef]
- Eriksson, B.; Lindberg, A.; Mullerova, H.; Ronmark, E.; Lundback, B. Association of heart diseases with COPD and restrictive lung function—Results from a population survey. Respir. Med. 2013, 107, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Grant, M.C.; Stone, A.; Wu, C.L.; Wick, E.C. A Meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: Is low tidal volume alone sufficient to protect healthy lungs? Ann. Surg. 2016, 263, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Ahn, H.J.; Kim, J.A.; Yang, M.; Heo, B.Y.; Choi, J.W.; Kim, Y.R.; Lee, S.H.; Jeong, H.; Choi, S.J.; et al. Driving Pressure during Thoracic Surgery: A Randomized Clinical Trial. Anesthesiology 2019, 130, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Similowski, T.; Milic-Emili, J.; Derenne, J.P. Respiratory mechanics during acute respiratory failure of chronic obstructive pulmonary disease. Lung. Biol. Health Dis. 1996, 92, 23–46. [Google Scholar]
- Celli, B.R. Pathophysiology of Chronic Obstructive Pulmonary Disease. In Mechanics of Breathing: Pathophysiology, Diagnosis and Treatment; Aliverti, A., Brusasco, V., Macklem, P.T., Pedotti, A., Eds.; Springer: Milano, Italy, 2002; pp. 218–231. [Google Scholar]
- Papandrinopoulou, D.; Tzouda, V.; Tsoukalas, G. Lung compliance and chronic obstructive pulmonary disease. Pulm. Med. 2012, 2012, 542769. [Google Scholar] [CrossRef]
- Young, C.C.; Harris, E.M.; Vacchiano, C.; Bodnar, S.; Bukowy, B.; Elliott, R.R.D.; Migliarese, J.; Ragains, C.; Trethewey, B.; Woodward, A.; et al. Lung-protective ventilation for the surgical patient: International expert panel-based consensus recommendations. Br. J. Anaesth. 2019. [Google Scholar] [CrossRef]
- Williams, E.C.; Motta-Ribeiro, G.C.; Vidal Melo, M.F. Driving Pressure and Transpulmonary Pressure: How Do We Guide Safe Mechanical Ventilation? Anesthesiology 2019, 131, 155–163. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, Z. Positive end expiratory pressure titration guided by plateau pressure in chronic obstructive pulmonary disease patients. Clin. Respir. J. 2018, 12, 674–680. [Google Scholar] [CrossRef]
- Nisanevich, V.; Felsenstein, I.; Almogy, G.; Weissman, C.; Einav, S.; Matot, I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005, 103, 25–32. [Google Scholar] [CrossRef]
- Holte, K.; Sharrock, N.E.; Kehlet, H. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 2002, 89, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Svensén, C.H.; Olsson, J.; Hahn, R.G. Intravascular Fluid Administration and Hemodynamic Performance During Open Abdominal Surgery. Anesth. Analg. 2006, 103, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Buggy, D.J. Intraoperative fluids: How much is too much? Br. J. Anaesth. 2012, 109, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.Y. In the hour of Sugammadex. Korean J. Anesthesiol. 2013, 64, 3–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cammu, G.; Schepens, T.; De Neve, N.; Wildemeersch, D.; Foubert, L.; Jorens, P.G. Diaphragmatic and intercostal electromyographic activity during neostigmine, sugammadex and neostigmine-sugammadex-enhanced recovery after neuromuscular blockade: A randomised controlled volunteer study. Eur. J. Anaesthesiol. 2017, 34, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ottenheijm, C.A.; Heunks, L.M.; Dekhuijzen, R.P. Diaphragm adaptations in patients with COPD. Respir. Res. 2008, 9, 12. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Zheng, Z.; Luo, Q.; Chen, R. The analysis of components that lead to increased work of breathing in chronic obstructive pulmonary disease patients. J. Thorac. Dis. 2016, 8, 2212–2218. [Google Scholar] [CrossRef]
- Stubbing, D.G.; Pengelly, L.D.; Morse, J.L.; Jones, N.L. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 49, 511–515. [Google Scholar] [CrossRef]
- Suter, P.M.; Fairley, H.B.; Isenberg, M.D. Effect of tidal volume and positive end-expiratory pressure on compliance during mechanical ventilation. Chest 1978, 73, 158–162. [Google Scholar] [CrossRef]
- Bigatello, L.; Pesenti, A. Respiratory Physiology for the Anesthesiologist. Anesthesiology 2019, 130, 1064–1077. [Google Scholar] [CrossRef]



| Without PPCs (n = 298) | With PPCs (n = 121) | p | |
|---|---|---|---|
| Patient factors | |||
| Age > 70 years | 135 (45.3) | 67 (55.4) | 0.067 |
| Male sex | 243 (81.5) | 107 (88.4) | 0.109 |
| Body mass index (kg/m2) | 23.7 (21.5–25.3) | 23.3 (22.0–25.2) | 0.774 |
| Diabetes | 79 (26.5) | 33 (27.3) | 0.903 |
| Hypertension | 152 (51.0) | 73 (60.3) | 0.085 |
| Hemoglobin (g/dL) | 13.3 (11.8–14.5) | 13.3 (11.9–14.2) | 0.674 |
| Neutrophil-to-lymphocyte ratio | 1.9 (1.4–2.8) | 1.9 (1.4–2.6) | 0.701 |
| Albumin (g/dL) | 4.3 (4.0–4.5) | 4.2 (4.0–4.5) | 0.449 |
| Creatinine (mg/dL) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.664 |
| ASA class 3 or 4 | 9 (3.0) | 7 (5.8) | 0.258 |
| Arrhythmia on electrocardiography | 16 (5.4) | 11 (9.1) | 0.188 |
| Malignancy | 196 (65.8) | 104 (86.0) | <0.001 |
| Smoking history | 0.101 | ||
| Never smoker | 108 (36.2) | 31 (25.6) | |
| Previous smoker | 142 (47.7) | 65 (53.7) | |
| Current smoker | 48 (16.1) | 25 (20.7) | |
| Bronchiectasis | 31 (10.4) | 20 (16.5) | 0.099 |
| Airflow limitation degree | 0.972 | ||
| Mild | 36 (12.1) | 15 (12.4) | |
| Moderate | 232 (77.9) | 93 (76.9) | |
| Severe to very severe | 30 (10.1) | 13 (10.7) | |
| Combined restrictive lung disease | 131 (44.0) | 62 (51.2) | 0.195 |
| Perioperative bronchodilator use | 66 (22.1) | 37 (30.6) | 0.080 |
| Procedure factors | |||
| Surgical site | <0.001 | ||
| Upper abdomen | 99 (33.1) | 78 (64.5) | |
| Lower abdomen | 96 (32.2) | 36 (29.8) | |
| Perineal | 103 (34.6) | 7 (5.8) | |
| Surgical method | 0.022 | ||
| Nonlaparoscopic | 189 (63.2) | 91 (75.2) | |
| Laparoscopic | 109 (36.6) | 30 (24.8) | |
| ARISCAT score | 34 (19–41) | 41 (41–19) | <0.001 |
| Intraoperative Variables | Without PPCs (n = 298) | With PPCs (n = 121) | p |
|---|---|---|---|
| Intubation grade moderate to difficult | 30 (10.1) | 6 (5.0) | 0.123 |
| Anesthetic maintenance agent | 0.083 | ||
| Sevoflurane | 183 (61.4) | 81 (66.9) | |
| Isoflurane | 15 (19.2) | 12 (9.9) | |
| Desflurane | 81 (27.2) | 23 (19.0) | |
| Propofol | 19 (6.4) | 5 (4.1) | |
| Neuromuscular blocking agent | <0.001 | ||
| Rocuronium | 126 (42.3) | 21 (17.4) | |
| Vecuronium | 133 (44.6) | 81 (66.9) | |
| Cisatracurium | 39 (13.0) | 19 (15.7) | |
| Sugammadex-induced neuromuscular blockade reversal | 52 (17.4) | 7 (5.8) | 0.002 |
| Mechanical ventilation parameters | |||
| Tidal volume (mL/kg IBW) | 8.1 (7.4–8.8) | 8.3 (7.6–9.0) | 0.142 |
| Low tidal volume ventilation | 143 (47.8) | 47 (38.8) | 0.089 |
| Peak pressure (cmH2O) | 15 (14–17) | 15.0 (13–17) | 0.081 |
| Plateau pressure (cmH2O) | 12 (10–14) | 12.0 (10–13) | 0.289 |
| PEEP (cmH2O) | 2 (2–3) | 2 (2–3) | 0.422 |
| PEEP ≥5 cmH2O | 31 (10.4) | 15 (12.4) | 0.605 |
| Respiratory rate (bpm) | 10 (9–12) | 10 (9–11) | 0.200 |
| Driving pressure | 9 (8–11) | 9 (8–11) | 0.430 |
| Dynamic compliance | 30.2 (26.2–34.2) | 31.9 (28.2–35.7) | 0.010 |
| Active airway humidification | 71 (23.8) | 27 (22.3) | 0.800 |
| Fluid therapy | |||
| Crystalloid infusion (mL/kg/h) | 5.6 (4.2–6.8) | 6.4 (4.9–7.6) | <0.001 |
| Colloid infusion | 72 (24.2) | 54 (44.6) | <0.001 |
| Red blood cell transfusion | 20 (6.7) | 17 (14.0) | 0.022 |
| Hemodynamic parameters | |||
| Estimated blood loss (mL) | 100 (50–200) | 200 (100–450) | <0.001 |
| MBP <60 cmH2O for >30 min | 14 (4.7) | 12 (9.9) | 0.071 |
| Continuous vasoactive drug use | 9 (3.0) | 11 (8.3) | 0.034 |
| Hypothermia | 210 (70.5) | 92 (76.0) | 0.280 |
| Opioid use for intraoperative pain control | 0.468 | ||
| None | 52 (17.4) | 18 (14.9) | |
| Fentanyl | 40 (13.4) | 15 (12.4) | |
| Hydromorphone | 120 (40.3) | 59 (48.8) | |
| Demerol | 45 (15.1) | 12 (9.9) | |
| Morphine | 41 (13.8) | 17 (14.0) | |
| Anesthesia time >3 h | 151 (50.7) | 97 (80.2) | <0.001 |
| Discharge to intensive care unit | 60 (20.1) | 59 (48.8) | <0.001 |
| OR | p | |
|---|---|---|
| Nonmodifiable factor | ||
| Age > 70 years | 1.86 (1.10–3.15) | 0.022 |
| Preoperative bronchiectasis | 2.27 (1.10–4.68) | 0.026 |
| Surgical site (vs. perineal) | ||
| Upper abdomen | 7.43 (3.02–18.29) | <0.001 |
| Lower abdomen | 3.40 (1.35–8.57) | 0.009 |
| Arrhythmia on electrocardiography | 2.38 (0.87–6.52) | 0.092 |
| Smoking history (vs. never smoker) | ||
| Previous smoker | 1.78 (0.99–3.17) | 0.055 |
| Current smoker | 1.98 (0.94–4.15) | 0.073 |
| Anesthesia time > 3 h | 2.75 (1.55–4.88) | 0.001 |
| Discharge to intensive care unit | 1.71 (0.96–3.05) | 0.066 |
| Modifiable factor | ||
| Low tidal volume ventilation | 0.50 (0.29–0.85) | 0.010 |
| Crystalloid infusion (mL/kg/h) | 1.13 (1.03–1.25) | 0.012 |
| Sugammadex-induced neuromuscular blockade reversal | 0.27 (0.11–0.69) | 0.006 |
| Without PPCs (n = 298) | With PPCs (n = 121) | p | |
|---|---|---|---|
| Prolonged mechanical ventilation > 24 h | 1 (0.3) | 3 (0.7) | 0.075 |
| Reintubation | 0 | 1 (0.8) | 0.289 |
| Postoperative length of hospital stay (h) | 159.4 (61.1–203.8) | 187.4 (161.8–251.7) | 0.071 |
| 30-day mortality | 1 (0.3) | 1 (0.8) | 0.495 |
| 90-day mortality | 4 (1.3) | 7 (5.8) | 0.016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Oh, E.J.; Han, S.; Shin, B.; Shin, S.H.; Im, Y.; Son, Y.H.; Park, H.Y. Intraoperative Anesthetic Management of Patients with Chronic Obstructive Pulmonary Disease to Decrease the Risk of Postoperative Pulmonary Complications after Abdominal Surgery. J. Clin. Med. 2020, 9, 150. https://doi.org/10.3390/jcm9010150
Park S, Oh EJ, Han S, Shin B, Shin SH, Im Y, Son YH, Park HY. Intraoperative Anesthetic Management of Patients with Chronic Obstructive Pulmonary Disease to Decrease the Risk of Postoperative Pulmonary Complications after Abdominal Surgery. Journal of Clinical Medicine. 2020; 9(1):150. https://doi.org/10.3390/jcm9010150
Chicago/Turabian StylePark, Sukhee, Eun Jung Oh, Sangbin Han, Beomsu Shin, Sun Hye Shin, Yunjoo Im, Yong Hoon Son, and Hye Yun Park. 2020. "Intraoperative Anesthetic Management of Patients with Chronic Obstructive Pulmonary Disease to Decrease the Risk of Postoperative Pulmonary Complications after Abdominal Surgery" Journal of Clinical Medicine 9, no. 1: 150. https://doi.org/10.3390/jcm9010150
APA StylePark, S., Oh, E. J., Han, S., Shin, B., Shin, S. H., Im, Y., Son, Y. H., & Park, H. Y. (2020). Intraoperative Anesthetic Management of Patients with Chronic Obstructive Pulmonary Disease to Decrease the Risk of Postoperative Pulmonary Complications after Abdominal Surgery. Journal of Clinical Medicine, 9(1), 150. https://doi.org/10.3390/jcm9010150





