Effects of Sacubitril/Valsartan on the Right Ventricular Arterial Coupling in Patients with Heart Failure with Reduced Ejection Fraction
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 18, 891–975. [Google Scholar]
- John, J.V.; McMurray, M.D.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar]
- Tan, N.Y.; Sangaralingham, L.R.; Sangaralingham, S.J.; Yao, X.; Shah, N.D.; Dunlay, S.M. Comparative effectiveness of sacubitril-valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC Heart Fail. 2020, 8, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Prescott, M.F.; Butler, J.; Felker, G.M.; Maisel, A.S.; McCague, K.; Camacho, A.; Piña, I.L.; Rocha, R.A.; Shah, A.M.; et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019, 322, 1–11. [Google Scholar] [CrossRef]
- Castrichini, M.; Manca, P.; Nuzzi, V.; Barbati, G.; De Luca, A.; Korcova, R.; Stolfo, D.; Di Lenarda, A.; Merlo, M.; Sinagra, G. Sacubitril/Valsartan induces global cardiac reverse remodeling in long-lasting heart failure with reduced ejection fraction: Standard and advanced echocardiographic evidences. J. Clin. Med. 2020, 9, 906. [Google Scholar] [CrossRef] [Green Version]
- Martens, P.; Beliën, H.; Dupont, M.; Vandervoort, P.; Mullens, W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc. Ther. 2018, 36, e12435. [Google Scholar] [CrossRef] [Green Version]
- Polito, M.V.; Silverio, A.; Rispoli, A.; Vitulano, G.; Auria, F.; De Angelis, E.; Loria, F.; Gigantino, A.; Bonadies, D.; Citro, R.; et al. Clinical and echocardiographic benefit of Sacubitril/Valsartan in a real-world population with HF with reduced ejection fraction. Sci. Rep. 2020, 20, 6665–6672. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, S.-J.; Shin, S.-H.; Hong, G.-R.; Lee, S.; Kim, M.; Yun, S.-C.; Song, J.-M.; Park, S.-W.; Kim, J.-J. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019, 139, 1354–1365. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R. Pulmonary hypertension in heart failure. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef]
- Ghio, S.; Raineri, C.; Scelsi, L.; Ašanin, M.; Polovina, M.; Seferovic, P. Pulmonary hypertension and right ventricular remodeling in HFpEF and HFrEF. Heart Fail. Rev. 2019, 25, 85–91. [Google Scholar] [CrossRef]
- Monitillo, F.; Di Terlizzi, V.; Gioia, M.I.; Barone, R.; Grande, D.; Parisi, G.; Brunetti, N.D.; Iacoviello, M. Right ventricular function in chronic heart failure: From the diagnosis to the therapeutic approach. J. Cardiovasc. Dev. Dis. 2020, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.; Dewachter, C.; McEntee, K.; Fesler, P.; Brimioulle, S.; Naeije, R. Early right ventriculo-arterial uncoupling in borderline pulmonary hypertension on experimental heart failure. J. Appl. Physiol. 2010, 109, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Shaw, L.K.; O’Connor, C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J. Am. Coll. Cardiol. 2002, 39, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Ghio, S.; Recusani, F.; Klersy, C.; Sebastiani, R.; Laudisa, M.L.; Campana, C.; Gavazzi, A.; Tavazzi, L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am. J. Cardiol. 2000, 85, 837–842. [Google Scholar] [CrossRef]
- Damy, T.; Kallvikbacka-Bennett, A.; Goode, K.; Khaleva, O.; LeWinter, C.; Hobkirk, J.; Nikitin, N.P.; Dubois-Randé, J.-L.; Hittinger, L.; Clark, A.L.; et al. Prevalence of, associations with, and prognostic value of Tricuspid Annular Plane Systolic Excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J. Card. Fail. 2012, 18, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, A.; Lewis, R.; Hickey, P.; Durrington, C.; Elliot, C.; Condliffe, R.; Sabroe, I.; Kiely, D.G. Pathophysiology and diagnosis of pulmonary hypertension due to left heart disease. Front. Med. 2018, 5, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Maeder, M.T.; Schoch, O.D.; Kleiner, R.; Joerg, L.; Weilenmann, D.; Hypertension, S.S.F.P. Pulmonary hypertension associated with left-sided heart disease. Swiss Med. Wkly. 2017, 147, w14395. [Google Scholar]
- Assad, T.R.; Hemnes, A.R.; Larkin, E.K.; Glazer, A.M.; Xu, M.; Wells, Q.S.; Farber-Eger, E.H.; Sheng, Q.; Shyr, Y.; Harrell, F.E.; et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J. Am. Coll. Cardiol. 2016, 68, 2525–2536. [Google Scholar] [CrossRef]
- Al-Omary, M.S.; Sugito, S.; Boyle, A.J.; Sverdlov, A.; Collins, N.J. Pulmonary hypertension due to left heart disease. Hypertension 2020, 75, 1397–1408. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Siwamogsatham, S.; Hayek, S.S.; Li, S.; Deka, A.; Marti, C.N.; Georgiopoulou, V.V.; Butler, J. Echocardiographic assessment of pulmonary artery systolic pressure and outcomes in ambulatory heart failure patients. J. Am. Heart Assoc. 2014, 3, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meluzín, J.; Spinarová, L.; Hude, P.; Krejci, J.; Kincl, V.; Panovsky, R.; Dušek, L. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J. Am. Soc. Echocardiogr. 2005, 18, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Boulate, D.; Mercier, O.; Guihaire, J.; Fadel, E.; Naeije, R.; Haddad, F.; Rischard, F. Pulmonary circulatory—Right ventricular uncoupling: New insights into pulmonary hypertension pathophysiology. In Pulmonary Hypertension; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 241–253. [Google Scholar]
- Teramoto, K.; Sengelov, M.; West, E.; Santos, M.; Nadruz, W.; Skali, H.; Shah, A.M. Association of pulmonary hypertension and right ventricular function with exercise capacity in heart failure. ESC Heart Fail. 2020, 7, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Monitillo, F.; Citarelli, G.; Leone, M.; Grande, D.; Antoncecchi, V.; Rizzo, C.; Terlizzese, P.; Romito, R.; Caldarola, P.; et al. Right ventriculo-arterial coupling assessed by two-dimensional strain: A new parameter of right ventricular function independently associated with prognosis in chronic heart failure patients. Int. J. Cardiol. 2017, 241, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Vonk-Noordegraaf, A.; Westerhof, N. Describing right ventricular function. Eur. Respir. J. 2013, 41, 1419–1423. [Google Scholar] [CrossRef] [Green Version]
- Brimioulle, S.; Wauthy, P.; Ewalenko, P.; Rondelet, B.; Vermeulen, F.; Kerbaul, F.; Naeije, R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am. J. Physiol. Circ. Physiol. 2003, 284, H1625–H1630. [Google Scholar] [CrossRef]
- Smolarek, D.; Gruchała, M.; Sobiczewski, W. Echocardiographic evaluation of right ventricular systolic function: The traditional and innovative approach. Cardiol. J. 2017, 24, 563–572. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; D’Ascenzi, F.; Mandoli, G.E.; Rizzo, L.; Sisti, N.; Carrucola, C.; Cameli, P.; Bigio, E.; Mondillo, S.; Cameli, M. Traditional and novel imaging of right ventricular function in patients with heart failure and reduced ejection fraction. Curr. Heart Fail. Rep. 2020, 17, 28–33. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R.; Arena, R.; Corrà, U.; Ghio, S.; Forfia, P.; Rossi, A.; Cahalin, L.P.; Bandera, F.; Temporelli, P. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest 2015, 148, 226–234. [Google Scholar] [CrossRef]
- Tatli, E.; Kurum, T.; Aktoz, M.; Buyuklu, M. Effects of carvedilol on right ventricular ejection fraction and cytokines levels in patients with systolic heart failure. Int. J. Cardiol. 2008, 125, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bom, T.; Winter, M.M.; Bouma, B.J.; Groenink, M.; Vliegen, H.W.; Pieper, P.G.; Van Dijk, A.P.J.; Sieswerda, G.T.; Roos-Hesselink, J.W.; Zwinderman, A.H.; et al. Effect of valsartan on systemic right ventricular function. Circulation 2013, 127, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therrien, J.; Provost, Y.; Harrison, J.; Connelly, M.; Kaemmerer, H.; Webb, G.D. Effect of angiotensin receptor blockade on systemic right ventricular function and size: A small, randomized, placebo-controlled study. Int. J. Cardiol. 2008, 129, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kia, D.S.; Benza, E.; Bachman, T.N.; Tushak, C.; Kim, K.; Simon, M.A. Angiotensin receptor-neprilysin inhibition attenuates right ventricular remodeling in pulmonary hypertension. J. Am. Heart Assoc. 2020, 9, e015708. [Google Scholar] [CrossRef]
- Correale, M.; Mallardi, A.; Mazzeo, P.; Tricarico, L.; Diella, C.; Romano, V.; Ferraretti, A.; Leopizzi, A.; Merolla, G.; Di Biase, M.; et al. Sacubitril/valsartan improves right ventricular function in a real-life population of patients with chronic heart failure: The daunia heart failure registry. Int. J. Cardiol. Heart Vasc. 2020, 27, 100486. [Google Scholar] [CrossRef]
- Vicent, L.; Ayesta, A.; Esteban-Fernández, A.; Gómez-Bueno, M.; De-Juan, J.; Díez-Villanueva, P.; Iniesta, Á.M.; Rojas-González, A.; Bover-Freire, R.; Iglesias, D.; et al. Sex influence on the efficacy and safety of Sacubitril/Valsartan. Cardiology 2019, 142, 73–78. [Google Scholar] [CrossRef]
- Bayard, G.; Da Costa, A.; Pierrard, R.; Roméyer-Bouchard, C.; Guichard, J.B.; Isaaz, K. Impact of sacubitril/valsartan on echo parameters in heart failure patients with reduced ejection fraction a prospective evaluation. Int. J. Cardiol. Heart Vasc. 2019, 25, 100418. [Google Scholar] [CrossRef]
- Almufleh, A.; Marbach, J.; Chih, S.; Stadnick, E.; Davies, R.; Liu, P.; Mielniczuk, L. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/Valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017, 7, 108–113. [Google Scholar]
| Variable | Overall Population (n = 163) |
|---|---|
| Age (mean ± SD) | 57.9 ± 12.3 years |
| Female sex (n, %) | 52 (31.9%) |
| Ischemic (n, %) | 83 (50.9%) |
| Hypertension (n, %) | 102 (62.5%) |
| Diabetes (n, %) | 79 (48.4%) |
| COPD (n, %) | 61(37.4%) |
| NYHA II (n, %) | 112 (68.7%) |
| NYHA III (n, %) | 51 (31.3%) |
| Systolic blood pressure (mean ± SD) | 119 ± 14.8 mmHg |
| Diastolic blood pressure (mean ± SD) | 72.7 ± 9.3 mmHg |
| Heart rate (mean ± SD) | 68.2 ± 14.6 bpm |
| LVEDV (mean ± SD) | 237.2 ± 87.6 mL |
| LVESV (mean ± SD) | 179.5 ± 65.3 mL |
| LVEF (mean ± SD) | 28.9 ± 6.4% |
| E/e’ average (mean ± SD) | 14.5 ± 4.8 cm/sec |
| LAVI | 37.6 ± 5.2 mL/m2 |
| Creatinine (mean ± SD) | 1.3 ± 1.1 mg/dL |
| e-GFR (mean ± SD) | 63.6 ± 15.2 mL/min |
| NT-proBNP (mean ± SD) | 1716 ± 954 pg/mL |
| Loop diuretic (n, %) | 118 (72.3%) |
| Furosemide dose (mean ± SD) | 75 ± 25 mg |
| Beta-blockers (n, %) | 163 (100%) |
| Carvedilol dose (mean ± SD) | 37.5 ± 612.5 mg |
| Bisoprolol dose (mean ± SD) | 5 ± 3.75 mg |
| ACEi/ARBs (n, %) | 158 (96.9%) |
| Ramipril dose (mean ± SD) | 5 ± 3.75 mg |
| Valsartan dose (mean ± SD) | 120 ± 80 mg |
| MRA (n, %) | 105 (64.4%) |
| Eplerenone dose (mean ± SD) | 25 ± 12.5 mg |
| Ivabradine (n, %) | 30 (18.4%) |
| Ivabradine dose (mean ± SD) | 10 ± 5 mg |
| Digoxin (n, %) | 35 (23.3%) |
| Digoxin dose (mean ± SD) | 0.09375 ± 0.0625 mg |
| Variable | Baseline | 1-Year Follow-Up | p | 2 Years Follow-Up | p |
|---|---|---|---|---|---|
| LVEDV (mean ± SD) | 237.2 ± 87.6 mL | 213.3 ± 64.8 mL | <0.05 | 208.4 + 52.4 mL | <0.05 |
| LVESV (mean ± SD) | 179.5 ± 65.3 mL | 165.4 ± 52.7 mL | <0.05 | 157.9 ± 45.2 mL | <0.05 |
| LVEF (mean ± SD) | 28.9 ± 6.4% | 31.5 ± 6.2% | <0.05 | 33.4% ± 4.8% | <0.01 |
| LAVI (mean ± SD) | 37.6 ± 5.2 mL/m2 | 34.1 ± 4.4 mL/m2 | <0.01 | 31.8 ± 3.9 mL/m2 | <0.01 |
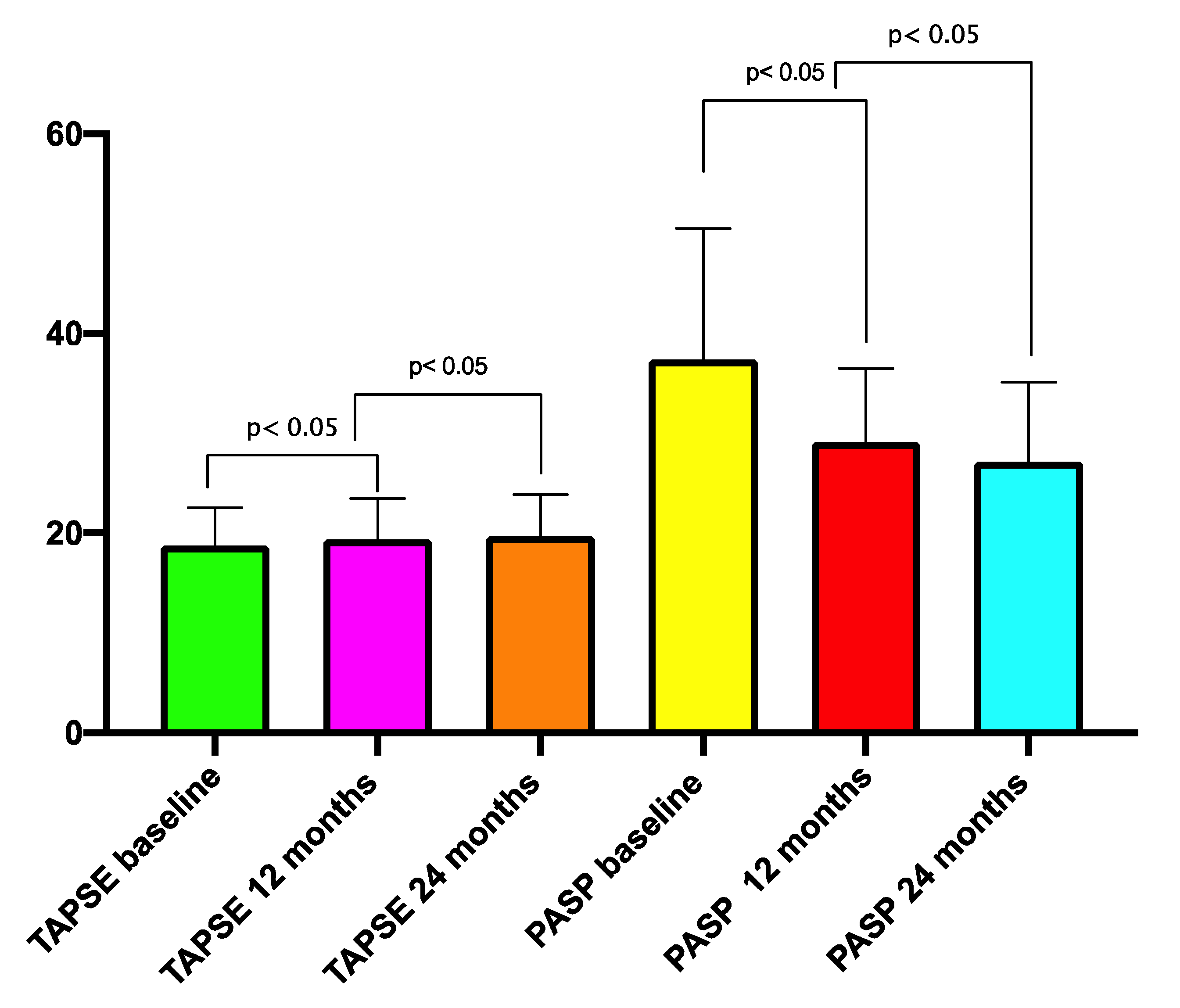
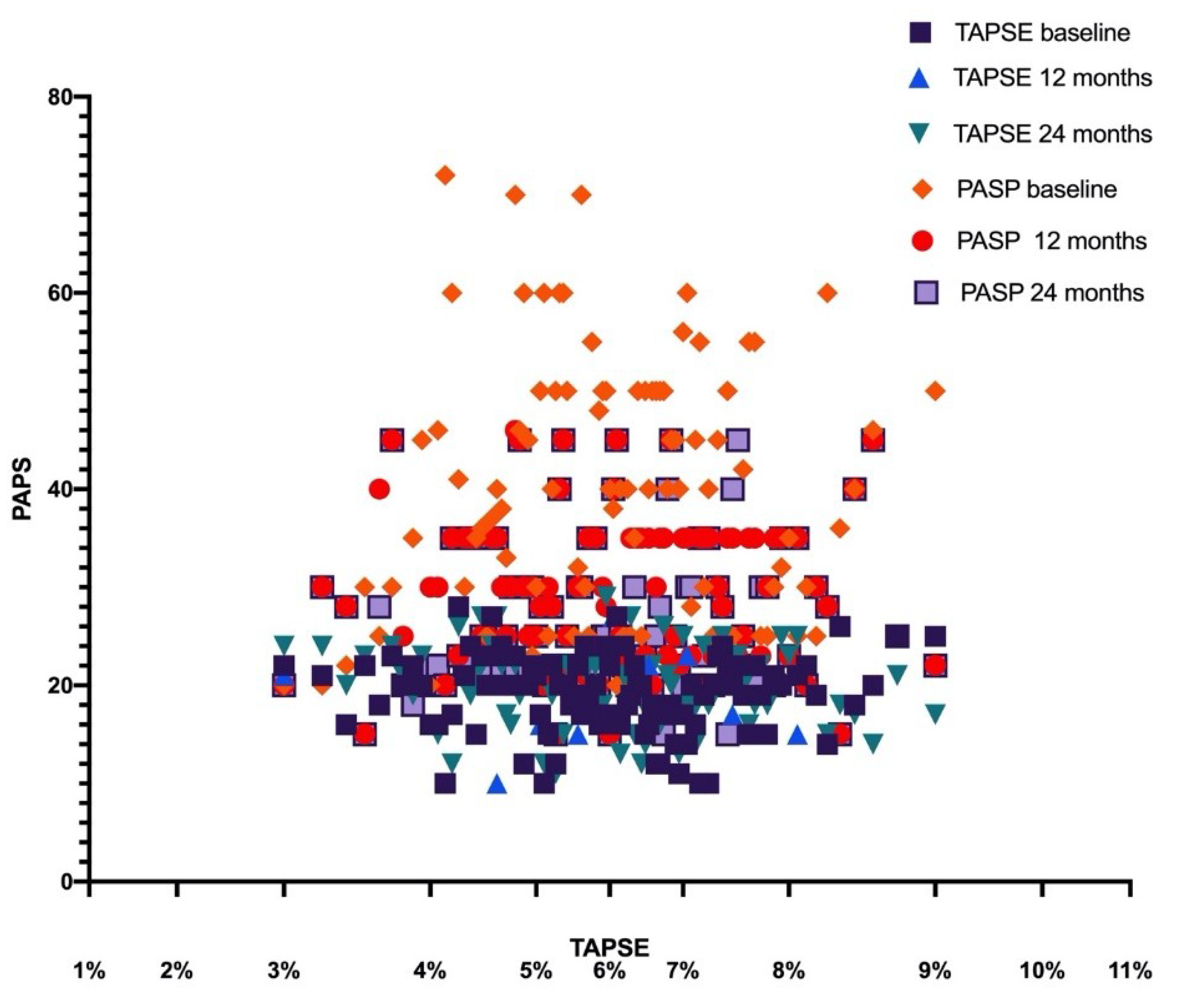
| TAPSE (mean ± SD) | 18.76 ± 3.7 mm | 19.3 ± 3.2 mm | <0.05 | 19.6 ± 6.8 mm | <0.05 |
| mPAP (mean ± SD) | 24.1 + 12.6 mmHg | 22.7 ± 10.9 mmHg | <0.05 | 20.8 + 11.3 mmHg | <0.05 |
| PASP (mean ± SD) | 38.3 ± 15.7 mmHg | 29.1 ± 14.8 mmHg | <0.01 | 27.3 ± 13.6 mmHg | <0.01 |
| Variable | Mean + SD | Β | t | p |
|---|---|---|---|---|
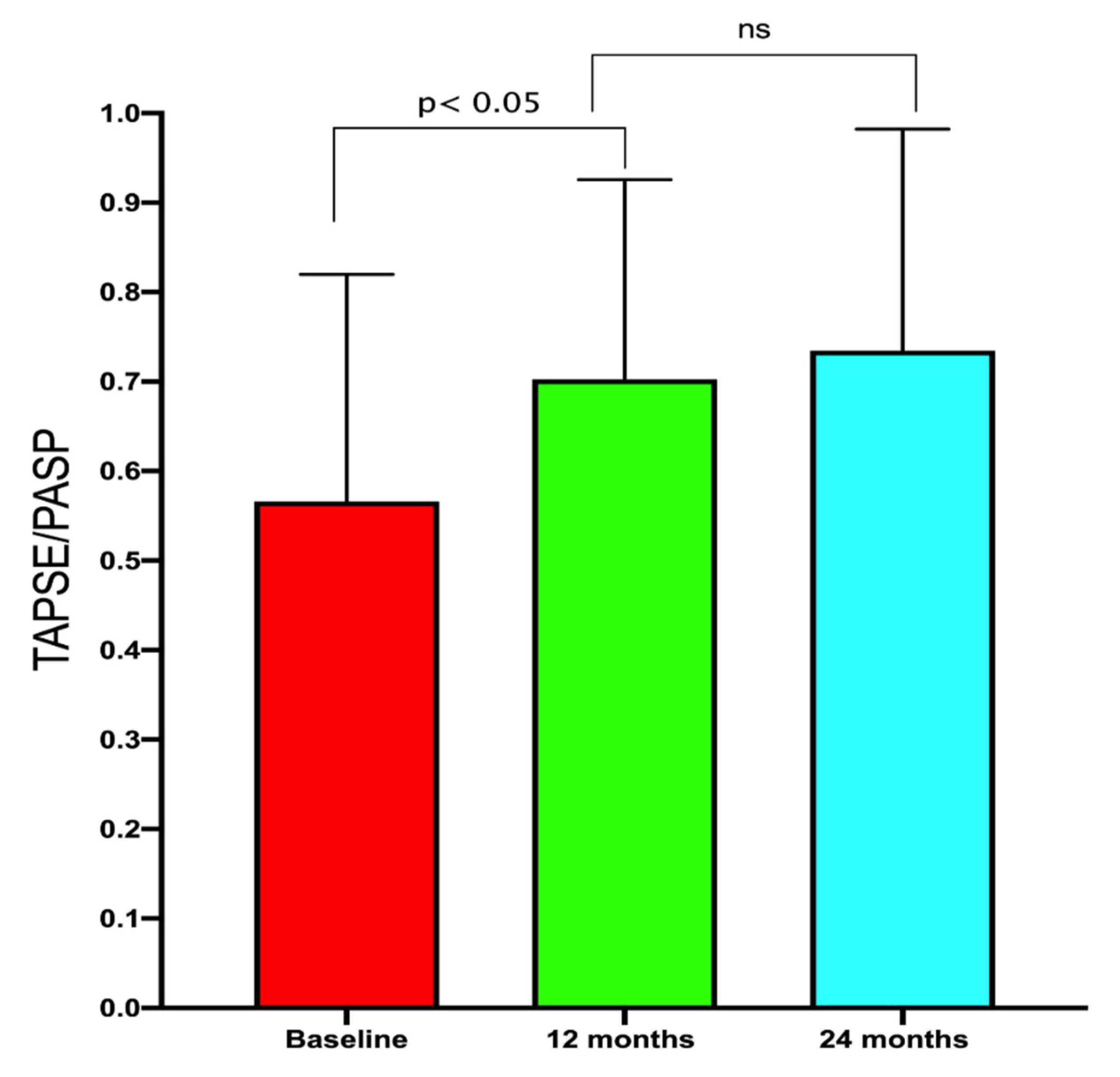
| Δ RV-PA coupling | 0.16 ± 0.03 | - | - | - |
| Δ LVEDV | 29.6 ± 15.8 mL | −0.058 | 0.190 | 0.849 |
| Δ LVESV | 21.68 ± 8.36 mL | 0.017 | 0.386 | 0.700 |
| Δ LVEF | 4.5 ± 0.9% | 0.186 | 0.391 | 0.697 |
| Δ E/e’ average | 3.58 ± 1.52 cm/s | −0.381 | 0.186 | 0.852 |
| Δ LAVI | 6.38 ± 2.5 mL/m2 | 3.075 | 0.2378 | 0.045 |
| Δ NT-pro BNP | 474.4 ± 254.6 pg/mL | −0.071 | 0.390 | 0.697 |
| Δ mPAP | 3.33 ± 1.65 | 0.014 | 0.869 | 0.993 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masarone, D.; Errigo, V.; Melillo, E.; Valente, F.; Gravino, R.; Verrengia, M.; Ammendola, E.; Vastarella, R.; Pacileo, G. Effects of Sacubitril/Valsartan on the Right Ventricular Arterial Coupling in Patients with Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2020, 9, 3159. https://doi.org/10.3390/jcm9103159
Masarone D, Errigo V, Melillo E, Valente F, Gravino R, Verrengia M, Ammendola E, Vastarella R, Pacileo G. Effects of Sacubitril/Valsartan on the Right Ventricular Arterial Coupling in Patients with Heart Failure with Reduced Ejection Fraction. Journal of Clinical Medicine. 2020; 9(10):3159. https://doi.org/10.3390/jcm9103159
Chicago/Turabian StyleMasarone, Daniele, Vittoria Errigo, Enrico Melillo, Fabio Valente, Rita Gravino, Marina Verrengia, Ernesto Ammendola, Rossella Vastarella, and Giuseppe Pacileo. 2020. "Effects of Sacubitril/Valsartan on the Right Ventricular Arterial Coupling in Patients with Heart Failure with Reduced Ejection Fraction" Journal of Clinical Medicine 9, no. 10: 3159. https://doi.org/10.3390/jcm9103159





