Impact of Right Atrial Physiology on Heart Failure and Adverse Events after Myocardial Infarction
Abstract
:1. Introduction
2. Methods
2.1. Study Population
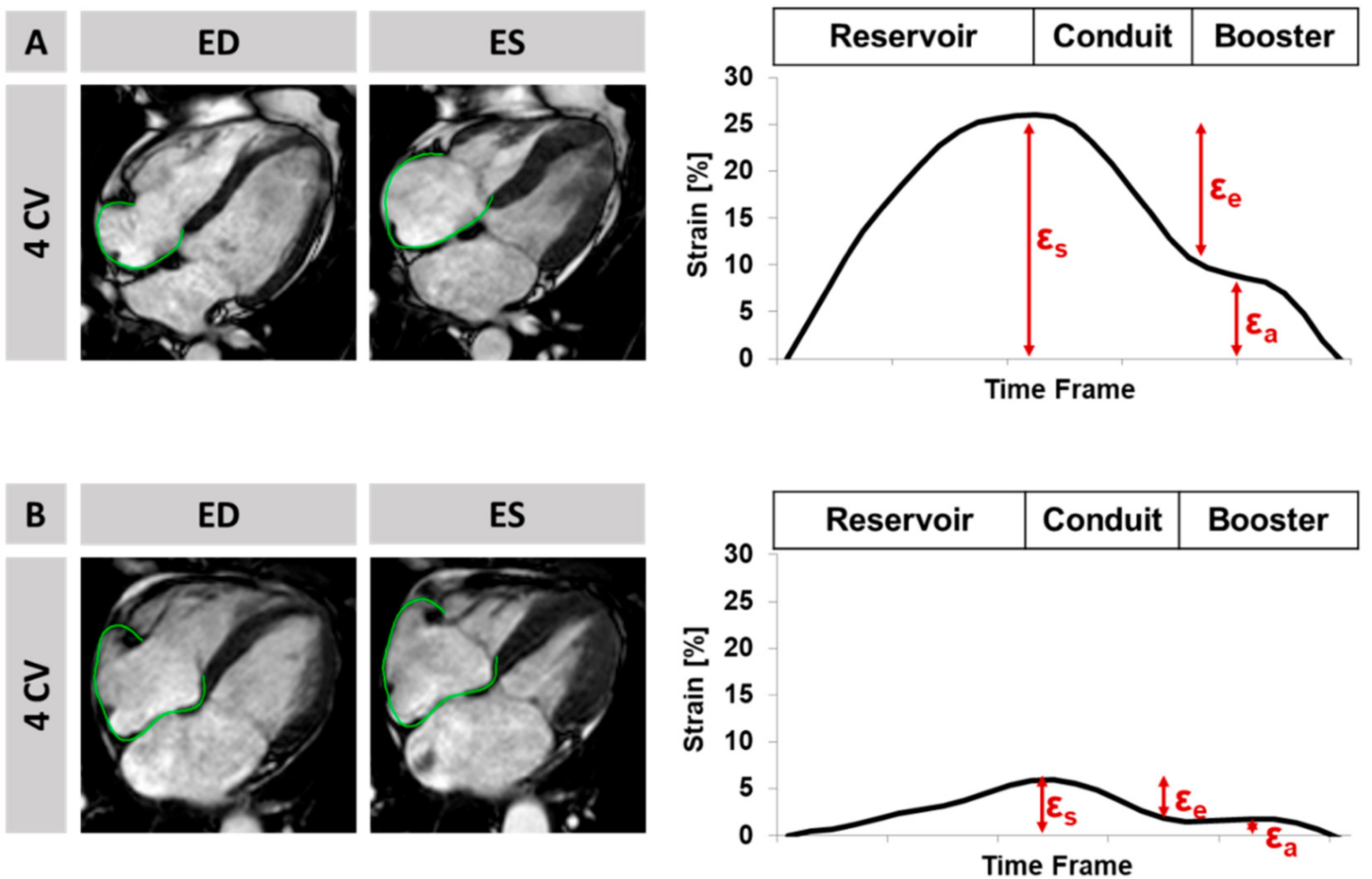
2.2. Cardiovascular Magnetic Resonance Imaging Protocol and Analyses
2.3. Clinical Endpoints
2.4. Statistics
3. Results
3.1. Study Population
3.2. Right Atrial Dysfunction
3.3. Risk Stratification
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, S.C.; Collins, A.; Ferrari, R.; Holmes, D.R.; Logstrup, S.; McGhie, D.V.; Ralston, J.; Sacco, R.L.; Stam, H.; Taubert, K.; et al. Our time: A call to save preventable death from cardiovascular disease (heart disease and stroke). Circulation 2012, 126, 2769–2775. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Eitel, I.; de Waha, S.; Wöhrle, J.; Fuernau, G.; Lurz, P.; Pauschinger, M.; Desch, S.; Schuler, G.; Thiele, H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Norris, R.M.; Brown, M.A.; Brandt, P.W.; Whitlock, R.M.; Wild, C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987, 76, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitel, I.; Stiermaier, T.; Lange, T.; Rommel, K.-P.; Koschalka, A.; Kowallick, J.T.; Lotz, J.; Kutty, S.; Gutberlet, M.; Hasenfuß, G.; et al. Cardiac Magnetic Resonance Myocardial Feature Tracking for Optimized Prediction of Cardiovascular Events Following Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Mangion, K.; Carrick, D.; Carberry, J.; Mahrous, A.; McComb, C.; Oldroyd, K.G.; Eteiba, H.; Lindsay, M.; McEntegart, M.; Hood, S.; et al. Circumferential Strain Predicts Major Adverse Cardiovascular Events Following an Acute ST-Segment-Elevation Myocardial Infarction. Radiology 2019, 290, 329–337. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Vahl, T.P.; Goliasch, G.; Picatoste, B.; Arias, T.; Ishikawa, K.; Njerve, I.U.; Sanz, J.; Narula, J.; Sengupta, P.P.; et al. Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation 2016, 133, 954–966. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Moller, J.E.; Hillis, G.S.; Oh, J.K.; Seward, J.B.; Reeder, G.S.; Wright, R.S.; Park, S.W.; Bailey, K.R.; Pellikka, P.A. Left atrial volume: A powerful predictor of survival after acute myocardial infarction. Circulation 2003, 107, 2207–2212. [Google Scholar] [CrossRef] [Green Version]
- Schuster, A.; Backhaus, S.J.; Stiermaier, T.; Navarra, J.-L.; Uhlig, J.; Rommel, K.-P.; Koschalka, A.; Kowallick, J.T.; Lotz, J.; Gutberlet, M.; et al. Left Atrial Function with MRI Enables Prediction of Cardiovascular Events after Myocardial Infarction: Insights from the AIDA STEMI and TATORT NSTEMI Trials. Radiology 2019, 293, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lee, A.P.-W.; Yu, C.-M. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr. Opin. Cardiol. 2014, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J. Cardiovascular magnetic resonance. Circulation 2010, 121, 692–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, A.; Morton, G.; Chiribiri, A.; Perera, D.; Vanoverschelde, J.-L.; Nagel, E. Imaging in the management of ischemic cardiomyopathy: Special focus on magnetic resonance. J. Am. Coll. Cardiol. 2012, 59, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Tadic, M. The right atrium, a forgotten cardiac chamber: An updated review of multimodality imaging. J. Clin. Ultrasound 2015, 43, 335–345. [Google Scholar] [CrossRef]
- Kowallick, J.T.; Kutty, S.; Edelmann, F.; Chiribiri, A.; Villa, A.; Steinmetz, M.; Sohns, J.M.; Staab, W.; Bettencourt, N.; Unterberg-Buchwald, C.; et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: A feasibility study. J. Cardiovasc. Magn. Reson. 2014, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Kowallick, J.T.; Morton, G.; Lamata, P.; Jogiya, R.; Kutty, S.; Hasenfuß, G.; Lotz, J.; Nagel, E.; Chiribiri, A.; Schuster, A. Quantification of atrial dynamics using cardiovascular magnetic resonance: Inter-study reproducibility. J. Cardiovasc. Magn. Reson. 2015, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Shinomiya, H.; Fukuda, N.; Takeichi, N.; Soeki, T.; Shinohara, H.; Yui, Y.; Tamura, Y.; Oki, T. Echocardiographic Assessment of Right Atrial Function in Patients with Myocardial Infarction with Reference to Obstructive Lesions of the Coronary Arteries. Jpn. Circ. J. 1998, 62, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Quraini, D.; Pandian, N.G.; Patel, A.R. Three-dimensional echocardiographic analysis of right atrial volume in normal and abnormal hearts: Comparison of biplane and multiplane methods. Echocardiography 2012, 29, 608–613. [Google Scholar] [CrossRef]
- Ersbøll, M.; Valeur, N.; Mogensen, U.M.; Andersen, M.J.; Møller, J.E.; Velazquez, E.J.; Hassager, C.; Søgaard, P.; Køber, L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 2013, 61, 2365–2373. [Google Scholar] [CrossRef] [Green Version]
- Ersbøll, M.; Andersen, M.J.; Valeur, N.; Mogensen, U.M.; Waziri, H.; Møller, J.E.; Hassager, C.; Søgaard, P.; Køber, L. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ. Cardiovasc. Imaging 2013, 6, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Söderberg, S.; Lindqvist, P. Association of Right Atrial Mechanics with Hemodynamics and Physical Capacity in Patients with Idiopathic Pulmonary Arterial Hypertension: Insight from a Single-Center Cohort in Northern Sweden. Echocardiography 2016, 33, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.M.; Dwyer, N.; Wahi, S.; Marwick, T.H. Association with right atrial strain with right atrial pressure: An invasive validation study. Int. J. Cardiovasc. Imaging 2018, 34, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Sun, B.; Shi, H.; Zhu, W.; Zhou, Q.; Jiang, Y.; Zhu, H.; Huang, G. Left atrial and right atrial deformation in patients with coronary artery disease: A velocity vector imaging-based study. PLoS ONE 2012, 7, e51204. [Google Scholar] [CrossRef]
- Mantziari, L.; Kamperidis, V.; Ventoulis, I.; Damvopoulou, E.; Giannakoulas, G.; Efthimiadis, G.; Paraskevaidis, S.; Vassilikos, V.; Ziakasm, A.; Karvounism, H.; et al. Increased Right Atrial Volume Index Predicts Low Duke Activity Status Index in Patients with Chronic Heart Failure. Hell. J. Cardiol. 2013, 54, 32–38. [Google Scholar]
- Thiele, H.; Wöhrle, J.; Hambrecht, R.; Rittger, H.; Birkemeyer, R.; Lauer, B.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Wiemer, M.; et al. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: A randomised trial. Lancet 2012, 379, 923–931. [Google Scholar] [CrossRef]
- Thiele, H.; de Waha, S.; Zeymer, U.; Desch, S.; Scheller, B.; Lauer, B.; Geisler, T.; Gawaz, M.; Gunkel, O.; Bruch, L.; et al. Effect of aspiration thrombectomy on microvascular obstruction in NSTEMI patients: The TATORT-NSTEMI trial. J. Am. Coll. Cardiol. 2014, 64, 1117–1124. [Google Scholar] [CrossRef]
- Eitel, I.; Wöhrle, J.; Suenkel, H.; Meissner, J.; Kerber, S.; Lauer, B.; Pauschinger, M.; Birkemeyer, R.; Axthelm, C.; Zimmermann, R.; et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: Cardiac magnetic resonance substudy of the AIDA STEMI trial. J. Am. Coll. Cardiol. 2013, 61, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Kowallick, J.T.; Morton, G.; Lamata, P.; Jogiya, R.; Kutty, S.; Lotz, J.; Hasenfuß, G.; Nagel, E.; Chiribiri, A.; Schuster, A. Inter-study reproducibility of left ventricular torsion and torsion rate quantification using MR myocardial feature tracking. J. Magn. Reson. Imaging 2016, 43, 128–137. [Google Scholar] [CrossRef]
- Morton, G.; Schuster, A.; Jogiya, R.; Kutty, S.; Beerbaum, P.; Nagel, E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J. Cardiovasc. Magn. Reson. 2012, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Schuster, A.; Stahnke, V.-C.; Unterberg-Buchwald, C.; Kowallick, J.T.; Lamata, P.; Steinmetz, M.; Kutty, S.; Fasshauer, M.; Staab, W.; Sohns, J.M.; et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: Intervendor agreement and considerations regarding reproducibility. Clin. Radiol. 2015, 70, 989–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowallick, J.T.; Silva Vieira, M.; Kutty, S.; Lotz, J.; Hasenfu, G.; Chiribiri, A.; Schuster, A. Left Atrial Performance in the Course of Hypertrophic Cardiomyopathy: Relation to Left Ventricular Hypertrophy and Fibrosis. Investig. Radiol. 2017, 52, 177–185. [Google Scholar] [CrossRef] [PubMed]
- de Waha, S.; Eitel, I.; Desch, S.; Scheller, B.; Böhm, M.; Lauer, B.; Gawaz, M.; Geisler, T.; Gunkel, O.; Bruch, L.; et al. Thrombus Aspiration in ThrOmbus containing culpRiT lesions in Non-ST-Elevation Myocardial Infarction (TATORT-NSTEMI): Study protocol for a randomized controlled trial. Trials 2013, 14, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin Bland, J.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Willens, H.J.; Fertel, D.P.; Qin, J.; Labrador, E.; Lowery, M.H. Effects of age and pulmonary arterial hypertension on the different phases of right atrial function. Int. J. Cardiovasc. Imaging 2008, 24, 703–710. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ma, C.; Guan, Z.; Liu, S.; Zhang, W.; Li, Y.; Yang, J. Evaluation of Left and Right Atrial Function in Patients with Coronary Slow-Flow Phenomenon Using Two-Dimensional Speckle Tracking Echocardiography. Echocardiography 2016, 33, 871–880. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Kocijancic, V.; Celic, V.; Vukomanovic, V. Does Left Ventricular Geometric Patterns Impact Right Atrial Phasic Function? Findings from the Hypertensive Population. Echocardiography 2016, 33, 1186–1194. [Google Scholar] [CrossRef]
- Von Roeder, M.; Kowallick, J.T.; Rommel, K.-P.; Blazek, S.; Besler, C.; Fengler, K.; Lotz, J.; Hasenfuß, G.; Lücke, C.; Gutberlet, M.; et al. Right atrial-right ventricular coupling in heart failure with preserved ejection fraction. Clin. Res. Cardiol. 2020, 109, 54–66. [Google Scholar] [CrossRef]
- Stillman, A.E.; Oudkerk, M.; Bluemke, D.A.; de Boer, M.J.; Bremerich, J.; Garcia, E.V.; Gutberlet, M.; van der Harst, P.; Hundley, W.G.; Jerosch-Herold, M.; et al. Imaging the myocardial ischemic cascade. Int. J. Cardiovasc. Imaging 2018, 34, 1249–1263. [Google Scholar] [CrossRef]
- Posina, K.; McLaughlin, J.; Rhee, P.; Li, L.; Cheng, J.; Schapiro, W.; Gulotta, R.J.; Berke, A.D.; Petrossian, G.A.; Reichek, N.; et al. Relationship of phasic left atrial volume and emptying function to left ventricular filling pressure: A cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2013, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- von Roeder, M.; Rommel, K.-P.; Kowallick, J.T.; Blazek, S.; Besler, C.; Fengler, K.; Lotz, J.; Hasenfuß, G.; Lücke, C.; Gutberlet, M.; et al. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients with Heart Failure and Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2017, 10, e005467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindan, M.; Kiotsekoglou, A.; Saha, S.K.; Camm, A.J. Right atrial myocardial deformation by two-dimensional speckle tracking echocardiography predicts recurrence in paroxysmal atrial fibrillation. J. Echocardiogr. 2017, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sicari, R. Right atrial function: A blind spot in a blind spot. Int. J. Cardiol. 2018, 255, 212. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dardeer, A.M.; Moody, W.E.; Edwards, N.C.; Hudsmith, L.E.; Steeds, R.P. Normal values for myocardial deformation within the right heart measured by feature-tracking cardiovascular magnetic resonance imaging. Int. J. Cardiol. 2018, 252, 220–223. [Google Scholar] [CrossRef] [Green Version]
- Backhaus, S.J.; Kowallick, J.T.; Stiermaier, T.; Lange, T.; Koschalka, A.; Navarra, J.-L.; Lotz, J.; Kutty, S.; Bigalke, B.; Gutberlet, M.; et al. Culprit vessel-related myocardial mechanics and prognostic implications following acute myocardial infarction. Clin. Res. Cardiol. 2019, 37, 267. [Google Scholar] [CrossRef]
- Goldstein, J.A. Acute right ventricular infarction: Insights for the interventional era. Curr. Probl. Cardiol. 2012, 37, 533–557. [Google Scholar] [CrossRef]
- Padeletti, M.; Cameli, M.; Lisi, M.; Zacà, V.; Tsioulpas, C.; Bernazzali, S.; Maccherini, M.; Mondillo, S. Right atrial speckle tracking analysis as a novel noninvasive method for pulmonary hemodynamics assessment in patients with chronic systolic heart failure. Echocardiography 2011, 28, 658–664. [Google Scholar] [CrossRef]



| Variable | All Patients n = 1031 | MACE n = 71 | No MACE n = 960 | p-Value |
|---|---|---|---|---|
| Age | 64 (53, 72) | 71 (60, 77) | 63 (52, 72) | <0.001 |
| Sex (m) | 774/1029 (75.2%) | 47/71 (66.2%) | 727/958 (75.9%) | 0.068 |
| Cardiovascular risk factors | ||||
| Active smoking | 415/951 (43.6%) | 20/64 (31.3%) | 395/887 (44.6%) | 0.039 |
| Hypertension | 728/1027 (70.6%) | 59/71 (83.1%) | 669/956 (70%) | 0.019 |
| Hyperlipoproteinemia | 387/1024 (37.8%) | 25/71 (35.2%) | 362/953 (38%) | 0.642 |
| Diabetes | 243/1027 (23.7%) | 23/71 (32.4) | 220/956 (23%) | 0.073 |
| Body mass index (kg/m²) | 27.5 (24.9, 30.4) | 27.7 (25.5, 31.1) | 27.5 (24.9, 30.3) | 0.433 |
| Previous myocardial infarction | 72/1027 (7%) | 4/71 (5.6%) | 68/956 (7.1%) | 0.638 |
| Previous PCI | 89/1028 (8.7%) | 5/71 (7%) | 84/957 (8.8%) | 0.616 |
| Previous CABG | 19/1028 (1.8%) | 2/69 (2.9%) | 17/957 (1.8%) | 0.530 |
| ST-segment elevation | 707/1029 (68.7%) | 48/71 (67.6%) | 659/958 (68.8%) | 0.836 |
| Systolic blood pressure (mmHg) | 133 (119, 150) | 130 (110, 150) | 134 (120, 150) | 0.166 |
| Diastolic blood pressure (mmHg) | 80 (70, 89) | 77 (65, 85) | 80 (70, 89) | 0.059 |
| Heart rate (beats/min) | 76 (67, 86) | 80 (70, 96) | 76 (66, 86) | 0.001 |
| Time symptoms to balloon * (min) | 180 (110, 310) | 194 (114, 390) | 180 (109, 306) | 0.279 |
| Door-to-balloon time * (min) | 30 (22, 42) | 28 (22.5, 40) | 30 (22, 42) | 0.490 |
| Killip class on admission | <0.001 | |||
| 1 | 911/1029 (88.7%) | 46/71 (64.8%) | 865/958 (90.3%) | |
| 2 | 81/1029 (7.9%) | 16/71 (22.5%) | 65/958 (6.8%) | |
| 3 | 22/1029 (2.1%) | 5/71 (7%) | 17/958 (1.8%) | |
| 4 | 15/1029 (1.5%) | 4/71 (5.6%) | 11/958 (1.1%) | |
| Diseased vessels | 0.025 | |||
| 1 | 517/1029 (50.3%) | 27/71 (38%) | 490/958 (51.1%) | |
| 2 | 311/1029 (30.3%) | 22/71 (31%) | 289/958 (30.2%) | |
| 3 | 201/1029 (19.6%) | 22/71 (31%) | 179/958 (18.7%) | |
| Affected artery | 0.337 | |||
| left anterior descending | 425/1029 (41.4%) | 37/71 (52.1%) | 388/958 (40.5%) | |
| left circumflex | 213/1029 (20.7%) | 14/71 (19.7%) | 199/958 (20.8%) | |
| left main | 4/1029 (0.4%) | 0/71 (0%) | 4/958 (0.4%) | |
| right coronary artery | 381/1029 (37.1%) | 20/71 (28.2%) | 361/958 (37.7%) | |
| bypass graft | 6/1029 (0.6%) | 0/71 (0%) | 6/958 (0.6%) | |
| TIMI flow grade before PCI | 0.607 | |||
| 0 | 515/1029 (50.1%) | 40/71 (56.3%) | 475/958 (49.6%) | |
| 1 | 111/1029 (10.8%) | 5/71 (7%) | 106/958 (11.1%) | |
| 2 | 215/1029 (20.9%) | 13/71 (18.3%) | 202/958 (21.1%) | |
| 3 | 188/1029 (18.3%) | 13/71 (18.3%) | 175/958 (18.3%) | |
| Stent implanted | 1005/1029 (97.9%) | 69/71 (97.2%) | 936/958 (97.7%) | 0.524 |
| TIMI flow grade after PCI | 0.294 | |||
| 0 | 20/1029 (1.9%) | 1/71 (1.4%) | 19/958 (2%) | |
| 1 | 21/1029 (2.0%) | 3/71 (4.2%) | 18/958 (1.9%) | |
| 2 | 76/1029 (7.4%) | 8/71 (11.3%) | 68/958 (7.1%) | |
| 3 | 912/1029 (88.8%) | 59/71 (83.1%) | 853/958 (89%) | |
| Medication | ||||
| Glycoprotein IIb/IIIa inhibitor | 729/1028 (70.9%) | 51/71 (71.8%) | 678/957 (70.8%) | 0.860 |
| Aspirin | 1027/1029 (99.8%) | 71/71 (100%) | 956/958 (99.8%) | 0.700 |
| Clopidogrel/Prasugrel/Ticagrelor | 1028/1028 (100%) | 71/71 (100%) | 957/957 (100%) | |
| Betablocker | 983/1028 (95.6%) | 69/71 (97.2%) | 914/957 (95.5%) | 0.505 |
| ACE-inhibitor/AT-1 antagonist | 947/1028 (92.1%) | 67/71 (94.4%) | 880/957 (92%) | 0.467 |
| Aldosterone antagonist | 133/1028 (12.9%) | 23/71 (32.4%) | 110/957 (11.5%) | <0.001 |
| Statin | 990/1028 (96.3%) | 69/71 (97.2%) | 921/957 (96.2%) | 0.684 |
| Time to MRI (days) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 0.06 |
| All Patients | MACE | No MACE | ||
|---|---|---|---|---|
| Functional parameter | median (IQR) | median (IQR) | median (IQR) | p |
| Infarct characteristics | ||||
| IS | 13.1 (5.20, 21.7) | 20.3 (9.83, 28.9) | 12.8 (5.15, 21.3) | 0.001 |
| AAR | 29.1 (20.1, 42.2) | 32.9 (24.2, 45.1) | 28.8 (20.0, 42.0) | 0.080 |
| MO | 0.33 (0.00, 1.92) | 0.80 (0.00, 2.53) | 0.29 (0.00, 1.90) | 0.060 |
| Left ventricle | ||||
| LV mass | 66.1 (57.4, 75.9) | 68.9 (58.9, 78.7) | 65.9 (57.2, 75.8) | 0.380 |
| EDV | 73.3 (62.5, 86.0) | 75.4 (67.0, 87.5) | 73.1 (62.1, 85.8) | 0.155 |
| ESV | 35.6 (27.8, 45.9) | 45.1 (31.6, 54.1) | 35.2 (27.6, 45.3) | <0.001 |
| EF | 50.5 (43.5, 57.5) | 41.2 (33.0, 52.2) | 51.0 (44.5, 57.6) | <0.001 |
| Strain | −16.6 (−12.5, −20.2) | −11.7 (−8.18, −17.1) | −16.8 (−13.0, −20.4) | <0.001 |
| Left atrium | ||||
| LAVI | 35.0 (26.6, 44.3) | 40.6 (28.7, 53.6) | 34.6 (26.6, 43.4) | 0.001 |
| LA Es | 20.9 (16.2, 25.7) | 16.2 (11.4, 21.1) | 21.2 (16.7, 26.1) | <0.001 |
| LA Ee | 8.69 (5.63, 11.7) | 6.92 (3.19, 8.73) | 8.83 (5.83, 11.9) | <0.001 |
| LA Ea | 11.5 (8.60, 15.3) | 9.96 (5.91, 12.7) | 11.7 (8.77, 15.5) | <0.001 |
| LA SRs | 0.88 (0.70, 1.08) | 0.79 (0.59, 0.93) | 0.90 (0.71, 1.10) | <0.001 |
| LA SRe | −0.55 (−0.38, −0.78) | −0.48 (−0.34, −0.67) | −0.56 (−0.39, −0.79) | 0.004 |
| LA SRa | −0,96 (−0.73, −1.25) | −0.84 (−0.59, −1.06) | −0.97 (−0.73, −1.26) | 0.001 |
| RV volumes | ||||
| RV mass | 22.2 (18.9, 26.2) | 20.8 (19.4, 24.9) | 22.2 (18.9, 26.4) | 0.247 |
| EDV | 60.9 (51.3, 71.4) | 59.8 (48.0, 68.2) | 61.0 (51.6, 71.5) | 0.122 |
| ESV | 23.1 (17.4, 31.2) | 23.1 (16.3, 35.6) | 23.1 (17.5, 30.8) | 0.878 |
| EF | 61.1 (54.2, 67.7) | 56.5 (46.1, 69.4) | 61.3 (54.6, 67.7) | 0.037 |
| Right atrium | ||||
| RAVI | 27.4 (20.7, 35.7) | 26.7 (18.7, 36.3) | 27.4 (20.8, 35.6) | 0.866 |
| RA Es | 24.4 (17.3, 32.4) | 22.1 (13.9, 30.7) | 24.8 (17.5, 32.5) | 0.061 |
| RA Ee | 10.9 (6.03, 16.5) | 8.88 (3.99, 13.9) | 11.1 (6.18, 16.7) | 0.006 |
| RA Ea | 12.3 (7.89, 17.5) | 11.4 (6.31, 18.6) | 12.3 (8, 17.4) | 0.579 |
| RA SRs | 1.11 (0.83, 1.43) | 0.98 (0.64, 1.43) | 1.11 (0.84, 1.43) | 0.049 |
| RA SRe | −0.54 (−0.33, −0.79) | −0.48 (−0.21, -0.65) | −0.55 (−0.34, −0.8) | 0.030 |
| RA SRa | −0.96 (−0.66, −1.37) | −0.89 (−0.52, −1.28) | −0.97 (−0.67, −1.37) | 0.118 |
| Mean Difference ± SD (%) | CoV (%) | ICC (95% CI) | |
|---|---|---|---|
| Intraobserver | |||
| RA Es | −2.05 ± 3.84 | 13.73 | 0.95 (0.86–0.98) |
| RA Ee | −0.17 ± 2.23 | 16.95 | 0.97 (0.94–0.99) |
| RA Ea | −1.98 ± 4.58 | 30.84 | 0.83 (0.62–0.92) |
| RA SRs | −0.08 ± 0.32 | 26.02 | 0.82 (0.63–0.92) |
| RA SRe | 0.02 ± 0.17 | 31.48 | 0.91 (0.80–0.96) |
| RA SRa | 0.08 ± 0.29 | 25.66 | 0.85 (0.69–0.93) |
| Interobserver | |||
| RA Es | 1.67 ± 6.23 | 23.86 | 0.87 (0.72–0.94) |
| RA Ee | 1.22 ± 4.14 | 33.15 | 0.88 (0.74–0.94) |
| RA Ea | 0.44 ± 3.44 | 25.26 | 0.93 (0.84–0.97) |
| RA SRs | 0.01 ± 0.29 | 25.44 | 0.84 (0.66–0.93) |
| RA SRe | 0.02 ± 0.20 | 37.04 | 0.88 (0.74–0.94) |
| RA SRa | −0.03 ± 0.29 | 27.10 | 0.86 (0.70–0.93) |
| Variable | Univariable Hazard Ratio (CI) | p |
|---|---|---|
| Age | 1.04 (1.02–1.06) | <0.001 |
| Hypertension | 2.07 (1.11–3.84) | 0.022 |
| LVEF | 0.94 (0.92–0.96) | <0.001 |
| RVEF | 0.97 (0.95–0.99) | 0.012 |
| Infarct Size | 1.03 (1.01–1.05) | 0.001 |
| Killip Class | 2.08 (1.64–2.64) | <0.001 |
| Number of diseased vessels | 1.46 (1.10–1.94) | 0.009 |
| Atrial fibrillation | 2.25 (1.20–4.23) | 0.011 |
| RA-Es | 0.98 (0.96–1.00) | 0.062 |
| RA-Ee | 0.95 (0.91–0.98) | 0.003 |
| RA-Ea | 1.00 (0.98–1.04) | 0.745 |
| RAVI | 1.01 (0.99–1.02) | 0.634 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, A.; Backhaus, S.J.; Stiermaier, T.; Navarra, J.-L.; Uhlig, J.; Rommel, K.-P.; Koschalka, A.; Kowallick, J.T.; Bigalke, B.; Kutty, S.; et al. Impact of Right Atrial Physiology on Heart Failure and Adverse Events after Myocardial Infarction. J. Clin. Med. 2020, 9, 210. https://doi.org/10.3390/jcm9010210
Schuster A, Backhaus SJ, Stiermaier T, Navarra J-L, Uhlig J, Rommel K-P, Koschalka A, Kowallick JT, Bigalke B, Kutty S, et al. Impact of Right Atrial Physiology on Heart Failure and Adverse Events after Myocardial Infarction. Journal of Clinical Medicine. 2020; 9(1):210. https://doi.org/10.3390/jcm9010210
Chicago/Turabian StyleSchuster, Andreas, Sören J. Backhaus, Thomas Stiermaier, Jenny-Lou Navarra, Johannes Uhlig, Karl-Philipp Rommel, Alexander Koschalka, Johannes T. Kowallick, Boris Bigalke, Shelby Kutty, and et al. 2020. "Impact of Right Atrial Physiology on Heart Failure and Adverse Events after Myocardial Infarction" Journal of Clinical Medicine 9, no. 1: 210. https://doi.org/10.3390/jcm9010210
APA StyleSchuster, A., Backhaus, S. J., Stiermaier, T., Navarra, J.-L., Uhlig, J., Rommel, K.-P., Koschalka, A., Kowallick, J. T., Bigalke, B., Kutty, S., Gutberlet, M., Hasenfuß, G., Thiele, H., & Eitel, I. (2020). Impact of Right Atrial Physiology on Heart Failure and Adverse Events after Myocardial Infarction. Journal of Clinical Medicine, 9(1), 210. https://doi.org/10.3390/jcm9010210






