Effect of Aerobic Exercise Training and Deconditioning on Oxidative Capacity and Muscle Mitochondrial Enzyme Machinery in Young and Elderly Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Individuals
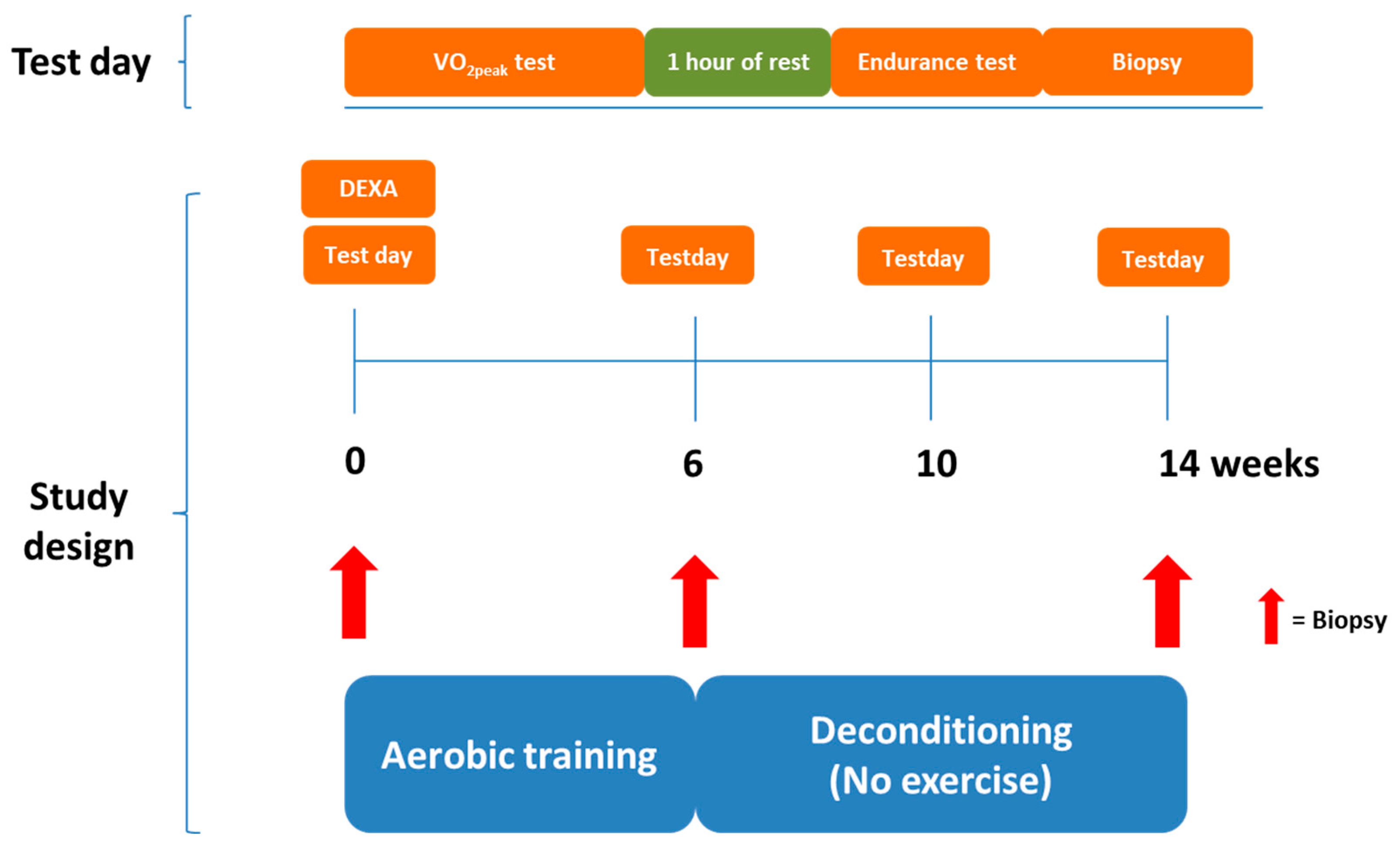
2.2. Study Design
2.3. DEXA Scanning
2.4. Maximal Oxygen Consumption Test
2.5. Endurance Test
2.6. Aerobic Exercise Training and Deconditioning Interventions
2.7. Skeletal Muscle Biopsies
2.8. Mitochondrial Enzyme Activities
2.9. Western Blotting Analysis
2.10. Bioplex Analysis
2.11. Statistical Analysis
3. Results
3.1. Anthropometry
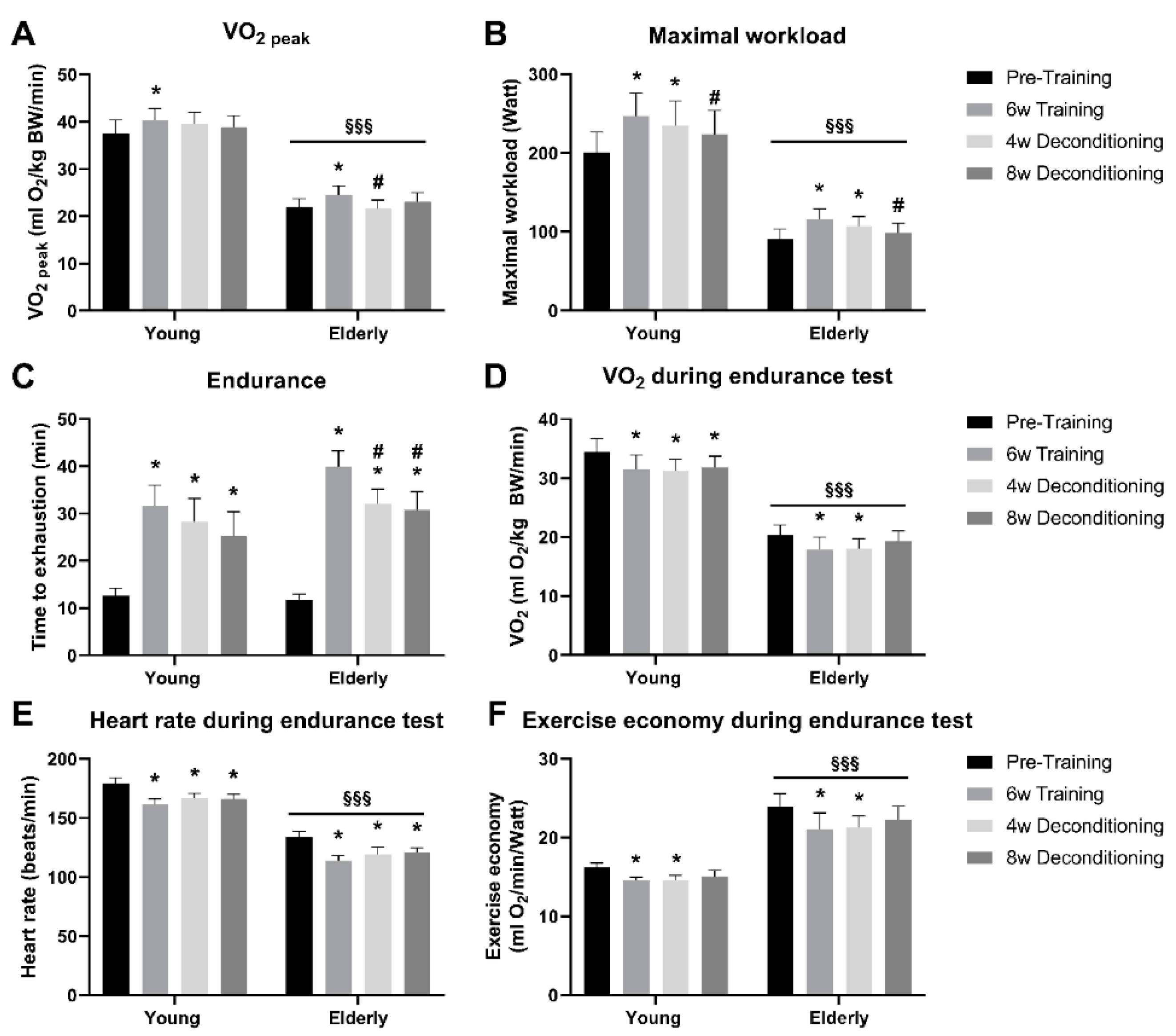
3.2. Functional Parameters of Aerobic Capacity
3.3. Mitochondrial Enzyme Activities
3.4. Apoptosis Markers: Cleaved Caspase 3 and Bcl2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuggle, N.; Shaw, S.; Dennison, E.; Cooper, C. Sarcopenia. Best Pr. Res. Clin. Rheumatol. 2017, 31, 218–242. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.N.; Joyner, M.J. Skeletal muscle mass and the reduction of VO2 max in trained older subjects. J. Appl. Physiol. 1997, 82, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Dat Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kohl, H.W., III; Paffenbarger, R.S., Jr.; Clark, D.G.; Cooper, K.H.; Gibbons, L.W. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989, 262, 2395–2401. [Google Scholar] [CrossRef]
- Posner, J.D.; McCully, K.K.; Landsberg, L.A.; Sands, L.; Tycenski, P.; Hofmann, M.T.; Wetterholt, K.L.; Shaw, C.E. Physical determinants of independence in mature women. Arch. Phys. Med. Rehabil. 1995, 76, 373–380. [Google Scholar] [CrossRef]
- Peterson, C.M.; Johannsen, D.L.; Ravussin, E. Skeletal Muscle Mitochondria and Aging: A Review. J. Aging Res. 2012, 2012, 1–20. [Google Scholar] [CrossRef]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef]
- Turner, D.L.; Hoppeler, H.; Claassen, H.; Vock, P.; Kayser, B.; Schena, F.; Ferretti, G. Effects of endurance training on oxidative capacity and structural composition of human arm and leg muscles. Acta Physiol. Scand. 1997, 161, 459–464. [Google Scholar] [CrossRef]
- Hoppeler, H.; Howald, H.; Conley, K.; Lindstedt, S.L.; Claassen, H.; Vock, P.; Weibel, E.R. Endurance training in humans: Aerobic capacity and structure of skeletal muscle. J. Appl. Physiol. 1985, 59, 320–327. [Google Scholar] [CrossRef]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Boil. Chem. 1967, 242, 2278–2282. [Google Scholar]
- Tarnopolsky, M.A.; Rennie, C.D.; Robertshaw, H.A.; Fedak-Tarnopolsky, S.N.; Devries, M.C.; Hamadeh, M.J. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R1271–R1278. [Google Scholar] [CrossRef] [PubMed]
- Chrøis, K.M.; Dohlmann, T.L.; Søgaard, D.; Hansen, C.V.; Dela, F.; Helge, J.W.; Larsen, S. Mitochondrial adaptations to high intensity interval training in older females and males. Eur. J. Sport Sci. 2019, 20, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Suer, M.K.; Wolff, C.A.; Harber, M.P. Markers of Human Skeletal Muscle Mitochondrial Biogenesis and Quality Control: Effects of Age and Aerobic Exercise Training. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 69, 371–378. [Google Scholar] [CrossRef]
- Menshikova, E.V.; Ritov, V.B.; Fairfull, L.; Ferrell, R.E.; Kelley, D.E.; Goodpaster, B.H. Effects of Exercise on Mitochondrial Content and Function in Aging Human Skeletal Muscle. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2006, 61, 534–540. [Google Scholar] [CrossRef]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Rizza, R.A.; Coenen-Schimke, J.M.; Nair, K.S. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003, 52, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Douglass, M.D.; Kaminsky, L.A.; Jemiolo, B.; Trappe, T.A.; Trappe, S.; Harber, M.P. Molecular Adaptations to Aerobic Exercise Training in Skeletal Muscle of Older Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2010, 65, 1201–1207. [Google Scholar] [CrossRef]
- Broskey, N.T.; Greggio, C.; Boss, A.; Boutant, M.; Dwyer, A.; Schlueter, L.; Hans, D.; Gremion, G.; Kreis, R.; Boesch, C.; et al. Skeletal Muscle Mitochondria in the Elderly: Effects of Physical Fitness and Exercise Training. J. Clin. Endocrinol. Metab. 2014, 99, 1852–1861. [Google Scholar] [CrossRef]
- Seals, D.R.; Hagberg, J.M.; Hurley, B.F.; Ehsani, A.A.; Holloszy, J.O. Endurance training in older men and women. I. Cardiovascular responses to exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1024–1029. [Google Scholar] [CrossRef]
- Seals, D.R.; Reiling, M.J. Effect of regular exercise on 24-hour arterial pressure in older hypertensive humans. Hypertension 1991, 18, 583–592. [Google Scholar] [CrossRef]
- De Vito, G.; Bernardi, M.; Forte, R.; Pulejo, C.; Figura, F. Effects of a low-intensity conditioning programme on V˙O2max and maximal instantaneous peak power in elderly women. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 80, 227–232. [Google Scholar] [CrossRef]
- Wang, E.; Næss, M.S.; Hoff, J.; Albert, T.L.; Pham, Q.; Richardson, R.S.; Helgerud, J. Exercise-training-induced changes in metabolic capacity with age: The role of central cardiovascular plasticity. AGE 2013, 36, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Örlander, J.; Aniansson, A. Effects of physical training on skeletal muscle metabolism and ultrastructure in 70 to 75-year-old men. Acta Physiol. Scand. 1980, 109, 149–154. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Thøgersen, F.D.; Thybo, K.; Vissing, J.; Krag, T.O.B.; Ruiz-Ruiz, C.; Risom, L.; Wibrand, F.; Høeg, L.D.; Kiens, B.; et al. Adaptations in Mitochondrial Enzymatic Activity Occurs Independent of Genomic Dosage in Response to Aerobic Exercise Training and Deconditioning in Human Skeletal Muscle. Cells 2019, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Martin, W.H.; Sinacore, D.R.; Joyner, M.J.; Hagberg, J.M.; Holloszy, J.O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. 1984, 57, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Martin, W.H.; Bloomfield, S.A.; Lowry, O.H.; Holloszy, J.O. Effects of detraining on responses to submaximal exercise. J. Appl. Physiol. 1985, 59, 853–859. [Google Scholar] [CrossRef]
- Klausen, K.; Andersen, L.B.; Pelle, I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol. Scand. 1981, 113, 9–16. [Google Scholar] [CrossRef]
- Jeppesen, T.D.; Schwartz, M.; Olsen, D.B.; Wibrand, F.; Krag, T.O.B.; Duno, M.; Hauerslev, S.; Vissing, J. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 2006, 129, 3402–3412. [Google Scholar] [CrossRef]
- Song, W.; Kwak, H.-B.; Lawler, J.M. Exercise Training Attenuates Age-Induced Changes in Apoptotic Signaling in Rat Skeletal Muscle. Antioxid. Redox Signal. 2006, 8, 517–528. [Google Scholar] [CrossRef]
- Kwak, H.-B.; Song, W.; Lawler, J.M. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006, 20, 791–793. [Google Scholar] [CrossRef]
- Chung, L.; Ng, Y.-C. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim. Biophys. Acta 2006, 1762, 103–109. [Google Scholar] [CrossRef]
- Dirks-Naylor, A.J.; Leeuwenburgh, C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic. Boil. Med. 2004, 36, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C. Role of Apoptosis in Sarcopenia. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M999–M1001. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, A.L.; Bonham, M.P.; Ghasem-Zadeh, A.; Evans, A.; Barber, E.M.; Day, K.; Kwok, A.; Truby, H. Reliability of Compartmental Body Composition Measures in Weight-Stable Adults Using GE iDXA: Implications for Research and Practice. Nutrients 2018, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.P. Energy cost calculations for exercise prescription: An update. Sports Med. 2000, 30, 17–22. [Google Scholar] [CrossRef]
- Bergstrom, J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest. 1975, 35, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Thøgersen, F.D.; Qadri, K.A.N.; Krag, T.; Sveen, M.-L.; Vissing, J.; Jeppesen, T.D. Preserved Capacity for Adaptations in Strength and Muscle Regulatory Factors in Elderly in Response to Resistance Exercise Training and Deconditioning. J. Clin. Med. 2020, 9, 2188. [Google Scholar] [CrossRef]
- Wibrand, F.; Jeppesen, T.D.; Frederiksen, A.L.; Olsen, D.B.; Duno, M.; Schwartz, M.; Vissing, J. Limited diagnostic value of enzyme analysis in patients with mitochondrial tRNA mutations. Muscle Nerve 2009, 41, 607–613. [Google Scholar] [CrossRef]
- Birchmachin, M.; Briggs, H.; Saborido, A.; Bindoff, L.A.; Turnbull, D. An Evaluation of the Measurement of the Activities of Complexes I-IV in the Respiratory Chain of Human Skeletal Muscle Mitochondria. Biochem. Med. Metab. Boil. 1994, 51, 35–42. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Talos, C.; Wiesmann, U.; Hoppel, C.L. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin. Chim. Acta 1994, 230, 177–187. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Madsen, A.B.; Kleinert, M.; Treebak, J.T.; Lundsgaard, A.-M.; Jensen, T.E.; Richter, E.A.; Wojtaszewski, J.F.; Kiens, B.; Frøsig, C. Regulation of autophagy in human skeletal muscle: Effects of exercise, exercise training and insulin stimulation. J. Physiol. 2016, 594, 745–761. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schrøder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Lundby, A.-K.M.; Jacobs, R.A.; Gehrig, S.; De Leur, J.; Hauser, M.; Bonne, T.C.; Flück, D.; Dandanell, S.; Kirk, N.; Kaech, A.; et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 2017, 222, e12905. [Google Scholar] [CrossRef]
- Ghosh, S.; Lertwattanarak, R.; Lefort, N.; Molina-Carrion, M.; Joya-Galeana, J.; Bowen, B.P.; Garduño-García, J.J.; Abdul-Ghani, M.; Richardson, A.; DeFronzo, R.A.; et al. Reduction in Reactive Oxygen Species Production by Mitochondria From Elderly Subjects With Normal and Impaired Glucose Tolerance. Diabetes 2011, 60, 2051–2060. [Google Scholar] [CrossRef]
- Boffoli, D.; Scacco, S.; Vergari, R.; Solarino, G.; Santacroce, G.; Papa, S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim. Biophys. Acta 1994, 1226, 73–82. [Google Scholar] [CrossRef]
- Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 15364–15369. [Google Scholar] [CrossRef] [PubMed]
- Tonkonogi, M.; Fernström, M.; Walsh, B.; Ji, L.L.; Rooyackers, O.; Hammarqvist, F.; Wernerman, J.; Sahlin, K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflügers Arch. 2003, 446, 261–269. [Google Scholar] [CrossRef]
- Crane, J.D.; Devries, M.C.; Safdar, A.; Hamadeh, M.J.; Tarnopolsky, M.A. The Effect of Aging on Human Skeletal Muscle Mitochondrial and Intramyocellular Lipid Ultrastructure. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 65, 119–128. [Google Scholar] [CrossRef]
- Brierley, E.; Johnson, M.; James, O.; Turnbull, D. Effects of physical activity and age on mitochondrial function. QJM Int. J. Med. 1996, 89, 251–258. [Google Scholar] [CrossRef]
- Barrientos, A.; Casademont, J.; Rötig, A.; Miro, O.; Urbano-Márquez, Á.; Rustin, P.; Cardellach, F. Absence of Relationship between the Level of Electron Transport Chain Activities and Aging in Human Skeletal Muscle. Biochem. Biophys. Res. Commun. 1996, 229, 536–539. [Google Scholar] [CrossRef]
- Gouspillou, G.; Sgarioto, N.; Kapchinsky, S.; Purves-Smith, F.; Norris, B.; Pion, C.H.; Barbat-Artigas, S.; Lemieux, F.; Taivassalo, T.; Morais, J.A.; et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2013, 28, 1621–1633. [Google Scholar] [CrossRef]
- Casuso, R.A.; Huertas, J.R. The emerging role of skeletal muscle mitochondrial dynamics in exercise and ageing. Ageing Res. Rev. 2020, 58, 101025. [Google Scholar] [CrossRef] [PubMed]
- Arribat, Y.; Broskey, N.T.; Greggio, C.; Boutant, M.; Alonso, S.C.; Kulkarni, S.S.; Lagarrigue, S.; Carnero, E.A.; Besson, C.; Canto, C.; et al. Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta Physiol. 2018, 225, e13179. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Yeo, D.-W.; Ji, L.L. Muscle immobilization activates mitophagy and disrupts mitochondrial dynamics in mice. Acta Physiol. 2016, 218, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Tryon, L.D.; Pauly, M.; Hood, D.A. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am. J. Physiol. Physiol. 2015, 308, C710–C719. [Google Scholar] [CrossRef]
- Carter, H.N.; Kim, Y.; Erlich, A.T.; Zarrin-Khat, D.; Hood, D.A.; Erlich, A.T. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J. Physiol. 2018, 596, 3567–3584. [Google Scholar] [CrossRef]


| Demographic Parameter | Young Group | Elderly Group |
|---|---|---|
| Age, years | 24 ± 3 | 80 ± 4 *** |
| Height, cm | 175 ± 13 | 169 ± 9 |
| Weight, kg | 70 ± 14 | 76 ± 14 |
| BMI, kg/m2 | 22.5 ± 2.5 | 26.5 ± 3.5 * |
| FFM, kg | 50.7 ± 14.3 | 48.4 ± 10.5 |
| FM, kg | 15.5 ± 6.0 | 26.0 ± 6.9 ** |
| Body fat, % | 24.2 ± 9.9 | 34.9 ± 7.7 * |
| VO2 peak, mL O2/kg/min | 37.5 ± 9.0 | 22.5 ± 6.1 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritzen, A.M.; Andersen, S.P.; Qadri, K.A.N.; Thøgersen, F.D.; Krag, T.; Ørngreen, M.C.; Vissing, J.; Jeppesen, T.D. Effect of Aerobic Exercise Training and Deconditioning on Oxidative Capacity and Muscle Mitochondrial Enzyme Machinery in Young and Elderly Individuals. J. Clin. Med. 2020, 9, 3113. https://doi.org/10.3390/jcm9103113
Fritzen AM, Andersen SP, Qadri KAN, Thøgersen FD, Krag T, Ørngreen MC, Vissing J, Jeppesen TD. Effect of Aerobic Exercise Training and Deconditioning on Oxidative Capacity and Muscle Mitochondrial Enzyme Machinery in Young and Elderly Individuals. Journal of Clinical Medicine. 2020; 9(10):3113. https://doi.org/10.3390/jcm9103113
Chicago/Turabian StyleFritzen, Andreas Mæchel, Søren Peter Andersen, Khaled Abdul Nasser Qadri, Frank D. Thøgersen, Thomas Krag, Mette C. Ørngreen, John Vissing, and Tina D. Jeppesen. 2020. "Effect of Aerobic Exercise Training and Deconditioning on Oxidative Capacity and Muscle Mitochondrial Enzyme Machinery in Young and Elderly Individuals" Journal of Clinical Medicine 9, no. 10: 3113. https://doi.org/10.3390/jcm9103113
APA StyleFritzen, A. M., Andersen, S. P., Qadri, K. A. N., Thøgersen, F. D., Krag, T., Ørngreen, M. C., Vissing, J., & Jeppesen, T. D. (2020). Effect of Aerobic Exercise Training and Deconditioning on Oxidative Capacity and Muscle Mitochondrial Enzyme Machinery in Young and Elderly Individuals. Journal of Clinical Medicine, 9(10), 3113. https://doi.org/10.3390/jcm9103113






