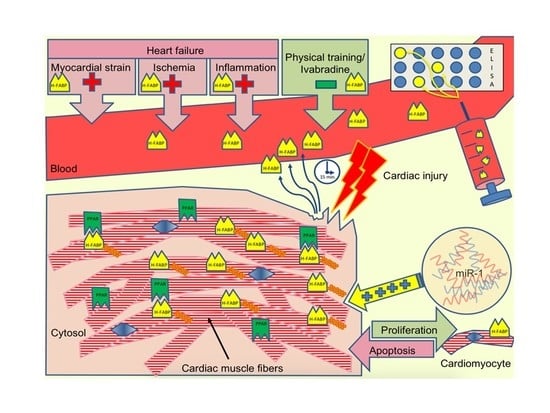
Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far?
Abstract
:1. Introduction
2. Methods
3. H-FABP as a Biomarker in Heart Failure
4. Discussion and Conclusion(s)
Author Contributions
Funding
Conflicts of Interest
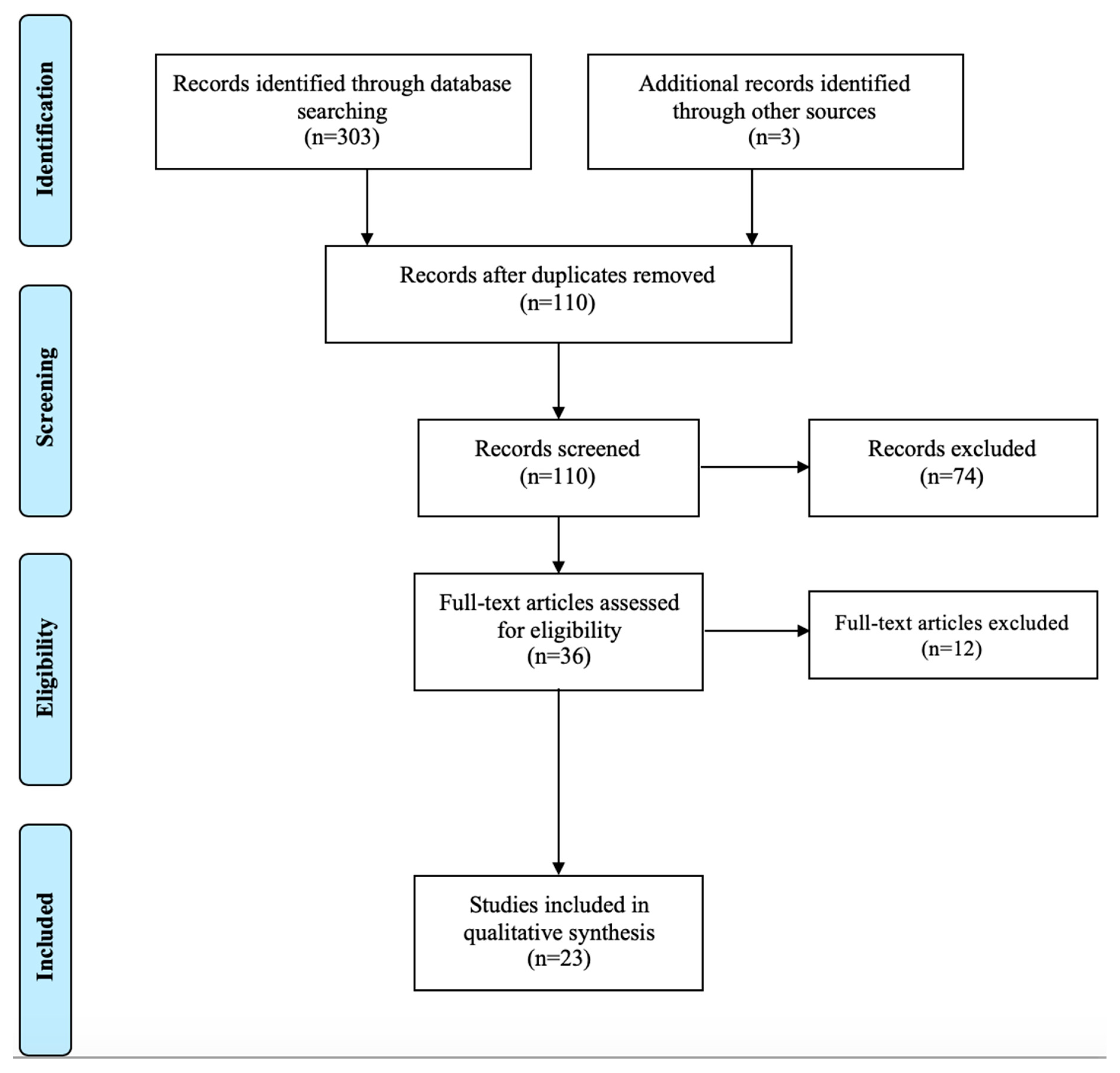
Appendix A. Flow Diagram
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- van Riet, E.E.S.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.J.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, N.M. The Cellular Fatty Acid Binding Proteins: Aspects of Structure, Regulation, and Function. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 111, pp. 143–184. ISBN 978-0-12-364511-1. [Google Scholar]
- Veerkamp, J.H.; Peeters, R.A.; Maatman, R.G. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim. Biophys. Acta 1991, 1081, 1–24. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Takahashi, H.; Hirayama, A.; Narumi, T.; Kadowaki, S.; Honda, Y.; Arimoto, T.; Shishido, T.; Miyamoto, T.; et al. Association of Heart-Type Fatty Acid-Binding Protein with Cardiovascular Risk Factors and All-Cause Mortality in the General Population: The Takahata Study. PLoS ONE 2014, 9, e94834. [Google Scholar] [CrossRef]
- Fatty Acid Binding Protein Family|HUGO GENE Nomenclature Committee. Available online: https://www.genenames.org/data/genegroup/#!/group/550 (accessed on 2 January 2019).
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Hostetler, H.A.; McIntosh, A.L.; Atshaves, B.P.; Storey, S.M.; Payne, H.R.; Kier, A.B.; Schroeder, F. L-FABP directly interacts with PPARalpha in cultured primary hepatocytes. J. Lipid Res. 2009, 50, 1663–1675. [Google Scholar] [CrossRef] [Green Version]
- Tan, N.-S.; Shaw, N.S.; Vinckenbosch, N.; Liu, P.; Yasmin, R.; Desvergne, B.; Wahli, W.; Noy, N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell. Biol. 2002, 22, 5114–5127. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Heart-type fatty acid-binding protein (H-FABP) and coronary heart disease. Indian Heart J. 2016, 68, 16–18. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Wu, L.; Wang, X.; Xin, Y.; Zan, L. Tissue expression analysis, cloning and characterization of the 5′-regulatory region of the bovine FABP3 gene. Mol. Biol. Rep. 2016, 43, 991–998. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kida, H.; Kagawa, Y.; Yasumoto, Y.; Miyazaki, H.; Islam, A.; Ogata, M.; Yanagawa, Y.; Mitsushima, D.; Fukunaga, K.; et al. FABP3 in the Anterior Cingulate Cortex Modulates the Methylation Status of the Glutamic Acid Decarboxylase67 Promoter Region. J. Neurosci. 2018, 38, 10411–10423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebetrau, C.; Nef, H.M.; Dörr, O.; Gaede, L.; Hoffmann, J.; Hahnel, A.; Rolf, A.; Troidl, C.; Lackner, K.J.; Keller, T.; et al. Release kinetics of early ischaemic biomarkers in a clinical model of acute myocardial infarction. Heart 2014, 100, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Tanaka, T.; Somiya, K.; Tsuji, R.; Okamoto, F.; Kawamura, K.; Ohkaru, Y.; Asayama, K.; Ishii, H. Human heart-type cytoplasmic fatty acid-binding protein as an indicator of acute myocardial infarction. Heart Vessel. 1995, 10, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, S.; Furuhashi, M.; Watanabe, Y.; Hoshina, K.; Fuseya, T.; Mita, T.; Okazaki, Y.; Koyama, M.; Tanaka, M.; Akasaka, H.; et al. Circulating Levels of Fatty Acid-Binding Protein Family and Metabolic Phenotype in the General Population. PLoS ONE 2013, 8, e81318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch, P.M.; Pogoryelova, O.; Goldstein, R.; Bennett, D.; Guglieri, M.; Straub, V.; Bushby, K.; Lochmüller, H.; Morris, C. Muscle-Derived Proteins as Serum Biomarkers for Monitoring Disease Progression in Three Forms of Muscular Dystrophy. J. Neuromuscul. Dis. 2015, 2, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunes, F.; Asik, M.; Temiz, A.; Vural, A.; Sen, H.; Binnetoglu, E.; Bozkurt, N.; Tekeli, Z.; Erbag, G.; Ukinc, K.; et al. Serum H-FABP levels in patients with hypothyroidism. Wien. Klin. Wochenschr. 2014, 126, 727–733. [Google Scholar] [CrossRef]
- Varrone, F.; Gargano, B.; Carullo, P.; Di Silvestre, D.; De Palma, A.; Grasso, L.; Di Somma, C.; Mauri, P.; Benazzi, L.; Franzone, A.; et al. The circulating level of FABP3 is an indirect biomarker of microRNA-1. J. Am. Coll. Cardiol. 2013, 61, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Fischer, T.A.; McNeil, P.L.; Khakee, R.; Finn, P.; Kelly, R.A.; Pfeffer, M.A.; Pfeffer, J.M. Cardiac Myocyte Membrane Wounding in the Abruptly Pressure-Overloaded Rat Heart Under High Wall Stress. Hypertension 1997, 30, 1041–1046. [Google Scholar] [CrossRef]
- Iida, M.; Yamazaki, M.; Honjo, H.; Kodama, I.; Kamiya, K. Predictive value of heart-type fatty acid-binding protein for left ventricular remodelling and clinical outcome of hypertensive patients with mild-to-moderate aortic valve diseases. J. Hum. Hypertens. 2007, 21, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; He, Y.; Wang, S.; Wong, G.T.; Irwin, M.G.; Xia, Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol. Sin. 2018, 39, 1155. [Google Scholar] [CrossRef]
- Kleine, A.H.; Glatz, J.F.C.; Van Nieuwenhoven, F.A.; Van der Vusse, G.J. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. In Lipid Metabolism in the Healthy and Disease Heart; van der Vusse, G.J., Stam, H., Eds.; Developments in Molecular and Cellular Biochemistry; Springer US: Boston, MA, USA, 1992; pp. 155–162. ISBN 978-1-4615-3514-0. [Google Scholar]
- Binas, B.; Danneberg, H.; McWhir, J.; Mullins, L.; Clark, A.J. Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J. 1999, 13, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binas, B.; Erol, E. FABPs as determinants of myocellular and hepatic fuel metabolism. Mol. Cell. Biochem. 2007, 299, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.K.; Kindler, P.M.; Cai, D.Q.; Chow, P.H.; Li, M.; Lee, K.K.H. Heart-type fatty acid binding proteins are upregulated during terminal differentiation of mouse cardiomyocytes, as revealed by proteomic analysis. Cell Tissue Res. 2004, 316, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Andreyev, O.; Hoyt, R.F.; Singh, A.; Hunt, T.; Horvath, K.A. Overexpression of FABP3 inhibits human bone marrow derived mesenchymal stem cell proliferation but enhances their survival in hypoxia. Exp. Cell Res. 2014, 323, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Hu, D.L.; Liu, Y.Q.; Zhang, Q.J.; Chen, F.K.; Kong, X.Q.; Cao, K.J.; Zhang, J.S.; Qian, L.M. Fabp3 inhibits proliferation and promotes apoptosis of embryonic myocardial cells. Cell Biochem. Biophys. 2011, 60, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Song, G.; Liu, Y.; Zhou, L.; Liu, H.; Kong, X.; Sheng, Y.; Cao, K.; Qian, L. Silencing of FABP3 inhibits proliferation and promotes apoptosis in embryonic carcinoma cells. Cell Biochem. Biophys. 2013, 66, 139–146. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Song, G.-X.; Liu, H.-L.; Wang, X.-J.; Shen, Y.-H.; Zhou, L.-J.; Jin, J.; Liu, M.; Shi, C.-M.; Qian, L.-M. Silencing of FABP3 leads to apoptosis-induced mitochondrial dysfunction and stimulates Wnt signaling in zebrafish. Mol. Med. Rep. 2013, 8, 806–812. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Jin, J.; Yang, Y.; Song, G.; Shen, Y.; Liu, H.; Liu, M.; Shi, C.; Qian, L. Knockdown of FABP3 impairs cardiac development in Zebrafish through the retinoic acid signaling pathway. Int. J. Mol. Sci. 2013, 14, 13826–13841. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Li, C.; Chen, Q.; Jin, Q.; Wu, L.; Lu, L.; Yan, X.; Chen, K. Fatty acid-binding protein 3 contributes to ischemic heart injury by regulating cardiac myocyte apoptosis and MAPK pathways. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H971–H984. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Zhou, W.; Zhao, Q.; Lü, Z. miR-192-5p mediates hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes via targeting of FABP3. J. Biochem. Mol. Toxicol. 2017, 31, e21873. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Q.J.; Wang, L.J.; Liu, Z.H.; Zhang, Q.; Yang, K.; Wang, H.B.; Yan, X.X.; Zhu, Z.B.; Du, R.; et al. Increment of HFABP Level in Coronary Artery In-Stent Restenosis Segments in Diabetic and Nondiabetic Minipigs: HFABP Overexpression Promotes Multiple Pathway-Related Inflammation, Growth and Migration in Human Vascular Smooth Muscle Cells. J. Vasc. Res. 2016, 53, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Arı, H.; Tokaç, M.; Alihanoğlu, Y.; Kıyıcı, A.; Kayrak, M.; Arı, M.; Sönmez, O.; Gök, H. Relationship between heart-type fatty acid-binding protein levels and coronary artery disease in exercise stress testing: An observational study. Anadolu Kardiyol. Derg. 2011, 11, 685–691. [Google Scholar] [PubMed] [Green Version]
- Lichtenauer, M.; Jirak, P.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Schernthaner, C.; Kraus, J.; Motloch, L.J.; Yilmaz, A.; et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur. J. Intern. Med. 2017, 44, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, F.; Sohmiya, K.; Ohkaru, Y.; Kawamura, K.; Asayama, K.; Kimura, H.; Nishimura, S.; Ishii, H.; Sunahara, N.; Tanaka, T. Human heart-type cytoplasmic fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. Clinical evaluation of H-FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin. Chem. Lab. Med. 2000, 38, 231–238. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Cristol, J.P.; Kuster, N.; Reynier, R.; Lefebvre, S.; Badiou, S.; Jreige, R.; Sebbane, M. Performances of the heart fatty acid protein assay for the rapid diagnosis of acute myocardial infarction in ED patients. Am. J. Emerg. Med. 2015, 33, 326–330. [Google Scholar] [CrossRef]
- Ruff, C.T.; Bonaca, M.P.; Kosowsky, J.M.; Conrad, M.J.; Murphy, S.A.; Jarolim, P.; Donahoe, S.M.; O’Donoghue, M.L.; Morrow, D.A. Evaluation of the diagnostic performance of heart-type fatty acid binding protein in the BWH-TIMI ED chest pain study. J. Thromb. Thrombolysis 2013, 36, 361–367. [Google Scholar] [CrossRef]
- Ho, S.-K.; Wu, Y.-W.; Tseng, W.-K.; Leu, H.-B.; Yin, W.-H.; Lin, T.-H.; Chang, K.-C.; Wang, J.-H.; Yeh, H.-I.; Wu, C.-C.; et al. The prognostic significance of heart-type fatty acid binding protein in patients with stable coronary heart disease. Sci. Rep. 2018, 8, 14410. [Google Scholar] [CrossRef]
- Body, R.; Burrows, G.; Carley, S.; Lewis, P.S. The Manchester Acute Coronary Syndromes (MACS) decision rule: Validation with a new automated assay for heart-type fatty acid binding protein. Emerg. Med. J. 2015, 32, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Schoenenberger, A.W.; Stallone, F.; Walz, B.; Bergner, M.; Twerenbold, R.; Reichlin, T.; Zogg, B.; Jaeger, C.; Erne, P.; Mueller, C. Incremental value of heart-type fatty acid-binding protein in suspected acute myocardial infarction early after symptom onset. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 185–192. [Google Scholar] [CrossRef]
- Bruins Slot, M.H.E.; Rutten, F.H.; van der Heijden, G.J.M.G.; Doevendans, P.A.; Mast, E.G.; Bredero, A.C.; van der Spoel, O.P.; Glatz, J.F.C.; Hoes, A.W. Diagnostic value of a heart-type fatty acid-binding protein (H-FABP) bedside test in suspected acute coronary syndrome in primary care. Int. J. Cardiol. 2013, 168, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Glatz, J.F.; Renneberg, R. Added value of H-FABP as a plasma biomarker for the early evaluation of suspected acute coronary syndrome. Clin. Lipidol. 2014, 9, 205–220. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [PubMed]
- Pufulete, M.; Maishman, R.; Dabner, L.; Higgins, J.P.T.; Rogers, C.A.; Dayer, M.; MacLeod, J.; Purdy, S.; Hollingworth, W.; Schou, M.; et al. B-type natriuretic peptide-guided therapy for heart failure (HF): A systematic review and meta-analysis of individual participant data (IPD) and aggregate data. Syst. Rev. 2018, 7, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisel, A.; Mueller, C.; Adams, K.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.F.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef]
- Demissei, B.G.; Valente, M.A.E.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Givertz, M.M.; et al. Optimizing clinical use of biomarkers in high-risk acute heart failure patients. Eur. J. Heart Fail. 2016, 18, 269–280. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Bellia, C.; Lo Sasso, B.; Ciaccio, M. Diagnostic and prognostic value of H-FABP in acute coronary syndrome: Still evidence to bring. Clin. Biochem. 2018, 58, 1–4. [Google Scholar] [CrossRef]
- Xu, L.-Q.; Yang, Y.-M.; Tong, H.; Xu, C.-F. Early Diagnostic Performance of Heart-Type Fatty Acid Binding Protein in Suspected Acute Myocardial Infarction: Evidence from a Meta-Analysis of Contemporary Studies. Heart Lung Circ. 2018, 27, 503–512. [Google Scholar] [CrossRef]
- Bajaj, A.; Rathor, P.; Sehgal, V.; Shetty, A.; Kabak, B.; Hosur, S. Risk stratification in acute pulmonary embolism with heart-type fatty acid-binding protein: A meta-analysis. J. Crit. Care 2015, 30, 1151-e1. [Google Scholar] [CrossRef]
- Qian, H.-Y.; Huang, J.; Yang, Y.-J.; Yang, Y.-M.; Li, Z.-Z.; Zhang, J.-M. Heart-type Fatty Acid Binding Protein in the Assessment of Acute Pulmonary Embolism. Am. J. Med. Sci. 2016, 352, 557–562. [Google Scholar] [CrossRef]
- Dellas, C.; Lobo, J.L.; Rivas, A.; Ballaz, A.; Portillo, A.K.; Nieto, R.; del Rey, J.M.; Zamorano, J.L.; Lankeit, M.; Jiménez, D. Risk stratification of acute pulmonary embolism based on clinical parameters, H-FABP and multidetector CT. Int. J. Cardiol. 2018, 265, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2019, 1–61. [Google Scholar] [CrossRef]
- van der Vusse, G.J.; Glatz, J.F.; Stam, H.C.; Reneman, R.S. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol. Rev. 1992, 72, 881–940. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takahashi, T.; Okuyama, H.; Takabatake, N.; Nozaki, N.; Hirono, O.; Tsunoda, Y.; Shishido, T.; et al. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ. J. 2005, 69, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Nozaki, N.; Hirono, O.; Watanabe, T.; Nitobe, J.; Miyashita, T.; Miyamoto, T.; Koyama, Y.; et al. Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ. J. 2008, 72, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Espeter, F.; Weiß, C.; Ahmad-Nejad, P.; Lang, S.; Brueckmann, M.; Akin, I.; Neumaier, M.; Borggrefe, M.; Behnes, M. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure-diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc. Disord. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishino, M.; Shishido, T.; Arimoto, T.; Takahashi, H.; Miyashita, T.; Miyamoto, T.; Nitobe, J.; Watanabe, T.; Kubota, I. Heart-Type Fatty Acid Binding Protein (H-FABP) in Acute Decompensated Heart Failure. J. Card. Fail. 2010, 16, S166. [Google Scholar] [CrossRef]
- Jirak, P.; Fejzic, D.; Paar, V.; Wernly, B.; Pistulli, R.; Rohm, I.; Jung, C.; Hoppe, U.C.; Schulze, P.C.; Lichtenauer, M.; et al. Influences of Ivabradine treatment on serum levels of cardiac biomarkers sST2, GDF-15, suPAR and H-FABP in patients with chronic heart failure. Acta Pharmacol. Sin. 2018, 39, 1189. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Wei, C.-P.; Ma, S.-C.; Zhang, Y.-F.; Qiao, L.-Y.; Li, D.-H.; Shan, R.-B. Effect of Carvedilol on Serum Heart-type Fatty Acid-binding Protein, Brain Natriuretic Peptide, and Cardiac Function in Patients with Chronic Heart Failure. J. Cardiovasc. Pharmacol. 2015, 65, 480–484. [Google Scholar] [CrossRef]
- Kazimierczyk, E.; Kazimierczyk, R.; Harasim-Symbor, E.; Kaminski, K.; Sobkowicz, B.; Chabowski, A.; Tycinska, A. Persistently elevated plasma heart-type fatty acid binding protein concentration is related with poor outcome in acute decompensated heart failure patients. Clin. Chim. Acta 2018, 487, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Uchiyama, S.; Sawatani, T.; Asai, K.; Shimizu, W. Worsening renal failure in patients with acute heart failure: The importance of cardiac biomarkers. ESC Heart Fail. 2019, 6, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutsuzawa, D.; Arimoto, T.; Watanabe, T.; Shishido, T.; Miyamoto, T.; Miyashita, T.; Takahashi, H.; Niizeki, T.; Takeishi, Y.; Kubota, I. Ongoing myocardial damage in patients with heart failure and preserved ejection fraction. J. Cardiol. 2012, 60, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, W.; Nickl, W.; Füth, R.; Lankisch, M.; Hess, G.; Zdunek, D.; Scheffold, T.; Barroso, M.C.; Tiroch, K.; Ziegler, D.; et al. High sensitive troponin T and heart fatty acid binding protein: Novel biomarker in heart failure with normal ejection fraction? A cross-sectional study. BMC Cardiovasc. Disord. 2011, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Abstracts Programme. Eur. J. Heart Fail. 2019, 21, 5–592. [CrossRef] [Green Version]
- Mirna, M.; Wernly, B.; Paar, V.; Jung, C.; Jirak, P.; Figulla, H.-R.; Kretzschmar, D.; Franz, M.; Hoppe, U.C.; Lichtenauer, M.; et al. Multi-biomarker analysis in patients after transcatheter aortic valve implantation (TAVI). Biomarkers 2018, 23, 773–780. [Google Scholar] [CrossRef]
- Otaki, Y.; Arimoto, T.; Takahashi, H.; Kadowaki, S.; Ishigaki, D.; Narumi, T.; Honda, Y.; Iwayama, T.; Nishiyama, S.; Shishido, T.; et al. Prognostic value of myocardial damage markers in patients with chronic heart failure with atrial fibrillation. Intern. Med. 2014, 53, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Rader, F.; Pujara, A.C.; Pattakos, G.; Rajeswaran, J.; Li, L.; Castel, L.; Chung, M.K.; Gillinov, A.M.; Costantini, O.; Van Wagoner, D.R.; et al. Perioperative heart-type fatty acid binding protein levels in atrial fibrillation after cardiac surgery. Heart Rhythm 2013, 10, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Shingu, Y.; Yokota, T.; Takada, S.; Niwano, H.; Ooka, T.; Katoh, H.; Tachibana, T.; Kubota, S.; Matsui, Y. Decreased gene expression of fatty acid binding protein 3 in the atrium of patients with new onset of atrial fibrillation in cardiac perioperative phase. J. Cardiol. 2018, 71, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Mirna, M.; Rohm, I.; Jirak, P.; Wernly, B.; Bäz, L.; Paar, V.; Kretzschmar, D.; Hoppe, U.C.; Schulze, P.C.; Lichtenauer, M.; et al. Analysis of Novel Cardiovascular Biomarkers in Patients With Pulmonary Hypertension (PH). Heart Lung Circ. 2019. [Google Scholar] [CrossRef]
- Zoair, A.; Mawlana, W.; Abo-Elenin, A.; Korrat, M. Serum Level of Heart-Type Fatty Acid Binding Protein (H-FABP) Before and After Treatment of Congestive Heart Failure in Children. Pediatr. Cardiol. 2015, 36, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Wang, W.-D.; Ma, S.-C.; Wang, L.-Y.; Qiao, L.-Y.; Zhang, L.-P. Changes of heart-type fatty acid-binding protein in children with chronic heart failure and its significance. Chin. J. Contemp. Pediatr. 2013, 15, 99–101. [Google Scholar]
- Hayabuchi, Y.; Inoue, M.; Watanabe, N.; Sakata, M.; Ohnishi, T.; Kagami, S. Serum concentration of heart-type fatty acid-binding protein in children and adolescents with congenital heart disease. Circ. J. 2011, 75, 1992–1997. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Shishido, T.; Watanabe, K.; Sugai, T.; Toshima, T.; Kinoshita, D.; Yokoyama, M.; Tamura, H.; Nishiyama, S.; Takahashi, H.; et al. Ventricular wall stress and silent myocardial damage are associated with pulse pressure in the general population. J. Clin. Hypertens. 2018, 20, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Uitterdijk, A.; Sneep, S.; van Duin, R.W.B.; Krabbendam-Peters, I.; Gorsse-Bakker, C.; Duncker, D.J.; van der Giessen, W.J.; van Beusekom, H.M.M. Serial measurement of hFABP and high-sensitivity troponin I post-PCI in STEMI: How fast and accurate can myocardial infarct size and no-reflow be predicted? Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1104–H1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalos, D.; Spinka, G.; Schneider, M.; Wernly, B.; Paar, V.; Hoppe, U.; Litschauer, B.; Strametz-Juranek, J.; Sponder, M. New Cardiovascular Biomarkers in Ischemic Heart Disease—GDF-15, A Probable Predictor for Ejection Fraction. J. Clin. Med. 2019, 8, 924. [Google Scholar] [CrossRef] [Green Version]
- Cabiati, M.; Caselli, C.; Caruso, R.; Prescimone, T.; Verde, A.; Botta, L.; Parodi, O.; Ry, S.D.; Giannessi, D. High peripheral levels of h-FABP are associated with poor prognosis in end-stage heart failure patients with mechanical circulatory support. Biomark. Med. 2013, 7, 481–492. [Google Scholar] [CrossRef]

| Main Findings | Study | Patient Number | Reference |
|---|---|---|---|
| High H-FABP (>4.3 ng/mL) and elevated BNP (>200 pg/mL) showed highest rates for cardiac death and cardiac events and were also independent predictors of cardiac events (H-FABP HR 5.416, p = 0.0002; BNP HR 2.411, p = 0.0463) | Prospective study for 534+/−350 days on CHF patients | 186 | Niizeki T. et al., 2005 [58] |
| Persistently high H-FABP levels at hospital discharge (>4.3 ng/mL) correlated with increased rates for CV events (HR 5.68) | Prospective study for 624+/−299 days on patients with CHF | 113 | Niizeki T. et al., 2008 [59] |
| Two-fold higher rate of primary CV events between high H-FABP (>4.143 ng/mL) vs. low H-FABP group (32% vs. 16% respectively) | Prospective multicenter study for 24 months on patients with stable coronary heart disease (SCHD) | 1071 | Ho S. et al., 2018 [40] |
| H-FABP levels of >5.7 ng/mL were correlated with significantly higher in-hospital mortality (6.7% vs. 0%, p < 0.05) and cardiac events | Study for 615 days on patients with ADHF | 134 | Ishino M. et al., 2010 [61] |
| Highest H-FABP level patient quartile showed increased all-cause mortality (HR: 2.1–2.5, p = 0.04) and AHF related rehospitalization rate (HR 2.8–8.3, p = 0.001); combining H-FABP & NT-proBNP improves diagnostic specificity and PPV to rule out AHF | Prospective study for up to five years on patients with acute dyspnea or peripheral edema with or without AHF | 401 | Hoffmann U. et al., 2015 [60] |
| Significant positive correlation between H-FABP with echocardiographic parameters, death and rehospitalization | Study on patients with ADHF | 77 | Kazimierczyk E. et al., 2018 [64] |
| Serum H-FABP levels were significantly higher in patients with true worsening renal failure | Retrospective study on patients with AHF | 281 | Shirakabe A. et al., 2019 [65] |
| H-FABP levels are significantly higher in patients with DCM and ICM; ejection fraction correlates inversely with H-FABP concentrations | Study on the diagnostic value of novel cardiac biomarkers in patients with HFrEF | 65 patients with DCM, 59 patients with ICM, 76 controls | Lichtenauer M. et al., 2017 [36] |
| Significantly higher levels of Troponin T and H-FABP in patients with asymptomatic LVDD and patients with HFnEF | Study on patients with HFnEF | 49 patients with HFnEF, 51 patients with asymptomatic LVDD, 30 controls | Dinh W. et al., 2011 [67] |
| Higher H-FABP-levels correlated with adverse CV events; H-FABP levels did not differ between patients with HFpEF and HFrEF between each NYHA functional class | Prospective study on patients with HFpEF with a median follow-up of 694 days | 151 patients with HFpEF, 162 patients with HFrEF as controls | Kutsuzawa D. et al., 2012 [66] |
| A greater rise in post-operative H-FABP levels is associated with AF after cardiac surgery | Prospective study on patients undergoing cardiac surgery | 63 | Rader F. et al., 2013 [71] |
| Optimal cut-off values for H-FABP as myocardial damage marker were higher in CHF patients with AF than in patients with SR (5.4 vs. 4.6 ng/mL) | Prospective study on patients with CHF and AF or CHF and SR with a median follow-up of 643/688 days | 402 | Otaki Y. et al., 2014 [70] |
| H-FABP levels correlate independently with age, NYHA-class, CK-MB, creatinine and arterial oxygen saturation | Study in children and adolescents with congenital heart disease | 238 | Hayabuchi Y. et al., 2011 [76] |
| Significant negative correlation between H-FABP levels and heart function (LVEF, CI, LVSF) | Study in pediatric patients with chronic HF | 36 patients and 30 healthy controls | Sun Y.P. et al., 2013 [75] |
| Significant positive correlation between increased H-FABP levels and severity of HF and adverse outcome | Prospective cohort study for 3 months on pediatric patients with HF | 30 patients and 20 healthy controls | Zoair A. et al., 2015 [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164. https://doi.org/10.3390/jcm9010164
Rezar R, Jirak P, Gschwandtner M, Derler R, Felder TK, Haslinger M, Kopp K, Seelmaier C, Granitz C, Hoppe UC, et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far? Journal of Clinical Medicine. 2020; 9(1):164. https://doi.org/10.3390/jcm9010164
Chicago/Turabian StyleRezar, Richard, Peter Jirak, Martha Gschwandtner, Rupert Derler, Thomas K. Felder, Michael Haslinger, Kristen Kopp, Clemens Seelmaier, Christina Granitz, Uta C. Hoppe, and et al. 2020. "Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far?" Journal of Clinical Medicine 9, no. 1: 164. https://doi.org/10.3390/jcm9010164
APA StyleRezar, R., Jirak, P., Gschwandtner, M., Derler, R., Felder, T. K., Haslinger, M., Kopp, K., Seelmaier, C., Granitz, C., Hoppe, U. C., & Lichtenauer, M. (2020). Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far? Journal of Clinical Medicine, 9(1), 164. https://doi.org/10.3390/jcm9010164