Hypercapnia: An Aggravating Factor in Asthma
Abstract
1. Introduction
2. CO2 Sensing and Respiration
2.1. Central CO2 Chemosensing
2.1.1. pH-Sensitive Ion Channel
2.1.2. CO2-Sensitive Connexin Protein
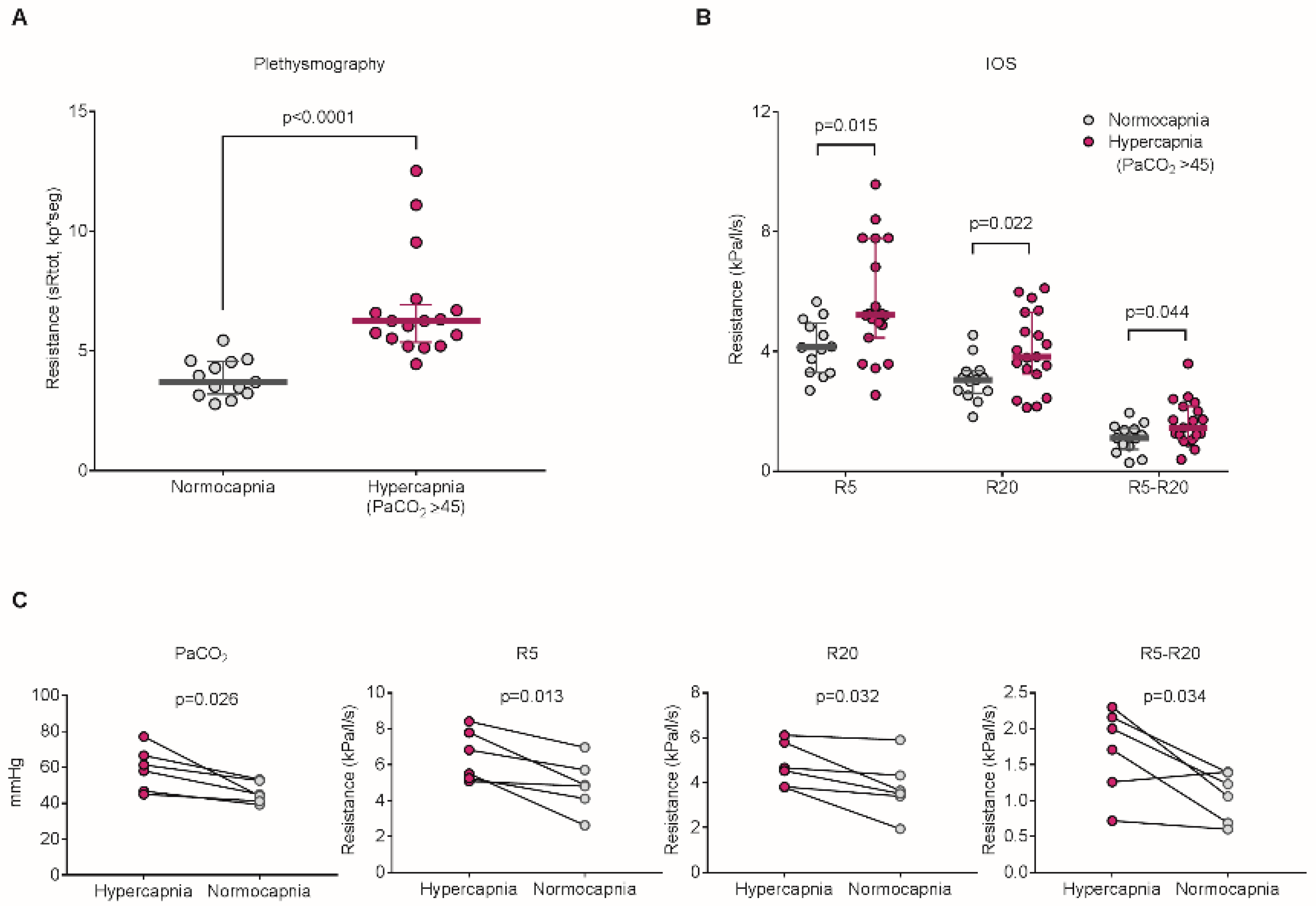
3. Hypercapnia in Asthmatic Patients
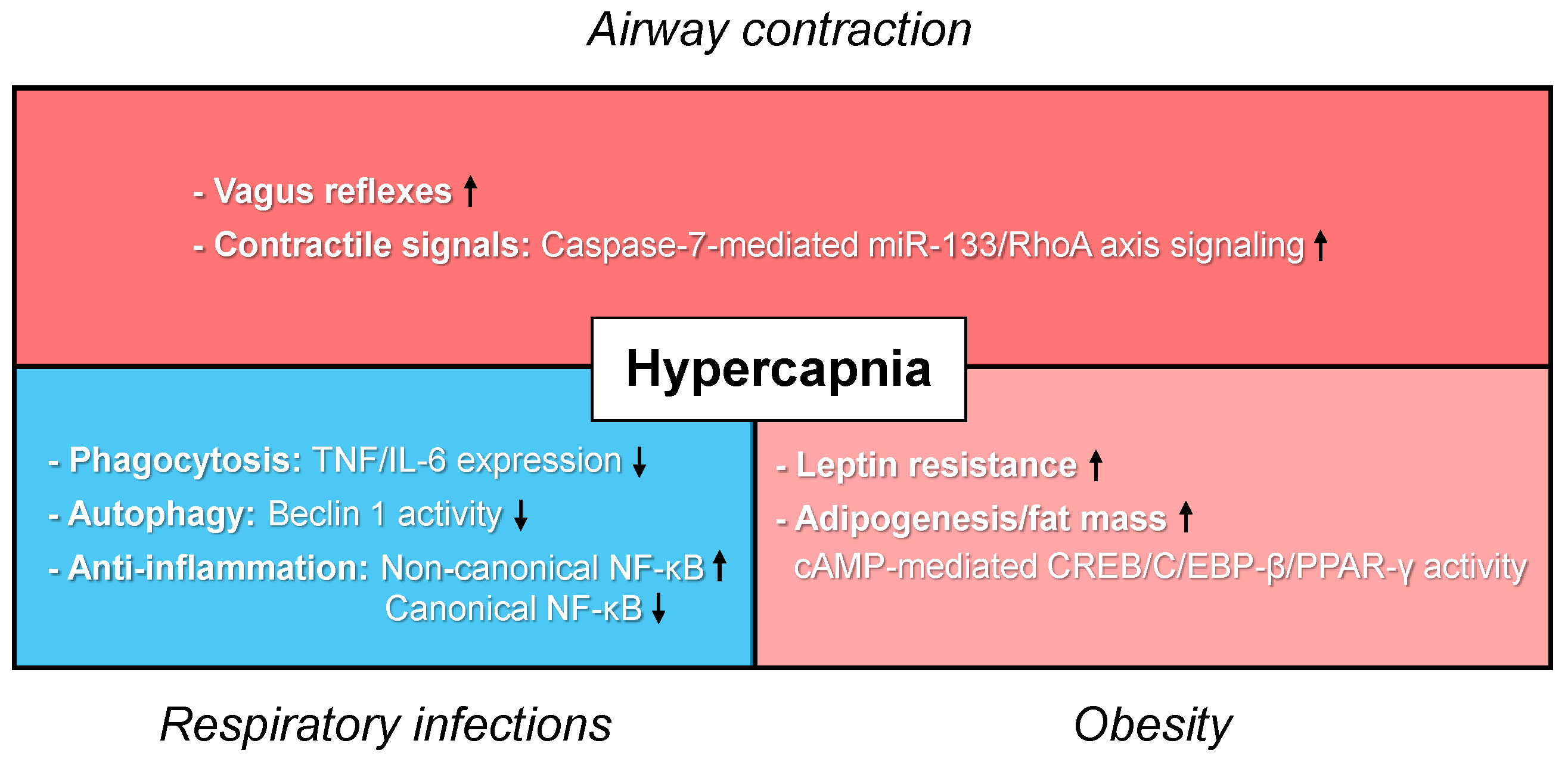
4. Detrimental Effects of Hypercapnia in Asthma
4.1. Lung Airways
4.1.1. Hypercapnia
4.1.2. Respiratory Acidosis
4.2. Innate Immunity
4.3. Adipogenesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GINA 2020. Available online: www.ginasthma.org (accessed on 11 September 2020).
- World Health Organization. Asthma. Available online: https://www.who.int/news-room/q-a-detail/asthma (accessed on 11 September 2020).
- Scala, R. Noninvasive ventilation in severe acute asthma? Still far from the truth. Respir. Care 2010, 55, 630–637. [Google Scholar] [PubMed]
- Green, E.; Jain, P.; Bernoth, M. Noninvasive ventilation for acute exacerbations of asthma: A systematic review of the literature. Aust. Crit. Care 2017, 30, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Keogh, B.; Chung, K.F.; Ayres, J.G.; Harrison, D.A.; Goldfrad, C.; Brady, A.R.; Rowan, K. Characteristics and outcome for admissions to adult, general critical care units with acute severe asthma: A secondary analysis of the ICNARC Case Mix Programme Database. Crit. Care 2004, 8, R112. [Google Scholar] [CrossRef] [PubMed]
- Stow, P.J.; Pilcher, D.; Wilson, J.; George, C.; Bailey, M.; Higlett, T.; Bellomo, R.; Hart, G.K.; the Australian & New Zealand Intensive Care Society Adult Patient Database Management Committee. Improved outcomes from acute severe asthma in Australian intensive care units (1996–2003). Thorax 2007, 62, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, M.; Lecuona, E.; Sznajder, J.I. Effects of hypercapnia on the lung. J. Physiol. 2017, 595, 2431–2437. [Google Scholar] [CrossRef]
- McFadden, E.R., Jr.; Lyons, H.A. Arterial-blood gas tension in asthma. N. Engl. J. Med. 1968, 278, 1027–1032. [Google Scholar] [CrossRef]
- Menitove, S.M.; Goldring, R.M. Combined ventilator and bicarbonate strategy in the management of status asthmaticus. Am. J. Med. 1983, 74, 898–901. [Google Scholar] [CrossRef]
- Darioli, R.; Perret, C. Mechanical controlled hypoventilation in status asthmaticus. Am. Rev. Respir. Dis. 1984, 129, 385–387. [Google Scholar]
- Mountain, R.D.; Sahn, S.A. Clinical features and outcome in patients with acute asthma presenting with hypercapnia. Am. Rev. Respir. Dis. 1988, 138, 535–539. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Factor, P.; Schwartz, D.E.; Sznajder, J.I. Severe status asthmaticus: Management with permissive hypercapnia and inhalation anesthesia. Crit. Care Med. 2002, 30, 477–480. [Google Scholar] [CrossRef]
- Adnet, F.; Plaisance, P.; Borron, S.W.; Levy, A.; Payen, D. Prolonged severe hypercapnia complicating near fatal asthma in a 35-year-old woman. Intensive Care Med. 1998, 24, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.T.; Spada, A.; Pratico, C.; Lucanto, T.; Santamaria, L.B. Hypercapnia: What is the limit in paediatric patients? A case of near-fatal asthma successfully treated by multipharmacological approach. Paediatr. Anaesth. 2004, 14, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, S.M.; Harrison, R. Subarachnoid hemorrhage in a child with status asthmaticus: Significance of permissive hypercapnia. Pediatr. Crit. Care Med. 2003, 4, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Tatsumi, H.; Goto, K.; Imaizumi, H.; Yoshida, S.; Kimijima, T.; Yamakage, M. Treatment of life-threatening hypercapnia with isoflurane in an infant with status asthmaticus. J. Anesth. 2014, 28, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Zender, H.O.; Eggimann, P.; Bulpa, P.; Chevrolet, J.C.; Jolliet, P. Quadriparesia following permissive hypercapnia and inhalational anesthesia in a patient with severe status asthmaticus. Intensive Care Med. 1996, 22, 1001. [Google Scholar] [CrossRef]
- Hickling, K.G.; Walsh, J.; Henderson, S.; Jackson, R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: A prospective study. Crit. Care Med. 1994, 22, 1568–1578. [Google Scholar] [CrossRef]
- Laffey, J.G.; Kavanagh, B.P. Carbon Dioxide and the Critically Ill—Too Little of a Good Thing? Lancet 1999, 354, 1283–1286. [Google Scholar] [CrossRef]
- ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Contreras, M.; Masterson, C.; Laffey, J.G. Permissive hypercapnia: What to remember. Curr. Opin. Anaesthesiol. 2015, 28, 26–37. [Google Scholar] [CrossRef]
- Nin, N.; Muriel, A.; Penuelas, O.; Brochard, L.; Lorente, J.A.; Ferguson, N.D.; Raymondos, K.; Rios, F.; Violi, D.A.; Thille, A.W.; et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, I.; Dada, L.A.; Briva, A.; Trejo, H.E.; Welch, L.C.; Chen, J.; Toth, P.T.; Lecuona, E.; Witters, L.A.; Schumacker, P.T.; et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Investig. 2008, 118, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.L.; Howell, H.A.; Nair, A.; Vohwinkel, C.U.; Welch, L.C.; Beitel, G.J.; Hauser, A.R.; Sznajder, J.I.; Sporn, P.H. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am. J. Respir. Cell Mol. Biol. 2013, 49, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, M.; Lecuona, E.; Angulo, M.; Homma, T.; Rodriguez, D.A.; Gonzalez-Gonzalez, F.J.; Welch, L.C.; Amarelle, L.; Kim, S.J.; Kaminski, N.; et al. Hypercapnia increases airway smooth muscle contractility via caspase-7-mediated miR-133a-RhoA signaling. Sci. Transl. Med. 2018, 10, eaat1662. [Google Scholar] [CrossRef]
- Bharat, A.; Graf, N.; Mullen, A.; Kanter, J.; Andrei, A.C.; Sporn, P.H.; DeCamp, M.M.; Sznajder, J.I. Pleural Hypercarbia After Lung Surgery Is Associated with Persistent Alveolopleural Fistulae. Chest 2016, 149, 220–227. [Google Scholar] [CrossRef]
- Bharat, A.; Angulo, M.; Sun, H.; Akbarpour, M.; Alberro, A.; Cheng, Y.; Shigemura, M.; Berdnikov, S.; Welch, L.C.; Kanter, J.A.; et al. High CO2 Levels Impair Lung Wound Healing. Am. J. Respir. Cell Mol. Biol. 2020, 63, 244–254. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Chen, F.; Gonzalez-Gonzalez, F.J.; Nair, A.; Dib, S.; Yemelyanov, A.; Gates, K.L.; Budinger, G.R.S.; Beitel, G.J.; Sporn, P.H.S. Hypercapnia Suppresses Macrophage Antiviral Activity and Increases Mortality of Influenza A Infection via Akt1. J. Immunol. 2020, 205, 489–501. [Google Scholar] [CrossRef]
- Jaitovich, A.; Angulo, M.; Lecuona, E.; Dada, L.A.; Welch, L.C.; Cheng, Y.; Gusarova, G.; Ceco, E.; Liu, C.; Shigemura, M.; et al. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1). J. Biol. Chem. 2015, 290, 9183–9194. [Google Scholar] [CrossRef]
- Korponay, T.C.; Balnis, J.; Vincent, C.E.; Singer, D.V.; Chopra, A.; Adam, A.P.; Ginnan, R.; Singer, H.A.; Jaitovich, A. High CO2 Downregulates Skeletal Muscle Protein Anabolism via AMP-activated Protein Kinase alpha2-mediated Depressed Ribosomal Biogenesis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 74–86. [Google Scholar] [CrossRef]
- Balnis, J.; Korponay, T.C.; Jaitovich, A. AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD). Int. J. Mol. Sci. 2020, 21, 955. [Google Scholar] [CrossRef]
- Wang, N.; Gates, K.L.; Trejo, H.; Favoreto, S., Jr.; Schleimer, R.P.; Sznajder, J.I.; Beitel, G.J.; Sporn, P.H. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010, 24, 2178–2190. [Google Scholar] [CrossRef] [PubMed]
- Cummins, E.P.; Oliver, K.M.; Lenihan, C.R.; Fitzpatrick, S.F.; Bruning, U.; Scholz, C.C.; Slattery, C.; Leonard, M.O.; McLoughlin, P.; Taylor, C.T. NF-kappaB links CO2 sensing to innate immunity and inflammation in mammalian cells. J. Immunol. 2010, 185, 4439–4445. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Lenihan, C.R.; Bruning, U.; Cheong, A.; Laffey, J.G.; McLoughlin, P.; Taylor, C.T.; Cummins, E.P. Hypercapnia induces cleavage and nuclear localization of RelB protein, giving insight into CO2 sensing and signaling. J. Biol. Chem. 2012, 287, 14004–14011. [Google Scholar] [CrossRef] [PubMed]
- Casalino-Matsuda, S.M.; Nair, A.; Beitel, G.J.; Gates, K.L.; Sporn, P.H. Hypercapnia Inhibits Autophagy and Bacterial Killing in Human Macrophages by Increasing Expression of Bcl-2 and Bcl-xL. J. Immunol. 2015, 194, 5388–5396. [Google Scholar] [CrossRef]
- Lu, Z.; Casalino-Matsuda, S.M.; Nair, A.; Buchbinder, A.; Budinger, G.R.S.; Sporn, P.H.S.; Gates, K.L. A role for heat shock factor 1 in hypercapnia-induced inhibition of inflammatory cytokine expression. FASEB J. 2018, 32, 3614–3622. [Google Scholar] [CrossRef]
- Li, G.; Zhou, D.; Vicencio, A.G.; Ryu, J.; Xue, J.; Kanaan, A.; Gavrialov, O.; Haddad, G.G. Effect of carbon dioxide on neonatal mouse lung: A genomic approach. J. Appl. Physiol. 2006, 101, 1556–1564. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Wang, N.; Ruhoff, P.T.; Matsuda, H.; Nlend, M.C.; Nair, A.; Szleifer, I.; Beitel, G.J.; Sznajder, J.I.; Sporn, P.H.S. Hypercapnia Alters Expression of Immune Response, Nucleosome Assembly and Lipid Metabolism Genes in Differentiated Human Bronchial Epithelial Cells. Sci. Rep. 2018, 8, 13508. [Google Scholar] [CrossRef]
- Shigemura, M.; Lecuona, E.; Angulo, M.; Dada, L.A.; Edwards, M.B.; Welch, L.C.; Casalino-Matsuda, S.M.; Sporn, P.H.S.; Vadasz, I.; Helenius, I.T.; et al. Elevated CO2 regulates the Wnt signaling pathway in mammals, Drosophila melanogaster and Caenorhabditis elegans. Sci. Rep. 2019, 9, 18251. [Google Scholar] [CrossRef]
- Briva, A.; Vadasz, I.; Lecuona, E.; Welch, L.C.; Chen, J.; Dada, L.A.; Trejo, H.E.; Dumasius, V.; Azzam, Z.S.; Myrianthefs, P.M.; et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE 2007, 2, e1238. [Google Scholar] [CrossRef]
- Vadasz, I.; Dada, L.A.; Briva, A.; Helenius, I.T.; Sharabi, K.; Welch, L.C.; Kelly, A.M.; Grzesik, B.A.; Budinger, G.R.; Liu, J.; et al. Evolutionary conserved role of c-Jun-N-terminal kinase in CO2-induced epithelial dysfunction. PLoS ONE 2012, 7, e46696. [Google Scholar] [CrossRef]
- Welch, L.C.; Lecuona, E.; Briva, A.; Trejo, H.E.; Dada, L.A.; Sznajder, J.I. Extracellular signal-regulated kinase (ERK) participates in the hypercapnia-induced Na, K-ATPase downregulation. FEBS Lett. 2010, 584, 3985–3989. [Google Scholar] [CrossRef] [PubMed]
- Dada, L.A.; Trejo Bittar, H.E.; Welch, L.C.; Vagin, O.; Deiss-Yehiely, N.; Kelly, A.M.; Baker, M.R.; Capri, J.; Cohn, W.; Whitelegge, J.P.; et al. High CO2 Leads to Na,K-ATPase Endocytosis via c-Jun Amino-Terminal Kinase-Induced LMO7b Phosphorylation. Mol. Cell. Biol. 2015, 35, 3962–3973. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lecuona, E.; Sun, H.; Chen, J.; Trejo, H.E.; Baker, M.A.; Sznajder, J.I. Protein kinase A-Ialpha regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO(2) concentrations. Am. J. Respir. Cell Mol. Biol. 2013, 48, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kryvenko, V.; Wessendorf, M.; Morty, R.E.; Herold, S.; Seeger, W.; Vagin, O.; Dada, L.A.; Sznajder, J.I.; Vadasz, I. Hypercapnia Impairs Na,K-ATPase Function by Inducing Endoplasmic Reticulum Retention of the beta-Subunit of the Enzyme in Alveolar Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 1467. [Google Scholar] [CrossRef] [PubMed]
- Doerr, C.H.; Gajic, O.; Berrios, J.C.; Caples, S.; Abdel, M.; Lymp, J.F.; Hubmayr, R.D. Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator-injured lungs. Am. J. Respir. Crit. Care Med. 2005, 171, 1371–1377. [Google Scholar] [CrossRef]
- O’Toole, D.; Hassett, P.; Contreras, M.; Higgins, B.D.; McKeown, S.T.; McAuley, D.F.; O’Brien, T.; Laffey, J.G. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax 2009, 64, 976–982. [Google Scholar] [CrossRef]
- Vohwinkel, C.U.; Lecuona, E.; Sun, H.; Sommer, N.; Vadász, I.; Chandel, N.S.; Sznajder, J.I. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011, 286, 37067–37076. [Google Scholar] [CrossRef]
- Cummins, E.P.; Strowitzki, M.J.; Taylor, C.T. Mechanisms and Consequences of Oxygen and Carbon Dioxide Sensing in Mammals. Physiol. Rev. 2020, 100, 463–488. [Google Scholar] [CrossRef]
- Monastersky, R. Global carbon dioxide levels near worrisome milestone. Nature 2013, 497, 13–14. [Google Scholar] [CrossRef]
- The Keeling Curve. Available online: https://scripps.ucsd.edu/programs/keelingcurve/ (accessed on 11 September 2020).
- Missner, A.; Pohl, P. 110 years of the Meyer-Overton rule: Predicting membrane permeability of gases and other small compounds. ChemPhysChem 2009, 10, 1405–1414. [Google Scholar] [CrossRef]
- Verkman, A.S. Role of aquaporins in lung liquid physiology. Respir. Physiol. Neurobiol. 2007, 159, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Musa-Aziz, R.; Chen, L.M.; Pelletier, M.F.; Boron, W.F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl. Acad. Sci. USA 2009, 106, 5406–5411. [Google Scholar] [CrossRef] [PubMed]
- Endeward, V.; Cartron, J.P.; Ripoche, P.; Gros, G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2008, 22, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Mitchell, R.A. Respiratory chemosensitivity in the medulla oblongata. J. Physiol. 1969, 202, 3P–4P. [Google Scholar]
- Mulkey, D.K.; Stornetta, R.L.; Weston, M.C.; Simmons, J.R.; Parker, A.; Bayliss, D.A.; Guyenet, P.G. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004, 7, 1360–1369. [Google Scholar] [CrossRef]
- Richerson, G.B.; Wang, W.; Tiwari, J.; Bradley, S.R. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir. Physiol. 2001, 129, 175–189. [Google Scholar] [CrossRef]
- Wang, W.; Richerson, G.B. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 1999, 90, 1001–1011. [Google Scholar] [CrossRef]
- Xu, F.; Frazier, D.T. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum 2002, 1, 35–40. [Google Scholar] [CrossRef]
- Smith, C.A.; Blain, G.M.; Henderson, K.S.; Dempsey, J.A. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2: Role of carotid body CO2. J. Physiol. 2015, 593, 4225–4243. [Google Scholar] [CrossRef]
- Bayliss, D.A.; Barhanin, J.; Gestreau, C.; Guyenet, P.G. The role of pH-sensitive TASK channels in central respiratory chemoreception. Pflugers Arch. 2015, 467, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Barhanin, J. Molecular physiology of pH-sensitive background K2P channels. Physiology 2011, 26, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Zhang, X.; Tadepalli, J.S.; Yu, L.; Gai, H.; Petit, J.; Pamulapati, R.T.; Jin, X.; Jiang, C. Involvement of TRP channels in the CO2 chemosensitivity of locus coeruleus neurons. J. Neurophysiol. 2011, 105, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Huckstepp, R.T.; id Bihi, R.; Eason, R.; Spyer, K.M.; Dicke, N.; Willecke, K.; Marina, N.; Gourine, A.V.; Dale, N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J. Physiol. 2010, 588, 3901–3920. [Google Scholar] [CrossRef]
- Meigh, L.; Greenhalgh, S.A.; Rodgers, T.L.; Cann, M.J.; Roper, D.I.; Dale, N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife 2013, 2, e01213. [Google Scholar] [CrossRef]
- Sullivan, W.J.; Dorman, P.J. The renal response to chronic respiratory acidosis. J. Clin. Investig. 1955, 34, 268–276. [Google Scholar] [CrossRef]
- Feihl, F.; Perret, C. Permissive hypercapnia. How permissive should we be? Am. J. Respir. Crit. Care Med. 1994, 150, 1722–1737. [Google Scholar] [CrossRef]
- Louie, S.; Morrissey, B.M.; Kenyon, N.J.; Albertson, T.E.; Avdalovic, M. The critically ill asthmatic—From ICU to discharge. Clin. Rev. Allergy Immunol. 2012, 43, 30–44. [Google Scholar] [CrossRef]
- Elsayegh, D.; Saito, S.; Eden, E.; Shapiro, J. Increasing severity of status asthmaticus in an urban medical intensive care unit. J. Hosp. Med. 2008, 3, 206–211. [Google Scholar] [CrossRef]
- Lumb, A.B. Nunn’s Applied Respiratory Physiology; foreword by Ronald Pearl; Elsevier: Edinburgh, UK, 2017. [Google Scholar]
- Rodrigo, C.; Rodrigo, G. Subarachnoid hemorrhage following permissive hypercapnia in a patient with severe acute asthma. Am. J. Emerg. Med. 1999, 17, 697–699. [Google Scholar] [CrossRef]
- Tang, W.C.; Weil, M.H.; Gazmuri, R.J.; Bisera, J.; Rackow, E.C. Reversible impairment of myocardial contractility due to hypercarbic acidosis in the isolated perfused rat heart. Crit. Care Med. 1991, 19, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Einthoven, W. Ueber die Wirkung der Bronchialmuskeln, nach einer neuen Methode untersucht, und über Asthma nervosum. Arch. Gesamte Physiol. Menschen Tiere 1892, 51, 367–445. [Google Scholar] [CrossRef]
- De Burgh Daly, M.; Lambertsen, D.C.; Schweitzer, A. The effects upon the bronchial musculature of altering the oxygen and carbon dioxide tensions of the blood perfusing the brain. J. Physiol. 1953, 119, 292–314. [Google Scholar] [CrossRef] [PubMed]
- Loofbourrow, G.N.; Wood, W.B.; Baird, I.L. Tracheal constriction in the dog. Am. J. Physiol. 1957, 191, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.A.; Widdicombe, J.G. Effect of changes in blood gas tensions and carotid sinus pressure on tracheal volume and total lung resistance to airflow. J. Physiol. 1962, 163, 13–33. [Google Scholar] [CrossRef]
- Green, M.; Widdicombe, J.G. The effects of ventilation of dogs with different gas mixtures on airway calibre and lung mechanics. J. Physiol. 1966, 186, 363–381. [Google Scholar] [CrossRef]
- Kondo, T.; Kobayashi, I.; Hayama, N.; Tazaki, G.; Ohta, Y. Role of cholinergic neural transmission on airway resistance in the dog. J. Auton. Nerv. Syst. 2000, 80, 64–70. [Google Scholar] [CrossRef]
- Dixon, W.E. Contributions to the physiology of the lungs: Part I. The bronchial muscles, their innervation, and the action of drugs upon them. J. Physiol. 1903, 29, 97–173. [Google Scholar] [CrossRef]
- Iscoe, S.; Fisher, J.T. Bronchomotor responses to hypoxia and hypercapnia in decerebrate cats. J. Appl. Physiol. 1995, 78, 117–123. [Google Scholar] [CrossRef]
- Sterling, G.M. The mechanism of decreased specific airway conductance in man during hypercapnia caused by inhalation of 7 percent CO2. Clin. Sci. 1969, 37, 539–548. [Google Scholar]
- Rodarte, J.R.; Hyatt, R.E. Effect of acute exposure to CO2 on lung mechanics in normal man. Respir. Physiol. 1973, 17, 135–145. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Swenson, E.W.; Finley, T.N.; Lategola, M.T.; Williams, J. Unilateral hypoventilation produced in dogs by occluding one pulmonary artery. J. Appl. Physiol. 1961, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Darke, C.S.; Astin, T.W. Differential ventilation in unilateral pulmonary artery occlusion. Thorax 1972, 27, 480–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Astin, T.W.; Barer, G.R.; Shaw, J.W.; Warren, P.M. The action of carbon dioxide on constricted airways. J. Physiol. 1973, 235, 607–623. [Google Scholar] [CrossRef]
- Sterling, G.M.; Holst, P.E.; Nadel, J.A. Effect of CO2 and pH on bronchoconstriction caused by serotonin vs. acetylcholine. J. Appl. Physiol. 1972, 32, 39–43. [Google Scholar] [CrossRef]
- Duckles, S.P.; Rayner, M.D.; Nadel, J.A. Effects of CO2 and pH on drug-induced contractions of airway smooth muscle. J. Pharmacol. Exp. Ther. 1974, 190, 472–481. [Google Scholar]
- Fisher, H.K.; Holton, P.; Buxton, R.S.; Nadel, J.A. Resistance to breathing during exercise-induced asthma attacks. Am. Rev. Respir. Dis. 1970, 101, 885–896. [Google Scholar]
- Fisher, H.K.; Hansen, T.A. Site of action of inhaled 6 per cent carbon dioxide in the lungs of asthmatic subjects before and after exercise. Am. Rev. Respir. Dis. 1976, 114, 861–870. [Google Scholar]
- Stephens, N.L.; Meyers, J.L.; Cherniack, R.M. Oxygen, carbon dioxide, H+ ion, and bronchial length-tension relationships. J. Appl. Physiol. 1968, 25, 376–383. [Google Scholar] [CrossRef]
- Croxton, T.L.; Lande, B.; Hirshman, C.A. Role of intracellular pH in relaxation of porcine tracheal smooth muscle by respiratory gases. Am. J. Physiol. 1995, 268, L207–L213. [Google Scholar] [CrossRef]
- Twort, C.H.; Cameron, I.R. Effects of PCO2, pH and extracellular calcium on contraction of airway smooth muscle from rats. Respir. Physiol. 1986, 66, 259–267. [Google Scholar] [CrossRef]
- Yamakage, M.; Lindeman, K.S.; Hirshman, C.A.; Croxton, T.L. Intracellular pH regulates voltage-dependent Ca2+ channels in porcine tracheal smooth muscle cells. Am. J. Physiol. 1995, 268, L642–L646. [Google Scholar] [CrossRef] [PubMed]
- El Mays, T.Y.; Saifeddine, M.; Choudhury, P.; Hollenberg, M.D.; Green, F.H. Carbon dioxide enhances substance P-induced epithelium-dependent bronchial smooth muscle relaxation in Sprague-Dawley rats. Can. J. Physiol. Pharmacol. 2011, 89, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, G.A.; Graf, P.D.; Nadel, J.A. Sympathetic versus parasympathetic nervous regulation of airways in dogs. J. Appl. Physiol. 1971, 31, 651–655. [Google Scholar] [CrossRef]
- Colebatch, H.J.; Halmagyi, D.F. Effect of Vagotomy and Vagal Stimulation on Lung Mechanics and Circulation. J. Appl. Physiol. 1963, 18, 881–887. [Google Scholar] [CrossRef]
- Woolcock, A.J.; Macklem, P.T.; Hogg, J.C.; Wilson, N.J.; Nadel, J.A.; Frank, N.R.; Brain, J. Effect of vagal stimulation on central and peripheral airways in dogs. J. Appl. Physiol. 1969, 26, 806–813. [Google Scholar] [CrossRef]
- Campbell, C.J.; Murtagh, J.A.; Raber, C.F. Chemical Agents and Reflex Control of Laryngeal Glottis. Ann. Otol. Rhinol. Laryngol. 1963, 72, 589–604. [Google Scholar] [CrossRef]
- Spann, R.W.; Hyatt, R.E. Factors affecting upper airway resistance in conscious man. J. Appl. Physiol. 1971, 31, 708–712. [Google Scholar] [CrossRef]
- Yamakage, M.; Kohro, S.; Yamauchi, M.; Namiki, A. The effects of extracellular pH on intracellular pH, Ca2+ and tension of canine tracheal smooth muscle strips. Life Sci. 1995, 56, PL175–PL180. [Google Scholar] [CrossRef]
- Wark, P.A.; Tooze, M.; Powell, H.; Parsons, K. Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology 2013, 18, 996–1002. [Google Scholar] [CrossRef]
- Oliver, B.G.; Robinson, P.; Peters, M.; Black, J. Viral infections and asthma: An inflammatory interface? Eur. Respir. J. 2014, 44, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Hojo, M.; Koketsu, R.; Watanabe, S.; Sato, A.; Chino, H.; Ro, S.; Masaki, H.; Hirashima, J.; Ishii, S.; et al. The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS ONE 2015, 10, e0123584. [Google Scholar] [CrossRef] [PubMed]
- Resiliac, J.; Grayson, M.H. Epidemiology of Infections and Development of Asthma. Immunol. Allergy Clin. N. Am. 2019, 39, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Laserna, E.; Sibila, O.; Aguilar, P.R.; Mortensen, E.M.; Anzueto, A.; Blanquer, J.M.; Sanz, F.; Rello, J.; Marcos, P.J.; Velez, M.I.; et al. Hypocapnia and hypercapnia are predictors for ICU admission and mortality in hospitalized patients with community-acquired pneumonia. Chest 2012, 142, 1193–1199. [Google Scholar] [CrossRef]
- Belkin, R.A.; Henig, N.R.; Singer, L.G.; Chaparro, C.; Rubenstein, R.C.; Xie, S.X.; Yee, J.Y.; Kotloff, R.M.; Lipson, D.A.; Bunin, G.R. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am. J. Respir. Crit. Care Med. 2006, 173, 659–666. [Google Scholar] [CrossRef]
- Sharabi, K.; Hurwitz, A.; Simon, A.J.; Beitel, G.J.; Morimoto, R.I.; Rechavi, G.; Sznajder, J.I.; Gruenbaum, Y. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 4024–4029. [Google Scholar] [CrossRef]
- Helenius, I.T.; Krupinski, T.; Turnbull, D.W.; Gruenbaum, Y.; Silverman, N.; Johnson, E.A.; Sporn, P.H.; Sznajder, J.I.; Beitel, G.J. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc. Natl. Acad. Sci. USA 2009, 106, 18710–18715. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Holguin, F.; Teague, W.G.; Brown, L.A. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J. Allergy Clin. Immunol. 2008, 121, 1372–1378. [Google Scholar] [CrossRef]
- Keogh, C.E.; Scholz, C.C.; Rodriguez, J.; Selfridge, A.C.; von Kriegsheim, A.; Cummins, E.P. Carbon dioxide-dependent regulation of NF-kappaB family members RelB and p100 gives molecular insight into CO2-dependent immune regulation. J. Biol. Chem. 2017, 292, 11561–11571. [Google Scholar] [CrossRef]
- Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001, 69, 522–530. [Google Scholar]
- Lang, C.J.; Dong, P.; Hosszu, E.K.; Doyle, I.R. Effect of CO2 on LPS-induced cytokine responses in rat alveolar macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L96–L103. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.E.; Kane, C.; Raff, H. Peripheral chemoreceptor control of fetal renin responses to hypoxia and hypercapnia. Circ. Res. 1990, 67, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Yilin, Z.; Yandong, N.; Faguang, J. Role of angiotensin-converting enzyme (ACE) and ACE2 in a rat model of smoke inhalation induced acute respiratory distress syndrome. Burns 2015, 41, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L. The Yin and Yang of ACE/ACE2 Pathways: The Rationale for the Use of Renin-Angiotensin System Inhibitors in COVID-19 Patients. Cells 2020, 9, 1704. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.G.; Lindberg, A.; Franklin, K.A.; Lundback, B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir. Med. 2001, 95, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Nyenhuis, S.M.; Weaver, T.E. Obstructive sleep apnea and asthma: Associations and treatment implications. Sleep Med. Rev. 2014, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.Z.; Subbarao, P.; Narang, I. The Bidirectional Relationship Between Asthma and Obstructive Sleep Apnea: Which Came First? J. Pediatr. 2016, 176, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.L.; Qin, Z.; Shen, H.; Jin, H.Y.; Wang, W.; Wang, Z.F. Association of Obstructive Sleep Apnea with Asthma: A Meta-Analysis. Sci. Rep. 2017, 7, 4088. [Google Scholar] [CrossRef]
- Redline, S.; Tishler, P.V.; Schluchter, M.; Aylor, J.; Clark, K.; Graham, G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am. J. Respir. Crit. Care Med. 1999, 159, 1527–1532. [Google Scholar] [CrossRef]
- Beuther, D.A.; Sutherland, E.R. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, P.; Padrao, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Moreira, A. Obesity increases the prevalence and the incidence of asthma and worsens asthma severity. Clin. Nutr. 2017, 36, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Mannino, D.; Brown, C.; Crocker, D.; Twum-Baah, N.; Holguin, F. Body mass index and asthma severity in the National Asthma Survey. Thorax 2008, 63, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mosen, D.M.; Schatz, M.; Magid, D.J.; Camargo, C.A., Jr. The relationship between obesity and asthma severity and control in adults. J. Allergy Clin. Immunol. 2008, 122, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Piper, A. Obesity hypoventilation syndrome: Weighing in on therapy options. Chest 2016, 149, 856–868. [Google Scholar]
- Kikuchi, R.; Tsuji, T.; Watanabe, O.; Yamaguchi, K.; Furukawa, K.; Nakamura, H.; Aoshiba, K. Hypercapnia Accelerates Adipogenesis: A Novel Role of High CO2 in Exacerbating Obesity. Am. J. Respir. Cell Mol. Biol. 2017, 57, 570–580. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Tankersley, C.; Kleeberger, S.; Russ, B.; Schwartz, A.; Smith, P. Modified control of breathing in genetically obese (ob/ob) mice. J. Appl. Physiol. 1996, 81, 716–723. [Google Scholar] [CrossRef]
- O’Donnell, C.P.; Schaub, C.D.; Haines, A.S.; Berkowitz, D.E.; Tankersley, C.G.; Schwartz, A.R.; Smith, P.L. Leptin prevents respiratory depression in obesity. Am. J. Respir. Crit. Care Med. 1999, 159, 1477–1484. [Google Scholar] [CrossRef]
- Caro, J.F.; Kolaczynski, J.W.; Nyce, M.R.; Ohannesian, J.P.; Opentanova, I.; Goldman, W.H.; Lynn, R.B.; Zhang, P.L.; Sinha, M.K.; Considine, R.V. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: A possible mechanism for leptin resistance. Lancet 1996, 348, 159–161. [Google Scholar] [CrossRef]
- Campo, A.; Fruhbeck, G.; Zulueta, J.J.; Iriarte, J.; Seijo, L.M.; Alcaide, A.B.; Galdiz, J.B.; Salvador, J. Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur. Respir. J. 2007, 30, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Köhnlein, T.; Windisch, W.; Köhler, D.; Drabik, A.; Geiseler, J.; Hartl, S.; Karg, O.; Laier-Groeneveld, G.; Nava, S.; Schönhofer, B.; et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med. 2014, 2, 698–705. [Google Scholar] [CrossRef]
- Murphy, P.B.; Rehal, S.; Arbane, G.; Bourke, S.; Calverley, P.M.A.; Crook, A.M.; Dowson, L.; Duffy, N.; Gibson, G.J.; Hughes, P.D.; et al. Effect of Home Noninvasive Ventilation With Oxygen Therapy vs Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation: A Randomized Clinical Trial. JAMA 2017, 317, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.M.; Villagra, A.; Blanch, L.; Fernandez, R. Non-invasive mechanical ventilation in status asthmaticus. Intensive Care Med. 2001, 27, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Tomii, K.; Seo, R.; Tachikawa, R.; Harada, Y.; Murase, K.; Kaji, R.; Takeshima, Y.; Hayashi, M.; Nishimura, T.; Ishihara, K. Impact of noninvasive ventilation (NIV) trial for various types of acute respiratory failure in the emergency department; decreased mortality and use of the ICU. Respir. Med. 2009, 103, 67–73. [Google Scholar] [CrossRef][Green Version]
- Nanchal, R.; Kumar, G.; Majumdar, T.; Taneja, A.; Patel, J.; Dagar, G.; Jacobs, E.R.; Whittle, J. Utilization of mechanical ventilation for asthma exacerbations: Analysis of a national database. Respir. Care 2014, 59, 644–653. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigemura, M.; Homma, T.; Sznajder, J.I. Hypercapnia: An Aggravating Factor in Asthma. J. Clin. Med. 2020, 9, 3207. https://doi.org/10.3390/jcm9103207
Shigemura M, Homma T, Sznajder JI. Hypercapnia: An Aggravating Factor in Asthma. Journal of Clinical Medicine. 2020; 9(10):3207. https://doi.org/10.3390/jcm9103207
Chicago/Turabian StyleShigemura, Masahiko, Tetsuya Homma, and Jacob I Sznajder. 2020. "Hypercapnia: An Aggravating Factor in Asthma" Journal of Clinical Medicine 9, no. 10: 3207. https://doi.org/10.3390/jcm9103207
APA StyleShigemura, M., Homma, T., & Sznajder, J. I. (2020). Hypercapnia: An Aggravating Factor in Asthma. Journal of Clinical Medicine, 9(10), 3207. https://doi.org/10.3390/jcm9103207




