Conjunctival Injection Reduction in Patients with Atopic Keratoconjunctivitis Due to Synergic Effect of Bovine Enteric-Coated Lactoferrin in 0.1% Tacrolimus Ophthalmic Suspension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Test Material
2.3. Patients
2.4. Evaluation Methods
2.4.1. Serum Analysis
2.4.2. Ocular Surface Evaluation
2.5. Sample Size and Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimecki, M.; Wlaszczyk, A.; Cheneau, P.; Brunel, A.S.; Mazurier, J.; Spik, G.; Kubler, A. Immunoregulatory effects of a nutritional preparation containing bovine lactoferrin taken orally by healthy individuals. Arch. Immunol. Ther. Exp. 1998, 46, 231–240. [Google Scholar]
- Sekine, K.; Ushida, Y.; Kuhara, T.; Iigo, M.; Baba-Toriyama, H.; Moore, M.A.; Murakoshi, M.; Satomi, Y.; Nishino, H.; Kakizoe, T.; et al. Inhibition of initiation and early stage development of aberrant crypt foci and enhanced natural killer activity in male rats administered bovine lactoferrin concomitantly with azoxymethane. Cancer Lett. 1997, 121, 211–216. [Google Scholar] [CrossRef]
- Kawakami, H.; Park, H.; Park, S.; Kuwata, H.; Shephard, R.J.; Aoyagi, Y. Effects of enteric-coated lactoferrin supplementation on the immune function of elderly indivisualsindividuals: A randomised, double-blind, placebo-controlled trial. Int. Dairy J. 2015, 47, 79–85. [Google Scholar] [CrossRef]
- Terreni, E.; Burgalassi, S.; Chetoni, P.; Tampucci, S.; Zucchetti, E.; Fais, R.; Ghelardi, E.; Lupetti, A.; Monti, D. Development and Characterization of a Novel Peptide-Loaded Antimicrobial Ocular Insert. Biomolecules 2020, 10, 664. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046–1060. [Google Scholar] [CrossRef]
- Hanstock, H.G.; Edwards, J.P.; Walsh, N.P. Tear Lactoferrin and Lysozyme as Clinically Relevant Biomarkers of Mucosal Immune Competence. Front. Immunol. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Rageh, A.A.; Ferrington, D.A.; Roehrich, H.; Yuan, C.; Terluk, M.R.; Nelson, E.F.; Montezuma, S.R. Lactoferrin expression in human and murine ocular tissue. Curr. Eye Res. 2016, 41, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [Green Version]
- Bielory, B.; Bielory, L. Atopic dermatitis and keratoconjunctivitis. Immunol. Allergy Clin. N. Am. 2010, 30, 323–336. [Google Scholar] [CrossRef]
- Fujishima, H.; Okada, N.; Dogru, M.; Baba, F.; Tomita, M.; Abe, J.; Matsumoto, K.; Saito, H. The role of Staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy 2012, 67, 799–803. [Google Scholar] [CrossRef]
- Ozcan, A.A.; Ersoz, T.R.; Dulger, E. Management of severe allergic conjunctivitis with topical cyclosporin a 0.05% eyedrops. Cornea 2007, 26, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Ebihara, N.; Fujishima, H.; Fukushima, A.; Kumagai, N.; Nakagawa, Y.; Namba, K.; Okamoto, S.; Shoji, J.; Takamura, E.; et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J. Ocul. Pharmacol. Ther. 2010, 26, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazu, H.; Shimizu, E.; Aketa, N.; Okada, N.; Fukagawa, K.; Fujishima, H. The efficacy of 0.1% tacrolimus ophthalmic suspension in the treatment of severe atopic keratoconjunctivitis. Ann. Allergy Asthma Immunol. 2019, 122, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Kligman, A.M.; Schreiner, V.; Stoudemayer, T. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res. Technol. 2001, 7, 73–77. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Ono, T.; Morishita, S.; Fujisaki, C.; Ohdera, M.; Murakoshi, M.; Iida, N.; Kato, H.; Miyashita, K.; Iigo, M.; Yoshida, T. Effects of pepsin and trypsin on the anti-adipogenic action of lactoferrin against pre-adipocytes derived from rat mesenteric fat. Br. J. Nutr. 2011, 105, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Yoshida, A.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Masuda, Y. Effect of tablets containing lactoferrin and lactoperoxidase on gingival health in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Periodontal. Res. 2019, 54, 702–708. [Google Scholar] [CrossRef]
- Oda, H.; Miyakawa, M.; Mizuki, M.; Misawa, Y.; Tsukahara, T.; Tanaka, M.; Yamauchi, K.; Abe, F.; Nomiyama, T. Effects of Lactoferrin on Subjective Skin Conditions in Winter: A Preliminary, Randomized, Double-Blinded, Placebo-Controlled Trial. Clin. Cosmet. Investig. Dermatol. 2019, 12, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Marquis, E.A.; Seidman, D.N.; Asta, M.; Woodward, C.; Ozolins, V. Mg segregation at Al/Al3Sc heterophase interfaces on an atomic scale: Experiments and computations. Phys. Rev. Lett. 2003, 91, 036101. [Google Scholar] [CrossRef] [Green Version]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-loaded contact lenses counteract cytotoxicity caused in vitro by keratoconic tears. Cont. Lens Anterior Eye 2019, 42, 253–257. [Google Scholar] [CrossRef]
- Legrand, D.; Mazurier, J. A critical review of the roles of host lactoferrin in immunity. Biometals 2010, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shau, H.; Kim, A.; Golub, S.H. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J. Leukoc. Biol. 1992, 51, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Wakabayashi, H.; Hashimoto, S.; Teraguchi, S.; Hayasawa, H.; Tomita, M. Effects of orally administered bovine lactoferrin on the immune system of healthy volunteers. Adv. Exp. Med. Biol. 1998, 443, 261–265. [Google Scholar] [PubMed]
- Grigorieva, D.V.; Gorudko, I.V.; Shamova, E.V.; Terekhova, M.S.; Maliushova, E.V.; Semak, I.V.; Cherenkevich, S.N.; Sokolov, A.V.; Timoshenko, A.V. Effects of recombinant human lactoferrin on calcium signaling and functional responses of human neutrophils. Arch. Biochem. Biophys. 2019, 675, 108122. [Google Scholar] [CrossRef]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008, 22, 2747–2757. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Nonnecke, E.; Lonnerdal, B.; Murray, J. The lactoferrin receptor may mediate the reduction of eosinophils in the duodenum of pigs consuming milk containing recombinant human lactoferrin. Biometals 2014, 27, 1031–1038. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lonnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Naidu, T.A.; Betageri, G.V.; Prasadarao, N.V.; Naidu, A.S. Inflammatory responses improve with milk ribonuclease-enriched lactoferrin supplementation in postmenopausal women. Inflamm. Res. 2010, 59, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.; Berenz, A.; Itell, H.L.; Conaway, M.; Blackman, A.; Nataro, J.P.; Permar, S.R. Dose Escalation Study of Bovine Lactoferrin in Preterm Infants: Getting the Dose Right. Biochem. Cell Biol. 2020, 1208–6002. [Google Scholar] [CrossRef]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev. Res. 2009, 2, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasibetti, E.; Bruni, N.; Bigliati, M.; Capucchio, M.T. Lactoferricin/verbascoside topical emulsion: A possible alternative treatment for atopic dermatitis in dogs. Nat. Prod. Res. 2018, 32, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.L.; West, N.P.; Cox, A.J.; Gebski, V.J.; Watts, A.M.; Dodds, A.; Fazekas de St Groth, B.; Cripps, A.W.; Shumack, S. Oral supplementation with bovine whey-derived Ig-rich fraction and lactoferrin improves SCORAD and DLQI in atopic dermatitis. J. Dermatol. Sci. 2017, 85, 143–146. [Google Scholar] [CrossRef]
- Shoji, J.; Ohashi, Y.; Fukushima, A.; Miyazaki, D.; Uchio, E.; Takamura, E.; Fujishima, H.; Namba, K.; Kumagai, N.; Ebihara, N.; et al. Topical Tacrolimus for Chronic Allergic Conjunctival Disease with and without Atopic Dermatitis. Curr. Eye Res. 2019, 44, 796–805. [Google Scholar] [CrossRef]
- Zhai, J.; Gu, J.; Yuan, J.; Chen, J. Tacrolimus in the treatment of ocular diseases. BioDrugs 2011, 25, 89–103. [Google Scholar] [CrossRef]
- Thaci, D.; Reitamo, S.; Gonzalez, E.M.A.; Moss, C.; Boccaletti, V.; Cainelli, T.; der Valk, P.; Buckova, H.; Sebstian, M.; Schuttelaar, M.L.; et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: Results of a randomized, multicentre, comparative study. Br. J. Dermatol. 2008, 159, 1348–1356. [Google Scholar] [CrossRef]

| 0 M | 3 M | ⊿ (0 M–3 M) | * p-value | ||||||||||
| 0 M | 3 M | ⊿ | |||||||||||
| Itching | Placebo | 0.8 | ± | 0.7 | 1.0 | ± | 0.7 | −0.2 | ± | 0.7 | 0.73 | 0.49 | 0.24 |
| eLF | 0.9 | ± | 0.6 | 0.9 | ± | 0.8 | 0.0 | ± | 0.5 | ||||
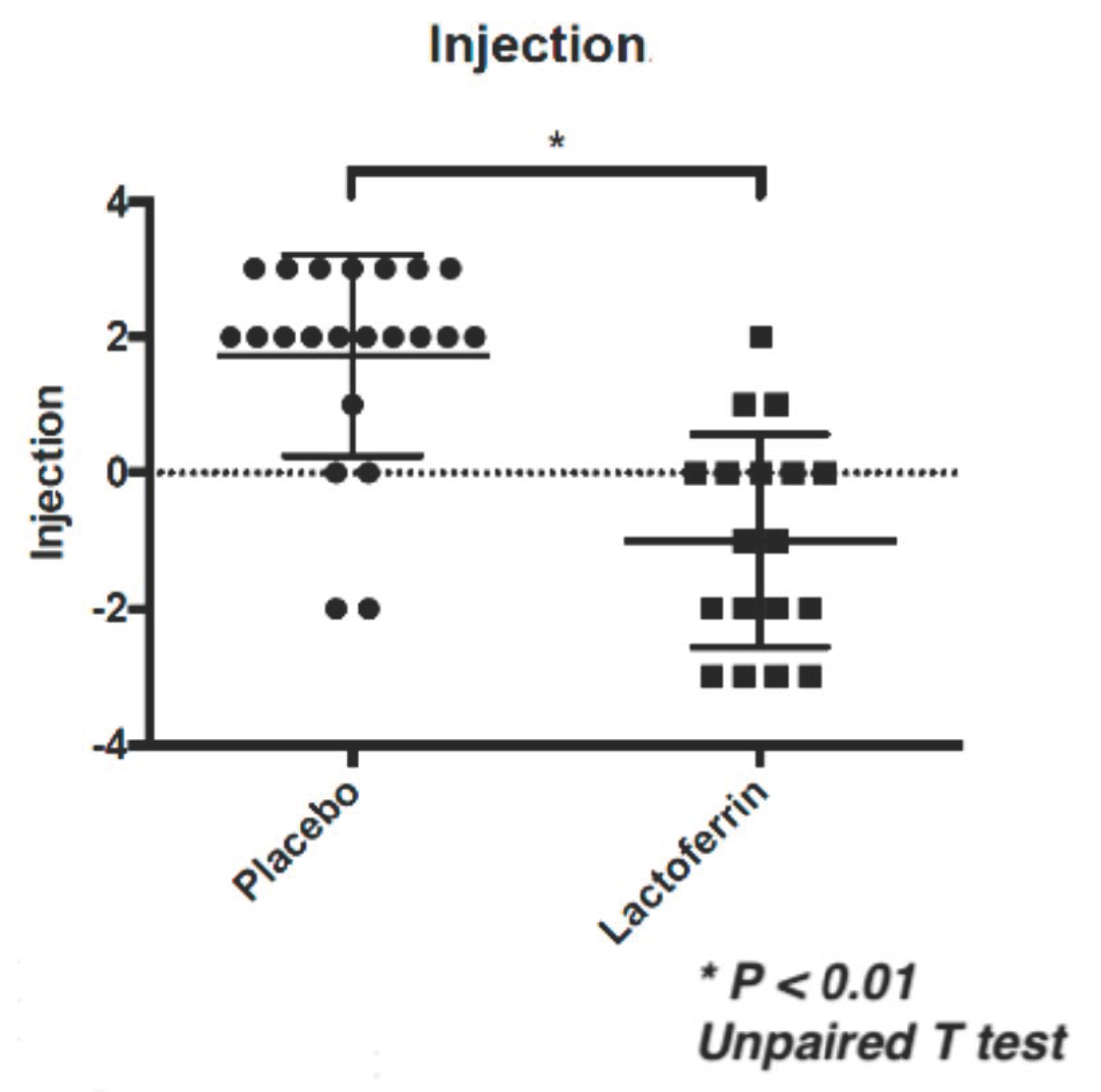
| Injection | Placebo | 1.3 | ± | 1.2 | 3.0 | ± | 1.8 | 1.7 | ± | 1.5 | <0.01 | <0.01 | <0.01 |
| eLF | 2.6 | ± | 1.7 | 1.6 | ± | 1.6 | −1.0 | ± | 1.6 | ||||
| Papillae | Placebo | 1.3 | ± | 0.6 | 1.2 | ± | 0.7 | 0.1 | ± | 0.8 | 0.71 | <0.01 | <0.01 |
| eLF | 1.4 | ± | 0.6 | 0.4 | ± | 0.5 | 0.9 | ± | 0.7 | ||||
| Corneal stain | Placebo | 0.2 | ± | 0.4 | 0.5 | ± | 0.7 | −0.2 | ± | 0.5 | 0.97 | 0.21 | 0.17 |
| eLF | 0.2 | ± | 0.4 | 0.2 | ± | 0.4 | 0.0 | ± | 0.5 | ||||
| Data are shown by Mean ± SD. * Unpaired T test or Mann-Whitney test. | |||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujishima, H.; Okada, N.; Matsumoto, K.; Shimizu, E.; Fukuda, S.; Tomita, M. Conjunctival Injection Reduction in Patients with Atopic Keratoconjunctivitis Due to Synergic Effect of Bovine Enteric-Coated Lactoferrin in 0.1% Tacrolimus Ophthalmic Suspension. J. Clin. Med. 2020, 9, 3093. https://doi.org/10.3390/jcm9103093
Fujishima H, Okada N, Matsumoto K, Shimizu E, Fukuda S, Tomita M. Conjunctival Injection Reduction in Patients with Atopic Keratoconjunctivitis Due to Synergic Effect of Bovine Enteric-Coated Lactoferrin in 0.1% Tacrolimus Ophthalmic Suspension. Journal of Clinical Medicine. 2020; 9(10):3093. https://doi.org/10.3390/jcm9103093
Chicago/Turabian StyleFujishima, Hiroshi, Naoko Okada, Kenji Matsumoto, Eisuke Shimizu, Shinji Fukuda, and Masaru Tomita. 2020. "Conjunctival Injection Reduction in Patients with Atopic Keratoconjunctivitis Due to Synergic Effect of Bovine Enteric-Coated Lactoferrin in 0.1% Tacrolimus Ophthalmic Suspension" Journal of Clinical Medicine 9, no. 10: 3093. https://doi.org/10.3390/jcm9103093





