Influence of Pneumonia on the Survival of Patients with COPD
Abstract
:1. Introduction
2. Patients and Methods
2.1. Design
2.2. Variables Collected
2.3. Statistical Analysis
2.4. Ethics Committee and Informed Consent
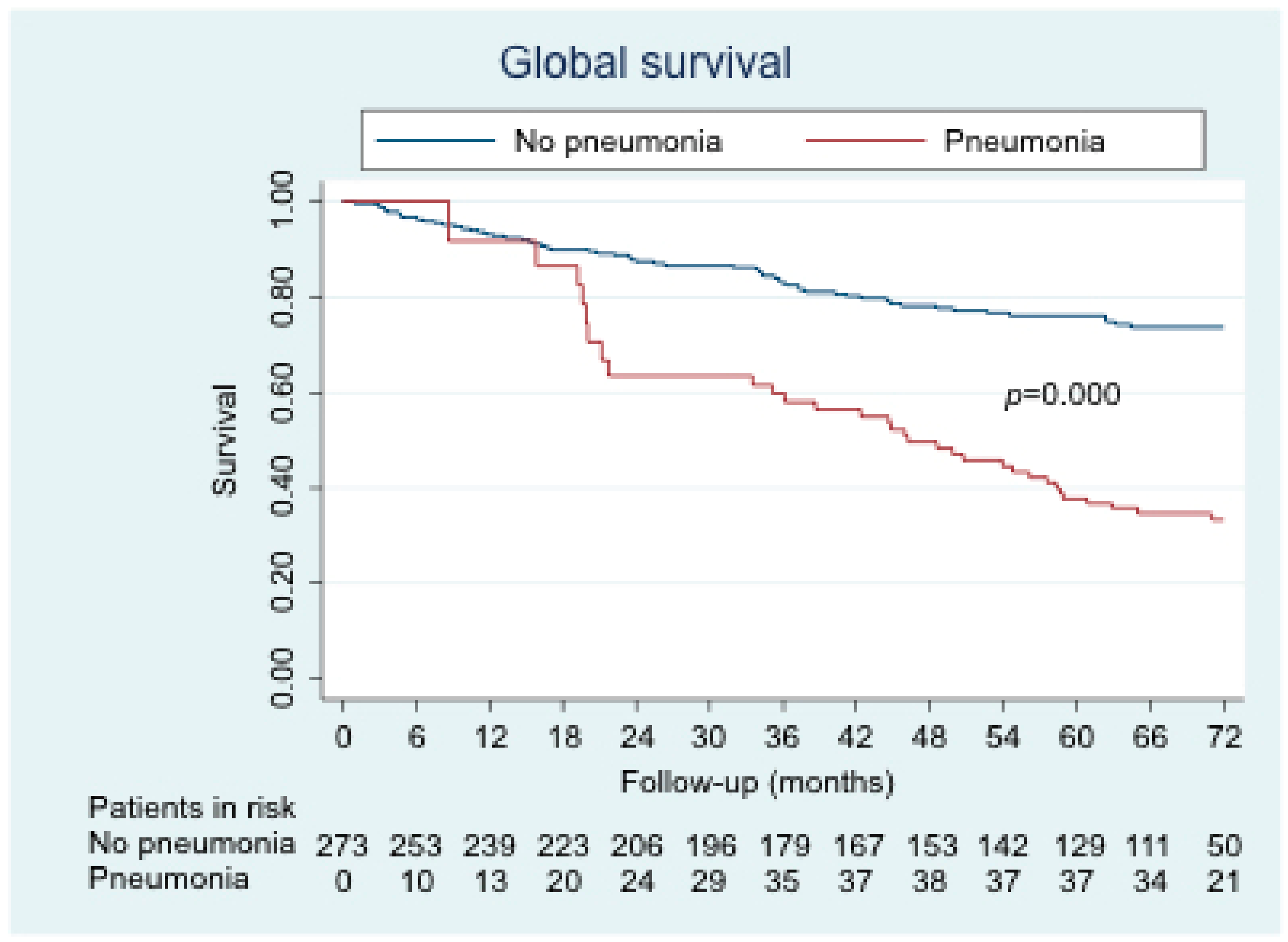
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almirall, J.; Bolibar, I.; Vidal, J.; Sauca, G.; Coll, P.; Niklasson, B. Epidemiology of community-acquired pneumonia in adults: A population-based study. Eur. Respir. J. 2000, 15, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Chacón García, A.; Ruigómez, A.; García Rodríguez, L.A. Incidencia de neumonía adquirida en la comunidad en la cohorte poblacional de la base de datos en atención primaria (BIFAP). Atención Primaria 2010, 42, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolome, M.; Almirall, J.; Morera, J.; Pera, G.; Ortun, V.; Bassa, J. A population-based study of the costs of care for community-acquired pneumonia. Eur. Respir. J. 2004, 23, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miguel-Díez, J.; Jiménez-García, R.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; de Miguel-Yanes, J.M.; Méndez-Bailón, M. Trends in hospitalizations for community-acquired pneumonia in Spain: 2004 to 2013. Eur. J. Intern. Med. 2017, 40, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Barberán, J.; Falguera, M.; Menéndez, R.; Molina, J.; Olaechea, P. Guía multidisciplinar para la valoración pronóstica, diagnóstico y tratamiento de la neumonía adquirida en la comunidad. Med. Clínica 2013, 140, 223.e1–223.e19. [Google Scholar] [CrossRef] [PubMed]
- Ancochea, J.; Badiola, C.; Duran-Tauleria, E.; Garcia Rio, F.; Miravitlles, M.; Muñoz, L. Estudio EPI-SCAN: Resumen del protocolo de un estudio para estimar la prevalencia de EPOC en personas de 40 a 80 años en España. Arch. Bronconeumol. 2009, 45, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Alfageme, I.; de Lucas, P.; Ancochea, J.; Miravitlles, M.; Soler-Cataluña, J.J.; García-Río, F. Nuevo estudio sobre la prevalencia de la EPOC en España: Resumen del protocolo EPISCAN II, 10 años después de EPISCAN. Arch. Bronconeumol. 2019, 55, 38–47. [Google Scholar] [CrossRef]
- Hernández Vázquez, J.; Ali García, I.; Jiménez-García, R.; Álvaro Meca, A.; López de Andrés, A.; Matesanz Ruiz, C. COPD phenotypes: Differences in survival. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2245–2251. [Google Scholar] [CrossRef] [Green Version]
- De Miguel Díez, J.; García, T.G.; Maestu, L.P. Comorbilidades de la EPOC. Arch. Bronconeumol. 2010, 46, 20–25. [Google Scholar] [CrossRef]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A. Guía española de la enfermedad pulmonar obstructiva crónica (GesEPOC) 2017. Tratamiento farmacológico en fase estable. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1995, 152, S77–S120. [Google Scholar]
- Boixeda, R.; Bacca, S.; Elias, L.; Capdevila, J.A.; Vilà, X.; Mauri, M. La neumonía como comorbilidad en la enfermedad pulmonar obstructiva crónica (EPOC). Diferencias entre la exacerbación aguda de la EPOC y la neumonía en los pacientes con EPOC. Arch. Bronconeumol. 2014, 50, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Bonnesen, B.; Egelund, G.B.; Jensen, A.V.; Andersen, S.; Petersen, P.T.; Rohde, G. Is chronic obstructive pulmonary disease a risk factor for death in patients with community acquired pneumonia? Infect. Dis. 2019, 51, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Sharafkhaneh, A.; Spiegelman, A.M.; Main, K.; Tavakoli-Tabasi, S.; Lan, C.; Musher, D. Mortality in Patients Admitted for Concurrent COPD Exacerbation and Pneumonia. COPD J. Chronic. Obstr. Pulm. Dis. 2017, 14, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, C.B.; Vietri, J.; Choate, R.; McDaniel, A.; Sato, R.; Ford, K.D. Patient-Reported Consequences of Community-Acquired Pneumonia in Patients with Chronic Obstructive Pulmonary Disease. Chronic. Obstr. Pulm. Dis. 2019, 6, 132–144. [Google Scholar]
- Yin, H.-L.; Yin, S.-Q.; Lin, Q.-Y.; Xu, Y.; Xu, H.-W.; Liu, T. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: A meta-analysis. Medicine (Baltimore) 2017, 96, e6836. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Available online: https://goldcopd.org (accessed on 25 May 2019).
- Gautam, S.S.; O’Toole, R.F. Convergence in the Epidemiology and Pathogenesis of COPD and Pneumonia. COPD J. Chronic. Obstr. Pulm. Dis. 2016, 13, 790–798. [Google Scholar] [CrossRef]
- Price, D.; Yawn, B.; Brusselle, G.; Rossi, A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim. Care Respir. J. 2012, 22, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Calverley, P.M.A.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W. Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2007, 356, 775–789. [Google Scholar] [CrossRef] [Green Version]
- Ernst, P.; Gonzalez, A.V.; Brassard, P.; Suissa, S. Inhaled Corticosteroid Use in Chronic Obstructive Pulmonary Disease and the Risk of Hospitalization for Pneumonia. Am. J. Respir. Crit. Care Med. 2007, 176, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Tashkin, D.; Zhang, X.; Radner, F.; Sjöbring, U.; Thorén, A. Budesonide and the risk of pneumonia: A meta-analysis of individual patient data. Lancet 2009, 374, 712–719. [Google Scholar] [CrossRef]
- Jebrak, G. Recommandations et prise en charge de la BPCO en France: Les recommandations sur la prise en charge de la BPCO ne sont pas suivies dans la vraie vie! Rev. Mal. Respir. 2010, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.E.M.; Smeenk, F.; Smeele, I.J.; van Schayck, C.P. Overtreatment with inhaled corticosteroids and diagnostic problems in primary care patients, an exploratory study. Fam. Pract. 2008, 25, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourbeau, J.; Sebaldt, R.J.; Day, A.; Bouchard, J.; Kaplan, A.; Hernandez, P. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: The CAGE study. Can. Respir. J. 2008, 15, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Rossi, A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir. Med. 2012, 106, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef]
- Gershon, A.S.; Mecredy, G.C.; Guan, J.; Victor, J.C.; Goldstein, R.; To, T. Quantifying comorbidity in individuals with COPD: A population study. Eur. Respir. J. 2015, 45, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Chittal, P.; Babu, A.S.; Lavie, C.J. Obesity Paradox: Does Fat Alter Outcomes in Chronic Obstructive Pulmonary Disease? COPD J. Chronic Obstr. Pulm. Dis. 2015, 12, 14–18. [Google Scholar] [CrossRef]
- Barbarito, N.; Mattia, E.D. Obesity paradox in chronic obstructive pulmonary disease: A result of airflow obstruction over-grading? Respir. Med. 2017, 126, 133. [Google Scholar] [CrossRef]
- Galesanu, R.G.; Bernard, S.; Marquis, K.; Lacasse, Y.; Poirier, P.; Bourbeau, J. Obesity and chronic obstructive pulmonary disease: Is fatter really better? Can. Respir. J. 2014, 21, 297–301. [Google Scholar] [CrossRef] [PubMed]

| Variable | All Phenotypes | Positive Bronchodilator Response | Non-Exacerbator | Exacerbator with Emphysema | Exacerbator with Chronic Bronchitis |
|---|---|---|---|---|---|
| Patients, n (%) | 273 (100.0) | 71 (26.0) | 135 (49.5) | 27 (9.9) | 40 (14.7) |
| Follow-up, months (IQR) | 68.15 (40.69–72.12) | 70.99 (54.13–73.45) | 66.55 (37.83–71.74) | 61.32 (35.33–71.94) | 41.65 (20.18–71.21) |
| Males, n (%) | 243 (89.0) | 63 (88.7) | 124 (91.8) | 22 (81.5) | 34 (85.0) |
| Age, years (SD) | 67.99 (10.62) | 63.44 (11.70) | 69.76 (9.33) | 67.44 (11.23) | 70.47 (10.10) |
| Weight, kg (SD) | 75.03 (16.89) | 77.01 (16.94) | 76.04 (17.75) | 71.85 (18.46) | 66,78 (10.85) |
| Height, m (SD) | 1.63 (0.08) | 1.65 (0.09) | 1.63 (0.07) | 1.61 (0.08) | 1.61 (0.08) |
| BMI, kg/m2 (SD) | 28.05 (5.49) | 28.15 (5.44) | 28.48 (5.83) | 27.46 (6.19) | 26.82 (3.55) |
| FEV1, % (SD) | 48.64 (12.59) | 53.71 (11.66) | 48.57 (12.52) | 44.66 (11.82) | 42.53 (11.55) |
| FVC, % (SD) | 73.18 (15.00) | 79.60 (13.19) | 71.74 (15.08) | 71.58 (14.09) | 67.72 (15.11) |
| Active smoking, n (%) | 92 (34%) | 35 (49.3) | 36 (26.7) | 7 (25.9) | 14 (35.0) |
| Comorbidity indexes, median (IQR) | |||||
| Charlson | 2 (1–4) | 2 (1–3) | 2 (1–4) | 2 (1–3) | 3 (1–4) |
| COTE | 1 (0–2) | 0 (0–2) | 1 (0–3) | 0 (0–3) | 1 (0–3) |
| Pharmacological treatment, n (%) | |||||
| LAMA | 254 (93.0) | 60 (84.5) | 129 (95.6) | 25 (92.6) | 40 (100.0) |
| LABA | 242 (88.6) | 66 (93.0) | 111 (82.2) | 26 (96.3) | 39 (97.5) |
| ICS | 212 (77.7) | 61 (84.5) | 91 (67.4) | 23 (85.2) | 37 (92.5) |
| Respiratory therapies, n (%) | |||||
| LTOT | 91 (33.3) | 14 (20.0) | 39 (28.9) | 13 (48.1) | 25 (62.5) |
| CPAP | 31 (11.4) | 10 (14.1) | 16 (11.9) | 3 (11.1) | 2 (5.0) |
| BiPAP | 14 (5.1) | 1 (1.4) | 6 (4.4) | 2 (7.4) | 5 (12.5) |
| Pneumococcal vaccination, n (%) | 160 (58.6) | 32 (45.1) | 86 (63.7) | 16 (59.3) | 26 (65.0) |
| Pneumonia, n (%) | 77 (28.2) | 15 (21.1) | 38 (28.1) | 5 (18.5) | 19 (47.5) |
| Death, n (%) | 93 (34.1) | 12 (16.9) | 49 (36.3) | 12 (44.4) | 20 (50.0) |
| Variable | Pneumonia | No Pneumonia | p-Value |
|---|---|---|---|
| Male, n (%) | 69 (89.6) | 174 (88.8) | 0.843 |
| Age, years (SD) | 71.65 (8.90) | 66.55 (10.91) | 0.000 *** |
| Weight, kg (SD) | 75.06 (15.20) | 75.02 (17.52) | 0.984 |
| Height, m (SD) | 1.62 (0.08) | 1.63 (0.80) | 0.140 |
| BMI, kg/m2 (DE) | 28.53 (5.35) | 27.87 (5.55) | 0.369 |
| Active smoking, n (%) | 21 (27.3) | 71 (36.2) | 0.200 |
| Pulmonary function, % (SD) | |||
| FEV1 | 47.37 (13.20) | 49.14 (12.34) | 0.297 |
| FVC | 70.81 (14.82) | 74.11 (15.01) | 0.102 |
| Phenotypes, n (%) | |||
| Positive bronchodilator response | 15 (19.5) | 56 (28.6) | 0.016 * |
| Nonexacerbator | 38 (49.4) | 97 (49.5) | |
| Exacerbator with emphysema | 5 (6.5) | 22 (11.2) | |
| Exacerbator with chronic bronchitis | 19 (24.7) | 21 (10.7) | |
| Pharmacological treatment, n (%) | |||
| LAMA | 75 (97.4) | 179 (91.3) | 0.076 |
| LABA | 71 (92.2) | 171 (87.2) | 0.245 |
| ICS | 66 (85.7) | 146 (74.5) | 0.045 * |
| Respiratory therapies, n (%) | |||
| LTOT | 34 (44.2) | 57 (29.1) | 0.017 * |
| CPAP | 5 (6.5) | 26 (13.3) | 0.139 |
| BiPAP | 6 (7.8) | 8 (4.1) | 0.229 |
| Pneumococcal vaccination, n (%) | 21 (27.3) | 139 (70.9) | 0.000 *** |
| Comorbidities, n (%) | Pneumonia | No Pneumonia | p-Value |
|---|---|---|---|
| Arterial hypertension | 41 (53.2) | 105 (53.6) | 0.961 |
| Dyslipidemia | 40 (51.9) | 68 (34.7) | 0.009 ** |
| Mellitus diabetes | 22 (28.6) | 44 (22.4) | 0.288 |
| Atrial fibrillation | 13 (16.9) | 22 (11.2) | 0.229 |
| Ischemic heart disease | 14 (18.2) | 24 (12.2) | 0.243 |
| Heart failure | 17 (22.1) | 20 (10.2) | 0.017 * |
| Peripheral vasculopathy | 5 (6.5) | 6 (3.1) | 0.302 |
| Cerebrovascular disease | 0 (0.0) | 1 (0.5) | 1.000 |
| Dementia | 1 (1.3) | 4 (2.0) | 1.000 |
| Connective tissue disease | 2 (2.6) | 1 (0.5) | 0.193 |
| Gastroduodenal ulcer | 6 (7.8) | 8 (4.1) | 0.229 |
| Liver disease | 4 (5.2) | 12 (6.1) | 1.000 |
| Hemiplegia | 0 (0.0) | 0 (0.0) | - |
| Chronic kidney disease | 9 (11.7) | 14 (7.1) | 0.232 |
| All neoplasia | 19 (24.7) | 56 (28.6) | 0.550 |
| Lung cancer | 6 (7.8) | 18 (9.2) | 0.816 |
| AIDS | 1 (1.3) | 0 (0.0) | 0.282 |
| Anxiety | 0 (0.0) | 4 (2.0) | 0.580 |
| Idiopathic pulmonary fibrosis | 0 (0.0) | 0 (0.0) | - |
| Cirrhosis | 0 (0.0) | 0 (0.0) | - |
| Comorbidity indices, median (IQR) | |||
| Charlson | 2 (1–4) | 2 (1–3) | 0.099 |
| COTE | 1 (1–3) | 0 (0–2) | 0.244 |
| Variable | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Pneumonia | 2.65 | 1.57–4.48 | 0.000 *** |
| Age | 1.08 | 1.03–1.09 | 0.000 *** |
| Male | 1.95 | 0.74–5.15 | 0.180 |
| BMI | 0.92 | 0.87–0.96 | 0.001 ** |
| FEV1 | 1.00 | 0.97–1.02 | 0.780 |
| FVC | 0.99 | 0.97–1.00 | 0.142 |
| Active smoking | 1.29 | 0.76–2.19 | 0.353 |
| Phenotypes | |||
| Exacerbator with emphysema | 2.27 | 0.97–5.33 | 0.060 |
| Exacerbator with chronic bronchitis | 2.12 | 0.97–4.62 | 0.059 |
| Nonexacerbator | 1.68 | 0.86–3.27 | 0.129 |
| Pneumococcal vaccination | 0.92 | 0.58–1.45 | 0.720 |
| Charlson | 1.31 | 1.17–1.47 | 0.000 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Hernández Vázquez, J.; Bellón Cano, J.M.; Gallo González, V.; Recio Moreno, B.; Cerezo Lajas, A.; Puente Maestu, L.; de Miguel Díez, J. Influence of Pneumonia on the Survival of Patients with COPD. J. Clin. Med. 2020, 9, 230. https://doi.org/10.3390/jcm9010230
Ji Z, Hernández Vázquez J, Bellón Cano JM, Gallo González V, Recio Moreno B, Cerezo Lajas A, Puente Maestu L, de Miguel Díez J. Influence of Pneumonia on the Survival of Patients with COPD. Journal of Clinical Medicine. 2020; 9(1):230. https://doi.org/10.3390/jcm9010230
Chicago/Turabian StyleJi, Zichen, Julio Hernández Vázquez, José María Bellón Cano, Virginia Gallo González, Beatriz Recio Moreno, Alicia Cerezo Lajas, Luis Puente Maestu, and Javier de Miguel Díez. 2020. "Influence of Pneumonia on the Survival of Patients with COPD" Journal of Clinical Medicine 9, no. 1: 230. https://doi.org/10.3390/jcm9010230





