A Decade of Progress in Deep Brain Stimulation of the Subcallosal Cingulate for the Treatment of Depression
Abstract
:1. Introduction
2. Outline of the Review
3. The Development of Deep Brain Stimulation as a Treatment for Depression
4. Clinical and Preclinical Studies of SCC DBS for the Treatment of Depression
4.1. Progress in the Development of SCC-DBS
4.2. Remission Rates
4.3. Significant Challenges in the Development of SCC-DBS
4.4. Adverse Effects
4.5. Stimulation Parameters
4.6. Electrode Implantation
4.7. Other Responses to DBS
5. Preclinical Studies of Electrical Stimulation in the Medial Prefrontal Cortex in Rodents
5.1. vmPFC Stimulation
5.2. Other Brain Targets
5.3. vmPFC-Linked Modulation of Other Structures
5.4. Synergism with Other Treatments
5.5. Other Biological Parameters Modulated
6. Potential Mechanisms of Stimulation-Induced Antidepressant-Like Activities
Neuroplasticity-Dependent Effects of Electrical Stimulation
(i) Neurogenesis is a Long-Term Cellular Change Brought About by Electrical Stimulation
(ii) Synaptic Plasticity is Altered More Rapidly by Electrical Stimulation than by Neurogenesis
(iii) Neurotrophin Signaling Underlies the Antidepressant-Like Effect of Electrical Stimulation
(iv) Potential Involvement of Glial Cells in Mediating the Outcome of Electrical Stimulation
Neuroplasticity-Independent Effects of Electrical Stimulation
(i) An Alternative Action of the Serotonergic System by Electrical Stimulation
(ii) Glutamatergic Neurotransmission is a Promising Target of Electrical Stimulation
7. Concerns and Limitations of the Electrical Stimulation Studies
8. Prospective Approaches to Enhance Deep Brain Stimulation Safety and Efficacy
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Collaborators. GBD 2017 Disease and Injury Incidence and Prevalence. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PloS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, P.E.; Fournier, A.-A.; Sisitsky, T.; Pike, C.T.; Kessler, R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 2015, 76, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Kendler, K.S.; Walters, E.E.; Neale, M.C.; Kessler, R.C.; Heath, A.C.; Eaves, L.J. The Structure of the Genetic and Environmental Risk Factors for Six Major Psychiatric Disorders in Women: Phobia, Generalized Anxiety Disorder, Panic Disorder, Bulimia, Major Depression, and Alcoholism. Arch. Gen. Psychiatry 1995, 52, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Gardner, C.O.; Lichtenstein, P. A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychol. Med. 2008, 38, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, M.; Rosanowski, F.; Eysholdt, U.; Kummer, P. Anxiety and depression in mothers of speech impaired children. Int. J. Pediatric Otorhinolaryngol. 2003, 67, 1337–1341. [Google Scholar] [CrossRef]
- Montero-Pedrazuela, A.; Venero, C.; Lavado-Autric, R.; Fernández-Lamo, I.; García-Verdugo, J.M.; Bernal, J.; Guadaño-Ferraz, A. Modulation of adult hippocampal neurogenesis by thyroid hormones: Implications in depressive-like behavior. Mol. Psychiatry 2006, 11, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo, P.T.; Falcão, A.A.; Verreschi, I.T.N.; Zanella, M.-T. The imbalance of sex-hormones related to depressive symptoms in obese men. Aging Male 2016, 19, 20–26. [Google Scholar] [CrossRef]
- Rohr, U.D. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002, 41, 25–46. [Google Scholar] [CrossRef]
- Malhi, G.S.; Bridges, P.K.; Malizia, A.L. Neurosurgery for mental disorders (NMD) A clinical worldwide perspective: Past, present and future. Int. J. Psychiatry Clin. Pract. 1997, 1, 119–129. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Alamo, C. Monoaminergic Neurotransmission: The History of the Discovery of Antidepressants from 1950s Until Today. Curr. Pharm. Des. 2009, 15, 1563–1586. [Google Scholar] [CrossRef] [Green Version]
- Baghai, T.C.; Blier, P.; Baldwin, D.S.; Bauer, M.; Goodwin, G.M.; Fountoulakis, K.N.; Kasper, S.; Leonard, B.E.; Malt, U.F.; Stein, D.; et al. General and comparative efficacy and effectiveness of antidepressants in the acute treatment of depressive disorders: A report by the WPA section of pharmacopsychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 207–245. [Google Scholar] [CrossRef]
- McInerney, S.J.; McNeely, H.E.; Geraci, J.; Giacobbe, P.; Rizvi, S.J.; Ceniti, A.K.; Cyriac, A.; Mayberg, H.S.; Lozano, A.M.; Kennedy, S.H. Neurocognitive Predictors of Response in Treatment Resistant Depression to Subcallosal Cingulate Gyrus Deep Brain Stimulation. Front. Hum. Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzheimer, P.E.; Husain, M.M.; Lisanby, S.H.; Taylor, S.F.; Whitworth, L.A.; McClintock, S.; Slavin, K.V.; Berman, J.; McKhann, G.M.; Patil, P.G.; et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017, 4, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Temel, Y.; Hescham, S.A.; Jahanshahi, A.; Janssen, M.L.; Tan, S.K.; van Overbeeke, J.J.; Ackermans, L.; Oosterloo, M.; Duits, A.; Leentjens, A.F.; et al. Neuromodulation in psychiatric disorders. Int. Rev. Neurobiol. 2012, 107, 283–314. [Google Scholar] [PubMed]
- Morishita, T.; Fayad, S.M.; Higuchi, M.-a.; Nestor, K.A.; Foote, K.D. Deep Brain Stimulation for Treatment-resistant Depression: Systematic Review of Clinical Outcomes. Neurotherapeutics 2014, 11, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, S.H.; Giacobbe, P.; Rizvi, S.J.; Placenza, F.M.; Nishikawa, Y.; Mayberg, H.S.; Lozano, A.M. Deep Brain Stimulation for Treatment-Resistant Depression: Follow-Up After 3 to 6 Years. Am. J. Psychiatry 2011, 168, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J. A history of deep brain stimulation: Technological innovation and the role of clinical assessment tools. Soc. Stud. Sci. 2013, 43, 707–728. [Google Scholar] [CrossRef] [Green Version]
- Benabid, A.L.; Pollak, P.; Louveau, A.; Henry, S.; de Rougemont, J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl. Neurophysiol. 1987, 50, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.A. Violence, mental illness, and the brain—A brief history of psychosurgery: Part 2—From the limbic system and cingulotomy to deep brain stimulation. Surg. Neurol. Int. 2013, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Seminowicz, D.; Goldapple, K.; Kennedy, S.H.; Mayberg, H.S. State and trait influences on mood regulation in bipolar disorder: Blood flow differences with an acute mood challenge. Biol. Psychiatry 2003, 54, 1274–1283. [Google Scholar] [CrossRef]
- Zald, D.H.; Mattson, D.L.; Pardo, J.V. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc. Natl. Acad. Sci. USA 2002, 99, 2450–2454. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, F.; Velasco, F.; Salin-Pascual, R.; Hernandez, J.A.; Velasco, M.; Criales, J.L.; Nicolini, H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery 2005, 57, 585–593. [Google Scholar] [CrossRef]
- Schlaepfer, T.E.; Cohen, M.X.; Frick, C.; Kosel, M.; Brodesser, D.; Axmacher, N.; Joe, A.Y.; Kreft, M.; Lenartz, D.; Sturm, V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008, 33, 368–377. [Google Scholar] [CrossRef]
- Schlaepfer, T.E.; Bewernick, B.H.; Kayser, S.; Madler, B.; Coenen, V.A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 2013, 73, 1204–1212. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Aziz, T.Z. Deep brain stimulation: Avoiding the errors of psychosurgery. JAMA 2009, 301, 1705–1707. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Mayberg, H.S. Neuromodulation for treatment-resistant depression. F1000 Med. Rep. 2012, 4, 22. [Google Scholar] [CrossRef]
- Philip, M.L.; Richard, H.T.; Jeffrey, V.R.; Paul, B.F. Brain Neuromodulation Techniques: A Review. Neuroscientist 2016, 22, 406–421. [Google Scholar]
- Blomstedt, P.; Hariz, M.I. Deep brain stimulation for movement disorders before DBS for movement disorders. Parkinsonism Relat. Disord. 2010, 16, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, L.; Linge, R.; Campa, L.; Valdizán, E.M.; Pazos, Á.; Díaz, Á.; Adell, A. Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex. Neuropharmacology 2016, 108, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.D.; Gabriels, L.A.; Malone, D.A.; Friehs, G.M.; Okun, M.S.; Shapira, N.A.; Foote, K.D.; Cosyns, P.R.; Kubu, C.S.; Malloy, P.F.; et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol. Psychiatry 2010, 15, 64–79. [Google Scholar]
- Kriston, L.; von Wolff, A. Not as golden as standards should be: Interpretation of the Hamilton Rating Scale for Depression. J. Affect. Disord. 2011, 128, 175–177. [Google Scholar] [CrossRef]
- Hamani, C.; Mayberg, H.; Stone, S.; Laxton, A.; Haber, S.; Lozano, A.M. The subcallosal cingulate gyrus in the context of major depression. Biol. Psychiatry 2011, 69, 301–308. [Google Scholar] [CrossRef]
- Andrade, P.; Noblesse, L.H.M.; Temel, Y.; Ackermans, L.; Lim, L.W.; Steinbusch, H.W.M.; Visser-Vandewalle, V. Neurostimulatory and ablative treatment options in major depressive disorder: A systematic review. Acta Neurochir. 2010, 152, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, P.B.; Laird, A.R.; Maller, J.; Daskalakis, Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008, 29, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Mayberg, S.H.; Brannan, K.S.; Mahurin, K.R.; Jerabek, A.P.; Brickman, S.J.; Tekell, L.J.; Silva, A.J.; McGinnis, G.S.; Glass, C.T.; Martin, T.C.; et al. Cingulate function in depression: A potential predictor of treatment response. NeuroReport 1997, 8, 1057–1061. [Google Scholar] [CrossRef]
- Sankar, T.; Chakravarty, M.M.; Jawa, N.; Li, S.X.; Giacobbe, P.; Kennedy, S.H.; Rizvi, S.J.; Mayberg, H.S.; Hamani, C.; Lozano, A.M. Neuroanatomical predictors of response to subcallosal cingulate deep brain stimulation for treatment-resistant depression. J. Psychiatry Neurosci. 2020, 45, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Riva-Posse, P.; Inman, C.S.; Choi, K.S.; Crowell, A.L.; Gross, R.E.; Hamann, S.; Mayberg, H.S. Autonomic arousal elicited by subcallosal cingulate stimulation is explained by white matter connectivity. Brain Stimul. 2019, 12, 743–751. [Google Scholar] [CrossRef]
- Eitan, R.; Fontaine, D.; Benoit, M.; Giordana, C.; Darmon, N.; Israel, Z.; Linesky, E.; Arkadir, D.; Ben-Naim, S.; Iserlles, M.; et al. One year double blind study of high vs low frequency subcallosal cingulate stimulation for depression. J. Psychiatr. Res. 2018, 96, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Merkl, A.; Aust, S.; Schneider, G.-H.; Visser-Vandewalle, V.; Horn, A.; Kühn, A.A.; Kuhn, J.; Bajbouj, M. Deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: A double-blinded randomized controlled study and long-term follow-up in eight patients. J. Affect. Disord. 2018, 227, 521–529. [Google Scholar] [CrossRef]
- Howell, B.; Choi, K.S.; Gunalan, K.; Rajendra, J.; Mayberg, H.S.; McIntyre, C.C. Quantifying the axonal pathways directly stimulated in therapeutic subcallosal cingulate deep brain stimulation. Hum. Brain Mapp. 2019, 40, 889–903. [Google Scholar] [CrossRef] [Green Version]
- Waters, A.C.; Veerakumar, A.; Choi, K.S.; Howell, B.; Tiruvadi, V.; Bijanki, K.R.; Crowell, A.; Riva-Posse, P.; Mayberg, H.S. Test-retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum. Brain Mapp. 2018, 39, 4844–4856. [Google Scholar] [CrossRef] [Green Version]
- Smart, O.; Choi, K.S.; Riva-Posse, P.; Tiruvadi, V.; Rajendra, J.; Waters, A.C.; Crowell, A.L.; Edwards, J.; Gross, R.E.; Mayberg, H.S. Initial Unilateral Exposure to Deep Brain Stimulation in Treatment-Resistant Depression Patients Alters Spectral Power in the Subcallosal Cingulate. Front. Comput. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.S.; Noecker, A.M.; Riva-Posse, P.; Rajendra, J.K.; Gross, R.E.; Mayberg, H.S.; McIntyre, C.C. Impact of brain shift on subcallosal cingulate deep brain stimulation. Brain Stimul. 2018, 11, 445–453. [Google Scholar] [CrossRef]
- Conen, S.; Matthews, J.C.; Patel, N.K.; Anton-Rodriguez, J.; Talbot, P.S. Acute and chronic changes in brain activity with deep brain stimulation for refractory depression. J. Psychopharmacol. 2018, 32, 430–440. [Google Scholar] [CrossRef]
- Riva-Posse, P.; Choi, K.S.; Holtzheimer, P.E.; Crowell, A.L.; Garlow, S.J.; Rajendra, J.K.; McIntyre, C.C.; Gross, R.E.; Mayberg, H.S. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: Prospective targeting in treatment-resistant depression. Mol. Psychiatry 2017. [Google Scholar] [CrossRef] [Green Version]
- Tsolaki, E.; Espinoza, R.; Pouratian, N. Using probabilistic tractography to target the subcallosal cingulate cortex in patients with treatment resistant depression. Psychiatry Res. Neuroimaging 2017, 261, 72–74. [Google Scholar] [CrossRef] [Green Version]
- Accolla, E.A.; Aust, S.; Merkl, A.; Schneider, G.-H.; Kühn, A.A.; Bajbouj, M.; Draganski, B. Deep brain stimulation of the posterior gyrus rectus region for treatment resistant depression. J. Affect. Disord. 2016, 194, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Richieri, R.; Borius, P.Y.; Lagrange, G.; Faget-Agius, C.; Guedj, E.; Mc Gonigal, A.; Régis, J.M.; Lançon, C.; Bartolomei, F. Unmasking Partial Seizure after Deep Brain Stimulation for Treatment-Resistant Depression: A Case Report. Brain Stimul. 2016, 9, 636–638. [Google Scholar] [CrossRef]
- Hilimire, M.R.; Mayberg, H.S.; Holtzheimer, P.E.; Broadway, J.M.; Parks, N.A.; DeVylder, J.E.; Corballis, P.M. Effects of Subcallosal Cingulate Deep Brain Stimulation on Negative Self-Bias in Patients with Treatment-Resistant Depression. Brain Stimul. 2015, 8, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Martin-Blanco, A.; Serra-Blasco, M.; Perez-Egea, R.; de Diego-Adelino, J.; Carceller-Sindreu, M.; Puigdemont, D.; Molet, J.; Alvarez, E.; Perez, V.; Portella, M.J. Immediate cerebral metabolic changes induced by discontinuation of deep brain stimulation of subcallosal cingulate gyrus in treatment-resistant depression. J. Affect. Disord. 2015, 173, 159–162. [Google Scholar] [CrossRef]
- Puigdemont, D.; Portella, M.J.; Pérez-Egea, R.; Molet, J.; Gironell, A.; de Diego-Adeliño, J.; Martín, A.; Rodríguez, R.; Àlvarez, E.; Artigas, F.; et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: A pilot study of relapse prevention. J. Psychiatry Neurosci. Jpn. 2015, 40, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Serra-Blasco, M.; de Vita, S.; Rodriguez, M.R.; de Diego-Adelino, J.; Puigdemont, D.; Martin-Blanco, A.; Perez-Egea, R.; Molet, J.; Alvarez, E.; Perez, V.; et al. Cognitive functioning after deep brain stimulation in subcallosal cingulate gyrus for treatment-resistant depression: An exploratory study. Psychiatry Res. 2015, 225, 341–346. [Google Scholar] [CrossRef]
- Choi, K.S.; Riva-Posse, P.; Gross, R.E.; Mayberg, H.S. Mapping the “Depression Switch” During Intraoperative Testing of Subcallosal Cingulate Deep Brain Stimulation. JAMA Neurol. 2015, 72, 1252–1260. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Giacobbe, P.; Tang, C.W.; Barr, M.S.; Rajji, T.; Kennedy, S.H.; Fitzgerald, P.B.; Lozano, A.M.; Wong, W.; Daskalakis, Z.J. Deep Brain Stimulation Modulates Gamma Oscillations and Theta-Gamma Coupling in Treatment Resistant Depression. Brain Stimul. 2015, 8, 1033–1042. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Pérez-Egea, R.; Romero-Grimaldi, C.; Puigdemont, D.; Molet, J.; Caso, J.R.; Mico, J.A.; Pérez, V.; Leza, J.C.; Berrocoso, E. Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Mol. Psychiatry 2013, 19, 607. [Google Scholar] [CrossRef]
- Merkl, A.; Schneider, G.-H.; Schönecker, T.; Aust, S.; Kühl, K.-P.; Kupsch, A.; Kühn, A.A.; Bajbouj, M. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp. Neurol. 2013, 249, 160–168. [Google Scholar] [CrossRef]
- Ramasubbu, R.; Anderson, S.; Haffenden, A.; Chavda, S.; Kiss, Z.H. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: A pilot study. J. Psychiatry Neurosci. 2013, 38, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, C.V.; Ezquiaga, E.; Navas, M.; Sola, R.G. Deep brain stimulation of the subcallosal cingulate for medication-resistant type I bipolar depression: Case report. Bipolar. Disord. 2013, 15, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Broadway, J.M.; Holtzheimer, P.E.; Hilimire, M.R.; Parks, N.A.; DeVylder, J.E.; Mayberg, H.S.; Corballis, P.M. Frontal Theta Cordance Predicts 6-Month Antidepressant Response to Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression: A Pilot Study. Neuropsychopharmacology 2012, 37, 1764–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamani, C.; Giacobbe, P.; Diwan, M.; Balbino, E.S.; Tong, J.; Bridgman, A.; Lipsman, N.; Lozano, A.M.; Kennedy, S.H.; Nobrega, J.N. Monoamine oxidase inhibitors potentiate the effects of deep brain stimulation. Am. J. Psychiatry 2012, 169, 1320–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzheimer, P.E.; Kelley, M.E.; Gross, R.E.; Filkowski, M.M.; Garlow, S.J.; Barrocas, A.; Wint, D.; Craighead, M.C.; Kozarsky, J.; Chismar, R.; et al. Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Unipolar and Bipolar Depression. Arch. Gen. Psychiatry 2012, 69, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Giacobbe, P.; Hamani, C.; Rizvi, S.J.; Kennedy, S.H.; Kolivakis, T.T.; Debonnel, G.; Sadikot, A.F.; Lam, R.W.; Howard, A.K.; et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J. Neurosurg. 2011, 116, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Puigdemont, D.; Pérez-Egea, R.; Portella, M.J.; Molet, J.; de Diego-Adeliño, J.; Gironell, A.; Radua, J.; Gómez-Anson, B.; Rodríguez, R.; Serra, M.; et al. Deep brain stimulation of the subcallosal cingulate gyrus: Further evidence in treatment-resistant major depression. Int. J. Neuropsychopharmacol. 2012, 15, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Guinjoan, S.M.; Mayberg, H.S.; Costanzo, E.Y.; Fahrer, R.D.; Tenca, E.; Antico, J.; Cerquetti, D.; Smyth, E.; Leiguarda, R.C.; Nemeroff, C.B. Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Holtzheimer, P.E., 3rd; Mayberg, H.S. Deep brain stimulation for treatment-resistant depression. Am. J. Psychiatry 2010, 167, 1437–1444. [Google Scholar] [CrossRef]
- Hamani, C.; Mayberg, H.; Snyder, B.; Giacobbe, P.; Kennedy, S.; Lozano, A.M. Deep brain stimulation of the subcallosal cingulate gyrus for depression: Anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J. Neurosurg. 2009, 111, 1209. [Google Scholar] [CrossRef] [Green Version]
- Puigdemont, D.; Portella, M.J.; Pérez-Egea, R.; de Diego-Adeliño, J.; Gironell, A.; Molet, J.; Duran-Sindreu, S.; Álvarez, E.; Pérez, V. Depressive Relapse After Initial Response to Subcallosal Cingulate Gyrus-Deep Brain Stimulation in a Patient with a Treatment-Resistant Depression: Electroconvulsive Therapy as a Feasible Strategy. Biol. Psychiatry 2009, 66, e11–e12. [Google Scholar] [CrossRef]
- Lozano, A.M.; Mayberg, H.S.; Giacobbe, P.; Hamani, C.; Craddock, R.C.; Kennedy, S.H. Subcallosal Cingulate Gyrus Deep Brain Stimulation for Treatment-Resistant Depression. Biol. Psychiatry 2008, 64, 461–467. [Google Scholar] [CrossRef] [PubMed]
- McNeely, E.H.; Mayberg, S.H.; Lozano, M.A.; Kennedy, H.S. Neuropsychological Impact of Cg25 Deep Brain Stimulation for Treatment-Resistant Depression: Preliminary Results Over 12 Months. J. Nerv. Ment. Dis. 2008, 196, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Neimat, J.S.; Hamani, C.; Giacobbe, P.; Merskey, H.; Kennedy, S.H.; Mayberg, H.S.; Lozano, A.M. Neural stimulation successfully treats depression in patients with prior ablative cingulotomy. Am. J. Psychiatry 2008, 165, 687. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.A.; Mikell, C.B.; Abosch, A.; Ben-Haim, S.; Buchanan, R.J.; Burton, A.W.; Carcieri, S.; Cosgrove, G.R.; D’Haese, P.-F.; Daskalakis, Z.J.; et al. Charting the road forward in psychiatric neurosurgery: Proceedings of the 2016 American Society for Stereotactic and Functional Neurosurgery workshop on neuromodulation for psychiatric disorders. J. Neurol. Neurosurg. Psychiatry 2018, 89, 886–896. [Google Scholar] [CrossRef]
- Moreines, J.L.; McClintock, S.M.; Kelley, M.E.; Holtzheimer, P.E.; Mayberg, H.S. Neuropsychological function before and after subcallosal cingulate deep brain stimulation in patients with treatment-resistant depression. Depress. Anxiety 2014, 31, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Nyandege, A.N.; Slattum, P.W.; Harpe, S.E. Risk of Fracture and the Concomitant Use of Bisphosphonates With Osteoporosis-Inducing Medications. Ann. Pharmacother. 2015, 49, 437–447. [Google Scholar] [CrossRef]
- Cox, G.R.; Callahan, P.; Churchill, R.; Hunot, V.; Merry, S.N.; Parker, A.G.; Hetrick, S.E. Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents. Cochrane Database Syst. Rev. 2014, 11, CD008324. [Google Scholar] [CrossRef] [Green Version]
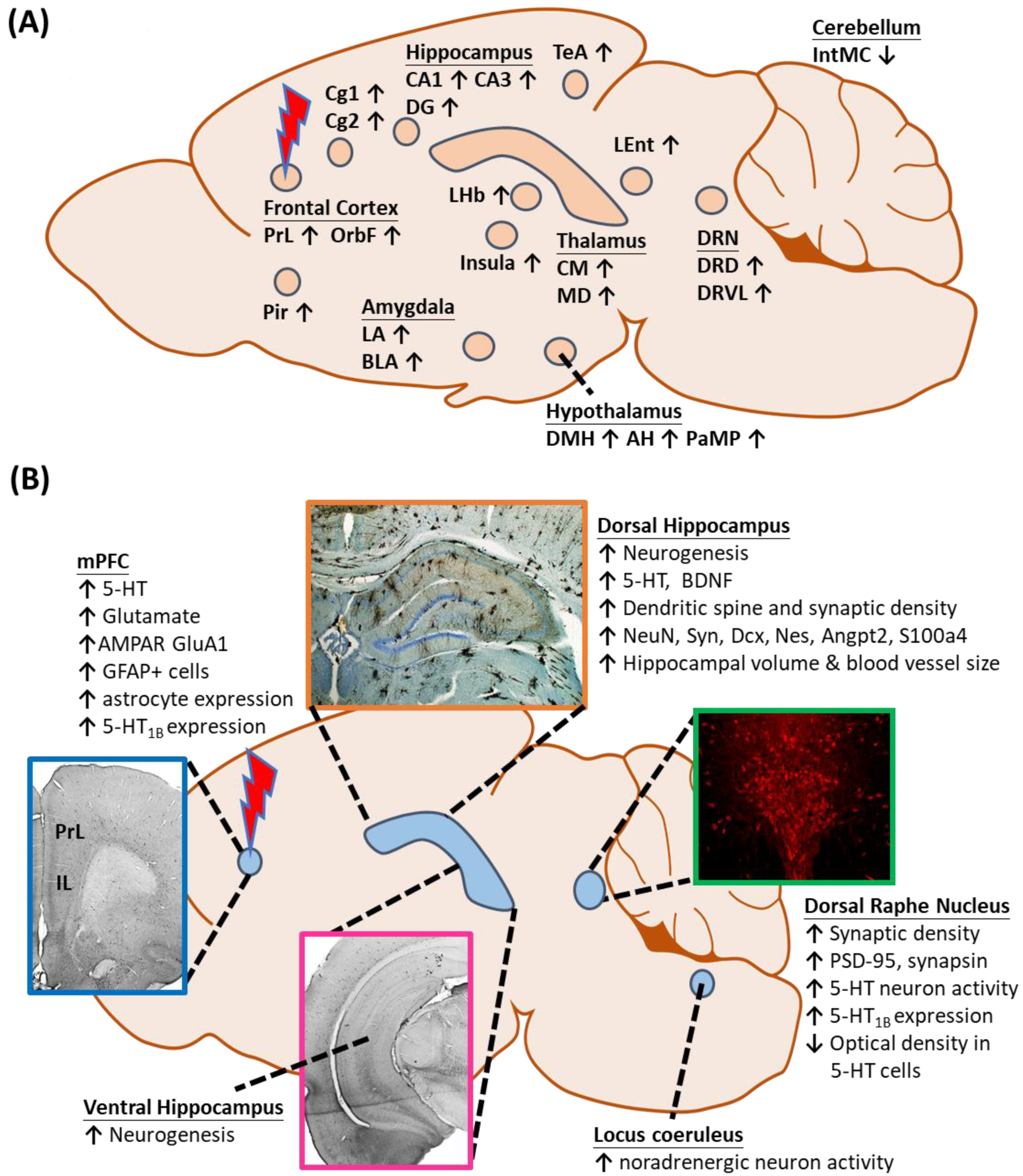
- Hamani, C.; Nobrega, J.N. Preclinical Studies Modeling Deep Brain Stimulation for Depression. Biol. Psychiatry 2012, 72, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Vertes, R.P. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 2006, 142, 1–20. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, L.; Castañé, A.; Pérez-Caballero, L.; Grifoll-Escoda, M.; López-Gil, X.; Campa, L.; Galofré, M.; Berrocoso, E.; Adell, A. Activation of AMPA Receptors Mediates the Antidepressant Action of Deep Brain Stimulation of the Infralimbic Prefrontal Cortex. Cereb. Cortex 2016, 26, 2778–2789. [Google Scholar] [CrossRef] [Green Version]
- Ferry, A.T.; Öngür, D.; An, X.; Price, J.L. Prefrontal cortical projections to the striatum in macaque monkeys: Evidence for an organization related to prefrontal networks. J. Comp. Neurol. 2000, 425, 447–470. [Google Scholar] [CrossRef]
- McFarland, N.R.; Haber, S.N. Thalamic Relay Nuclei of the Basal Ganglia Form Both Reciprocal and Nonreciprocal Cortical Connections, Linking Multiple Frontal Cortical Areas. J. Neurosci. 2002, 22, 8117–8132. [Google Scholar] [CrossRef] [Green Version]
- Parthoens, J.; Verhaeghe, J.; Stroobants, S.; Staelens, S. Deep Brain Stimulation of the Prelimbic Medial Prefrontal Cortex: Quantification of the Effect on Glucose Metabolism in the Rat Brain Using [18 F] FDG MicroPET. Mol. Imaging Biol. 2014, 16, 838–845. [Google Scholar] [CrossRef]
- Hajós, M.; Richards, C.D.; Székely, A.D.; Sharp, T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience 1998, 87, 95–108. [Google Scholar] [CrossRef]
- Wood, M.; Adil, O.; Wallace, T.; Fourman, S.; Wilson, S.P.; Herman, J.P.; Myers, B. Infralimbic prefrontal cortex structural and functional connectivity with the limbic forebrain: A combined viral genetic and optogenetic analysis. Brain Struct. Funct. 2019, 224, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Deutch, A.Y.; Roth, R.H.; Bunney, B.S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989, 290, 213–242. [Google Scholar] [CrossRef] [PubMed]
- Chandler, L.J.; Gass, J.T. The plasticity of extinction: Contribution of the prefrontal cortex in treating addiction though inhibitory learning. Front. Psychiatry 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Uylings, H.B.; van Eden, C.G. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog. Brain Res. 1990, 85, 31–62. [Google Scholar] [PubMed]
- Lim, L.W.; Tan, S.K.; Groenewegen, H.J.; Temel, Y. Electrical brain stimulation in depression: Which target(s)? Biol. Psychiatry 2011, 69, e5–e6, author reply e7–e8. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Diwan, M.; Macedo, C.E.; Brandao, M.L.; Shumake, J.; Gonzalez-Lima, F.; Raymond, R.; Lozano, A.M.; Fletcher, P.J.; Nobrega, J.N. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry 2010, 67, 117–124. [Google Scholar] [CrossRef]
- Hamani, C.; Machado, D.C.; Hipólide, D.C.; Dubiela, F.P.; Suchecki, D.; Macedo, C.E.; Tescarollo, F.; Martins, U.; Covolan, L.; Nobrega, J.N. Deep Brain Stimulation Reverses Anhedonic-Like Behavior in a Chronic Model of Depression: Role of Serotonin and Brain Derived Neurotrophic Factor. Biol. Psychiatry 2012, 71, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamani, C.; Diwan, M.; Isabella, S.; Lozano, A.M.; Nobrega, J.N. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J. Psychiatr. Res. 2010, 44, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Edemann-Callesen, H.; Voget, M.; Empl, L.; Vogel, M.; Wieske, F.; Rummel, J.; Heinz, A.; Mathé, A.A.; Hadar, R.; Winter, C. Medial Forebrain Bundle Deep Brain Stimulation has Symptom-specific Anti-depressant Effects in Rats and as Opposed to Ventromedial Prefrontal Cortex Stimulation Interacts With the Reward System. Brain Stimul. 2015, 8, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Rea, E.; Rummel, J.; Schmidt, T.T.; Hadar, R.; Heinz, A.; Mathé, A.A.; Winter, C. Anti-Anhedonic Effect of Deep Brain Stimulation of the Prefrontal Cortex and the Dopaminergic Reward System in a Genetic Rat Model of Depression: An Intracranial Self-Stimulation Paradigm Study. Brain Stimul. 2014, 7, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bruchim-Samuel, M.; Lax, E.; Gazit, T.; Friedman, A.; Ahdoot, H.; Bairachnaya, M.; Pinhasov, A.; Yadid, G. Electrical stimulation of the vmPFC serves as a remote control to affect VTA activity and improve depressive-like behavior. Exp. Neurol. 2016, 283, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bregman, T.; Nona, C.; Volle, J.; Diwan, M.; Raymond, R.; Fletcher, P.J.; Nobrega, J.N.; Hamani, C. Deep brain stimulation induces antidepressant-like effects in serotonin transporter knockout mice. Brain Stimul. 2018, 11, 423–425. [Google Scholar] [CrossRef]
- Bambico, F.R.; Bregman, T.; Diwan, M.; Li, J.; Darvish-Ghane, S.; Li, Z.; Laver, B.; Amorim, B.O.; Covolan, L.; Nobrega, J.N.; et al. Neuroplasticity-dependent and -independent mechanisms of chronic deep brain stimulation in stressed rats. Transl. Psychiatry 2015, 5, e674. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Jain, N.; Vyas, A.; Lim, L.W. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. eLife 2015, 4, e04803. [Google Scholar] [CrossRef]
- Hamani, C.; Amorim, B.O.; Wheeler, A.L.; Diwan, M.; Driesslein, K.; Covolan, L.; Butson, C.R.; Nobrega, J.N. Deep brain stimulation in rats: Different targets induce similar antidepressant-like effects but influence different circuits. Neurobiol. Dis. 2014, 71, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Bregman, T.; Reznikov, R.; Diwan, M.; Raymond, R.; Butson, C.R.; Nobrega, J.N.; Hamani, C. Antidepressant-like Effects of Medial Forebrain Bundle Deep Brain Stimulation in Rats are not Associated With Accumbens Dopamine Release. Brain Stimul. 2015, 8, 708–713. [Google Scholar] [CrossRef]
- Lim, L.W.; Prickaerts, J.; Huguet, G.; Kadar, E.; Hartung, H.; Sharp, T.; Temel, Y. Electrical stimulation alleviates depressive-like behaviors of rats: Investigation of brain targets and potential mechanisms. Transl. Psychiatry 2015, 5, e535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etiévant, A.; Oosterhof, C.; Bétry, C.; Abrial, E.; Novo-Perez, M.; Rovera, R.; Scarna, H.; Devader, C.; Mazella, J.; Wegener, G.; et al. Astroglial Control of the Antidepressant-Like Effects of Prefrontal Cortex Deep Brain Stimulation. EBioMedicine 2015, 2, 898–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerakumar, A.; Challis, C.; Gupta, P.; Da, J.; Upadhyay, A.; Beck, S.G.; Berton, O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol. Psychiatry 2014, 76, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, Y.; Lim, L.W.; Mitra, R. Enriched Environment Facilitates Anxiolytic Efficacy Driven by Deep-Brain Stimulation of Medial Prefrontal Cortex. Front. Behav. Neurosci. 2018, 12, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creed, M.C.; Hamani, C.; Nobrega, J.N. Effects of repeated deep brain stimulation on depressive- and anxiety-like behavior in rats: Comparing entopeduncular and subthalamic nuclei. Brain Stimul. 2013, 6, 506–514. [Google Scholar] [CrossRef]
- Meng, H.; Wang, Y.; Huang, M.; Lin, W.; Wang, S.; Zhang, B. Chronic deep brain stimulation of the lateral habenula nucleus in a rat model of depression. Brain Res. 2011, 1422, 32–38. [Google Scholar] [CrossRef]
- Lim, L.W.; Janssen, M.L.F.; Kocabicak, E.; Temel, Y. The antidepressant effects of ventromedial prefrontal cortex stimulation is associated with neural activation in the medial part of the subthalamic nucleus. Behav. Brain Res. 2015, 279, 17–21. [Google Scholar] [CrossRef]
- Reznikov, R.; Bambico, F.R.; Diwan, M.; Raymond, R.J.; Nashed, M.G.; Nobrega, J.N.; Hamani, C. Prefrontal Cortex Deep Brain Stimulation Improves Fear and Anxiety-Like Behavior and Reduces Basolateral Amygdala Activity in a Preclinical Model of Posttraumatic Stress Disorder. Neuropsychopharmacology 2017, 43, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Torres-Sanchez, S.; Perez-Caballero, L.; Mico, J.A.; Celada, P.; Berrocoso, E. Effect of Deep Brain Stimulation of the ventromedial prefrontal cortex on the noradrenergic system in rats. Brain Stimul. 2018, 11, 222–230. [Google Scholar] [CrossRef]
- Insel, N.; Pilkiw, M.; Nobrega, J.N.; Hutchison, W.D.; Takehara-Nishiuchi, K.; Hamani, C. Chronic deep brain stimulation of the rat ventral medial prefrontal cortex disrupts hippocampal–prefrontal coherence. Exp. Neurol. 2015, 269, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Laver, B.; Diwan, M.; Nobrega, J.N.; Hamani, C. Augmentative therapies do not potentiate the antidepressant-like effects of deep brain stimulation in rats. J. Affect. Disord. 2014, 161, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Rummel, J.; Voget, M.; Hadar, R.; Ewing, S.; Sohr, R.; Klein, J.; Sartorius, A.; Heinz, A.; Mathé, A.A.; Vollmayr, B.; et al. Testing different paradigms to optimize antidepressant deep brain stimulation in different rat models of depression. J. Psychiatr. Res. 2016, 81, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lehto, L.J.; Filip, P.; Laakso, H.; Sierra, A.; Slopsema, J.P.; Johnson, M.D.; Eberly, L.E.; Low, W.C.; Grohn, O.; Tanila, H.; et al. Tuning Neuromodulation Effects by Orientation Selective Deep Brain Stimulation in the Rat Medial Frontal Cortex. Front. Neurosci. 2018, 12, 899. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, L.; Soto-Montenegro, M.L.; Hidalgo-Figueroa, M.; Mico, J.A.; Desco, M.; Berrocoso, E. Deep brain stimulation electrode insertion and depression: Patterns of activity and modulation by analgesics. Brain Stimul. 2018, 11, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Sun, Z.; Shi, D.; Wang, M.; Jia, J.; He, Y.; Xue, F.; Ren, Y.; Yang, J.; Ma, X. Effects of different patterns of electric stimulation of the ventromedial prefrontal cortex on hippocampal–prefrontal coherence in a rat model of depression. Behav. Brain Res. 2019, 356, 179–188. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Lason, M.; Niemczyk, M.; Willner, P. The role of prefrontal cortex dopamine D2 and D3 receptors in the mechanism of action of venlafaxine and deep brain stimulation in animal models of treatment-responsive and treatment-resistant depression. J. Psychopharmacol. 2019, 33, 748–756. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Lason, M.; Tota-Glowczyk, K.; Niemczyk, M.; Litwa, E.; Willner, P. Rapid antidepressant effects of deep brain stimulation of the pre-frontal cortex in an animal model of treatment-resistant depression. J. Psychopharmacol 2018, 32, 1133–1140. [Google Scholar] [CrossRef]
- Volle, J.; Bregman, T.; Scott, B.; Diwan, M.; Raymond, R.; Fletcher, P.J.; Nobrega, J.N.; Hamani, C. Deep brain stimulation and fluoxetine exert different long-term changes in the serotonergic system. Neuropharmacology 2018, 135, 63–72. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Vertes, R.P.; Hoover, W.B.; Szigeti-Buck, K.; Leranth, C. Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Res. Bull. 2007, 71, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.; Bregman, T.; Voget, M.; Raymond, R.; Hadar, R.; Nobrega, J.N.; Hamani, C. Acute high frequency stimulation of the prefrontal cortex or nucleus accumbens does not increase hippocampal neurogenesis in rats. J. Psychiatr. Res. 2015, 68, 27–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.C.; Wellman, C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004, 60, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Magariños, A.M.; McEwen, B.S.; Flügge, G.; Fuchs, E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 3534. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, G.M. The dendritic spine: A multifunctional integrative unit. J. Neurophysiol. 1996, 75, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Bezchlibnyk, Y.B.; Stone, S.S.D.; Hamani, C.; Lozano, A.M. High frequency stimulation of the infralimbic cortex induces morphological changes in rat hippocampal neurons. Brain Stimul. 2017, 10, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, M.M.; Hamani, C.; Martinez-Canabal, A.; Ellegood, J.; Laliberté, C.; Nobrega, J.N.; Sankar, T.; Lozano, A.M.; Frankland, P.W.; Lerch, J.P. Deep brain stimulation of the ventromedial prefrontal cortex causes reorganization of neuronal processes and vasculature. NeuroImage 2016, 125, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Sheng, M. Dendritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880. [Google Scholar] [CrossRef]
- Kuem-Ju, L.; Sung-Jin, K.; Suk-Won, K.; Song-Hyen, C.; You-Chan, S.; Sang-Ha, P.; Bo-Hyun, M.; Eujin, C.; Min-Soo, L.; Sang-Hyun, C.; et al. Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Exp. Amp; Mol. Med. 2006, 38, 44. [Google Scholar]
- Gulyaeva, N.V. Molecular Mechanisms of Neuroplasticity: An Expanding Universe. Biochem. Mosc. 2017, 82, 237–242. [Google Scholar] [CrossRef]
- Chan, J.P.; Cordeira, J.; Calderon, G.A.; Iyer, L.K.; Rios, M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol. Cell. Neurosci. 2008, 39, 372–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, L.F. Neurotrophin-Regulated Signalling Pathways. Philos. Trans. Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlezon, W.A.; Duman, R.S.; Nestler, E.J. The many faces of CREB. Trends Neurosci. 2005, 28, 436–445. [Google Scholar] [CrossRef]
- Öngür, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef] [Green Version]
- Bowley, M.P.; Drevets, W.C.; Öngür, D.; Price, J.L. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry 2002, 52, 404–412. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Uranova, N.A.; Orlovskaya, D.D. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr. Res. 2007, 94, 273–280. [Google Scholar] [CrossRef]
- Stockmeier, C.A.; Mahajan, G.J.; Konick, L.C.; Overholser, J.C.; Jurjus, G.J.; Meltzer, H.Y.; Uylings, H.B.; Friedman, L.; Rajkowska, G. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 2004, 56, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Dubey, P.; Stockmeier, C.A.; Krishnan, K.R. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol. Psychiatry 2005, 58, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Airan, R.D.; Meltzer, L.A.; Roy, M.; Gong, Y.; Chen, H.; Deisseroth, K. High-Speed Imaging Reveals Neurophysiological Links to Behavior in an Animal Model of Depression. Science 2007, 317, 819. [Google Scholar] [CrossRef] [Green Version]
- Srejic, L.R.; Hamani, C.; Hutchison, W.D. High-frequency stimulation of the medial prefrontal cortex decreases cellular firing in the dorsal raphe. Eur. J. Neurosci. 2015, 41, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celada, P.; Puig, M.; Casanovas, J.; Guillazo, G.; Artigas, F. Control of Dorsal Raphe Serotonergic Neurons by the Medial Prefrontal Cortex: Involvement of Serotonin-1A, GABA sub(A), and Glutamate Receptors. J. Neurosci. 2001, 21, 9917–9929. [Google Scholar] [CrossRef] [Green Version]
- Nambu, A.; Tokuno, H.; Takada, M. Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’ pathway. Neurosci. Res. 2002, 43, 111–117. [Google Scholar] [CrossRef]
- Anderson, R.J.; Frye, M.A.; Abulseoud, O.A.; Lee, K.H.; McGillivray, J.A.; Berk, M.; Tye, S.J. Deep brain stimulation for treatment-resistant depression: Efficacy, safety and mechanisms of action. Neurosci. Biobehav. Rev. 2012, 36, 1920–1933. [Google Scholar] [CrossRef]
- Deeb, W.; Giordano, J.J.; Rossi, P.J.; Mogilner, A.Y.; Gunduz, A.; Judy, J.W.; Klassen, B.T.; Butson, C.R.; Van Horne, C.; Deny, D.; et al. Proceedings of the Fourth Annual Deep Brain Stimulation Think Tank: A Review of Emerging Issues and Technologies. Front. Integr. Neurosci. 2016, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Schermer, M. Ethical Issues in Deep Brain Stimulation. Front. Integr. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [Green Version]
- Bell, E.; Mathieu, G.; Racine, E. Preparing the ethical future of deep brain stimulation. Surg. Neurol. 2009, 72, 577. [Google Scholar] [CrossRef]
| Authors | Main Inclusion Criteria | No. of Patients | Stimulation Target & DBS Design | Stimulation Parameters | Clinical Evaluation | Major Outcomes | Adverse Effects |
|---|---|---|---|---|---|---|---|
| Sankar et al. 2020 [40] | TRD MDE; Current MDE ≥ 12 months; HRSD-17 score ≥ 20; Non-responsive (NR) ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20. | 27 | Target SCG | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3–6 V Pulse Width 90 μs | Volumetric analysis, Whole brain grey and white matter analysis | Left and average SCG volume significantly higher in responders compared to non-responders. Right and average amygdala volume significantly higher in responders compared to non-responders. Left, right, and average thalamus volume significantly higher in responders compared to non-responders. Brain grey matter volume significantly lower in responders compared to non-responders. Ratio of grey to white matter volume significantly higher in responders compared to non-responders. | N.A. |
| Riva-Posse et al. (2019) [41] | TRD MDE; Current MDE ≥ 12 months; HRSD-17 score ≥ 20; Non-responsive (NR) ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF < 50. | 9 | Target SCC Study Design Intraoperative Sessions: 6 min session of 3 min stimulation ON and 3 min stimulation OFF. Number of sessions: 12 trials (one at each of the eight available contacts. Four per hemisphere, plus four sham trials). Sham-controlled, double-blinded trials (one case w/single blind trial) | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 6 mA Pulse Width 90 μs | ECG, EDA, MRI Volume of Tissue Activated, Structural Connectivity Analysis | Autonomic changes with SCC-DBS correspond to salient behavioral responses. Distant effects of SCC-DBS in the midcingulate cortex. Increase in heart rate was only seen with left SCC-DBS. No significant relationship with skin conductance. These findings aid in the optimal selection of contacts and parameters in SCC-DBS surgery. | N.A. |
| Eitan et al. (2018) [42] | Both sexes, 21–70 years; Non-psychotic MDD; First MDE onset before 45 years old with current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; MADRS ≥ 22; GAF < 50; MMSE > 24; No changes in current antidepressant treatments ≥ 4 wks prior to study. | 9 | Target BA25 Study Design Double-blind, randomized. Two groups: High- OR low-frequency DBS for 12 months from 1 month after electrode implantation. | Implantation Bilateral Stimulation Monopolar Frequency 20 Hz or 130 Hz Amplitude 4–8 mA Pulse Width 91 μs | MADRS, HRSD-17, QIDS-SR, Q-LES-Q, GAF, HAM-A, CGI, PGI, CANTAB battery. | Four out of nine patients responded ◊ at the end of DBS (≥40% reduction in MADRS from baseline). The effect of DBS at 6–12 months was higher than DBS at 1–6 months. High-frequency DBS showed higher efficacy than low-frequency DBS. Non-responders crossed over after first 6 months of DBS. | Severe One patient overdosed on medication (dothiepin and valium). |
| Merkl et al. (2018) [43] | Diagnosed MDD and disease lasted for >2 years; HAMD-24 score ≥ 20; ATHF Score ≥ 3; TRD: NR ≥ 2 antidepressant therapies; Failed to respond to antidepressants and ECT; No changes in current antidepressant treatments ≥6 wks prior to study. | 8 | Target SCG Study Design Randomized; Two groups: sham-DBS (delayed onset) OR non-delayed onset group for the first 8 weeks in a blinded manner. Open-label DBS afterwards for up to 28 months. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 5–7 V Pulse Width 90 μs | HAMD-24, BDI, MADRS. | Three out of eight patients responded ** after 6 months DBS; three out of seven with the same criteria after 12 months. Two out of six responded ** at the end of DBS, follow-up at 28 months. Two out of six patients reached remission □ at the end of DBS, follow-up at 28 months. This study showed a delayed response in patients; no significant antidepressant effects between sham and active stimulation compared to baseline. | Non-severe Headache; Pain; Scalp tingling; Dizziness; Light hypomania; Inconvenient movement; Severe NIL |
| Howell et al. (2018) [44] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 6 | Target SCC (Cingulum Bundle and Forceps Minor) | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 4 V Pulse Width 90 μs | HDRS-17, MRI Field-cable modeling (non-VTA-based analysis) | All of the subjects responded. Left and right cingulum bundles as well as forceps minor are the most likely therapeutic targets. Right cingulum bundle activation beyond a threshold may protract recovery. Uncinate fasciculus and frontal pole were activated to a lesser extent, may not be necessary for anti-depressive effect of SCC-DBS. Time to a stable response (TSR) was 8–189 days, 1-year HDRS-17 was 2–11. Field cable modeling was more accurate than volume of activated tissue at approximating axonal activation. Overstimulation of CB-DBS can be detrimental to the recovery process. | N.A. |
| Waters et al. (2018) [45] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 4 | Target SCC Study Design Single-blinded, Session 3 min | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3–5 V Pulse Width 90 μs | HDRS-17, EEG | Symptom severity scores decreased. three out of four patients in remission (HDRS-17 ≤ 7). Test-retest reliability across four repeated measures over 14 months met or exceeded standards for valid test construction in three out of four patients for cortical-evoked responses studied. | N.A. |
| Smart et al. (2018) [46] | TRD patients enrolled from two separate clinical trials for Deep Brain Stimulation. Trial 1 Both sexes aged 18–70 years; Diagnosis of a Major Depressive Episode or Bipolar Type II—current episode depressed, Current episode duration of at least 1 year, Non-responsive (NR) ≥ 4 antidepressant therapies. Trial 2 Both sexes aged 25–70 years; Current depressive episode of at least 2 years duration OR a history of more than four lifetime depressive episodes, Non-responsive (NR) ≥ 4 antidepressant therapies. | 14 | Target SCC Study Design Double-blinded. Intraoperative behavioral testing: Frequency: 130 Hz Pulse width: 90 μs Current: 6 mA Eight patients continued SCC local field potential. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 6–8 mA for St. Jude Medical devices, 3.5–5 V for Medtronic devices Pulse Width 90 μs | HDRS-17, MRI, LFP. | 11 of 14 patients met the criteria for DBS antidepressant response by 6 months. Of the three 6 month non-responders, one responded after the 6 month study endpoint but without a contact change (Patient 2), one responded after a contact switch in the left hemisphere (Patient 7), and one remained a non-responder (Patient 6). Mean baseline HDRS-17 of 23.8 and SD of 2.8; HDRS-17 of 9.6 and SD of 4.5 at month 6; 19.9 weeks for stable response with SD of 20 weeks. Precision on the left may be more important than precision on the right, which is supported by theta decreases. | N.A. |
| Choi et al. (2018) [47] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 15 | Target SCC Study Design Patients went through SCC-DBS, followed by MRI scans. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 6 mA Pulse Width 90 μs | HDRS-17, MRI, DWI, Volume of Tissue Activated | Significant differences in the pathway activation changes over time between remitters and non-remitters. Non-remitters had significantly larger net changes in their pathway activation connection in both the near and long term relative to the initial plan. | N.A. |
| Conen et al. (2018) [48] | TRD (unipolar); NR ≥ 4 antidepressant therapies; MADRS Score ≥ 22. | 7 | Targets SCC followed by Ventral Anterior Capsule, nucleus accumbens (separately, unless patient in remission, and later combined, for non-responding patients). Study Design DBS was applied sequentially for 3 months per region, for a total period ranging from 16–45 months. | N.A. | MADRS, HAM-D 17, GAF. | Remitters had higher regional cerebral blood flow in the baseline prefrontal cortex and subsequent tests when compared to non-remitters and non-responders. Chronic DBS increased prefrontal cortex regional cerebral blood flow. Remitted patients had higher prefrontal cerebral blood flow at baseline. | N.A. |
| Holtzheimer et al. (2017) [15] | Both sexes aged 21–70 years; Unipolar, non-psychotic MDD First MDE onset before 45 years old with current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies, MADRS Score > 22; GAF < 50; MMSE > 24; No changes in current antidepressant treatments ≥ 4 wks prior to study. | 60 (DBS) 30 (Sham) | Target SCC Study Design DBS or sham stimulation 2 weeks after implantation for 6 months in randomized and double-blind manner. Two groups: DBS or sham then both groups received open-label stimulation for 6 months or 2 years. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 4–8 mA Pulse Width 91 μs | MADRS, GAF HRSD-17, 30-item Inventory of Depressive Symptomatology, QIDS-SR, WSAS, PGI, CGI, QOL, HAM-A. | Insignificant difference in response * between sham and DBS at the end of the 6-month double-blind phase. 38 patients responded * and 20 remitted □ after 6 month DBS. In 2 years of open-label active DBS, 48% achieved antidepressant response and 25% achieved remission—clinically meaningful long-term outcomes. | Severe Eight of 40 events reported related to device or surgery: six infections (in five patients), one skin erosion over the extension wires, and one postoperative seizure. |
| McInerney et al. (2017) [14] | Current MDE ≥ 12 months; HRSD-17 Score ≥ 20; NR ≥ 4 antidepressant therapies. | 20 | Target SCG Study Design DBS for 12 months open-label | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3.5–5 V Pulse Width 90 μs | Wisconsin Card Sorting Task (WCST), Hopkins Verbal Learning Test, Controlled Oral Word Association Test (COWA), Finger Tap Test, Stroop Test, HRSD-17. | Significant reduction in HRSD-17 from baseline to experimental follow-up. Baseline scores differed significantly between responders and non-responders. 11 patients responded ** and nine were non-responders. WCST Test results indicated that the total errors were predictive of responsiveness to DBS. No significant deterioration in cognition and psychomotor speed. Improvements in verbal memory and verbal fluency. | N.A. |
| Riva-Posse et al. (2018) [49] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 11 | Target SCC Study Design DBS from 4 weeks after surgery and lasted for 6 months, open-label. Stimulation contacts were changed in non-responders and they were stimulated for 6 more months. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 6–8 mA Pulse Width 91 μs | HRSD-17 | Eight out of 11 responded ** and six remitted □□ after 6 month DBS. Nine responded ** and six remitted □□ after 12 month DBS. Two did not respond throughout the study. Tractography-based surgery reduced variability in the effects of stimulation on patient-specific brain circuitry. | N.A. |
| Tsolaki et al. (2017) [50] | TRD | 2 | Target SCC | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 8 mA Pulse Width 91 μs | MRI, DTI, CT, FSL Probabilistic tractography, Volume of Tissue Activated, MADRS. | One patient was a responder (81% change in MADRS score). Responder’s contacts were closer to the Tractography-guided optimized target (TOT), unlike the non-responder. | N.A. |
| Accolla et al. (2016) [51] | MDD; NR to treatments; Currently in a depressive episode as in DSM-IV Axis I disorders; HAMD-24 score of ≥ 20. | 5 | Target BA25 Study Design Double-blind. Each homologous electrode pair was activated separately on 5 consecutive days, then antidepressant effects was assessed 24 h later. Open-label DBS for up to 24 months. Pre- and post-DBS MRI images were taken. | Implantation N.A. Stimulation Monopolar Frequency 130 Hz Amplitude 5 V Pulse Width 90 μs | HAMD-24, BDI. | Four out of five patients did not show sustained response ** to DBS (also ≥50% reduction in DBI). One patient responded ** to DBS of the bilateral posterior gyrus rectus instead of the intended target (BA25). | N.A. |
| Richieri et al. (2016) [52] | Diagnosed MDD; Severe cognitive defects and relapsed after ECT. | 1 | Target BA25 Study Design DBS at Day 5 after electrode implantation. | Implantation Bilateral Stimulation Bipolar Frequency 130 Hz Amplitude 4.2 V Pulse Width 90 μs | QIDS SR-16 | Remitted (QIDS SR-16 3/48) at 1 month after DBS and maintained at the end of DBS. | Seizure |
| Hilimire et al. (2015) [53] | Both sexes aged 18–70 years; Current MDE ≥ 12 months, Non-responsive (NR) ≥ 4 antidepressant therapies, HRSD-17 score ≥ 20, GAF score ≤ 50. | 7 | Target SCC Study Design DBS for 6 months, open-label. Behavioral testing and electrophysiological recording (i) before electrode implantation, (ii) after 1 month DBS and (iii) after 6 month DBS. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 4–8 mA Pulse Width 91 μs | HDRS-17, Emotional self-referential task, EEG recording. | Reduced proportion of negative self-descriptive words compared to baseline after 1 month and 6 month DBS. Significant reduction in P1 amplitude compared to baseline (for negative word self-description) after 1 month and 6 month DBS, and P3 amplitudes at 6 month DBS only Reduced depression severity. | N.A. |
| Martin-Blanco et al. (2015) [54] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 7 | Target SCG Study Design Chronic DBS for 9 months on average for clinical stabilization. A PET scan was acquired (i) during active stimulation and (ii) after 48 h of inactive stimulation. | Implantation Bilateral Stimulation Monopolar Frequency 135 Hz Amplitude 3.5–5 V Pulse Width 120–210 μs | HAMD-17, PET | Decreased metabolism in BA24, BA6, caudate putamen after 48 h DBS. This study suggests metabolic changes spread after longer periods of no stimulation. No clinical changes were detected according to HAMD-17. | N.A. |
| Puigdemont et al. (2015) [55] | Severe TRD; Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 5 | Target SCG Study Design Randomized, Double-blind. After stable clinical remission to DBS, patients were allocated to two groups, one with (i) 3 month DBS-ON, then (ii) 3 month sham stimulation (ON-OFF arm) or OFF-ON arm and the other, vice-versa. | Implantation Bilateral Stimulation Monopolar Frequency 130–135 Hz Amplitude 3.5–5 V Pulse Width 120–240 μs | Volume of Tissue Activated, HRSD-17 | Active stimulation: four of five patients were remitted patients. Sham stimulation: Only two patients remained in remission, another two relapsed, and one showed a progressive worsening without reaching relapse criteria. | N.A. |
| Serra-Blasco et al. (2015) [56] | Treatment-Resistant Depression (TRD) Group Resistant to pharmacological treatment; min. stage IV of Thase-Rush scale; HDRS score ≥ 18. First Episode MDD (FE MDD) Group HDRS score ≥ 14; Newly diagnosed MDD. | 16 | Target SCG Study Design DBS began at 48 h postoperative and ended when each patient had stabilized response for at least three consecutive visits, tests conducted before surgery, and 12 months after DBS treatment. | Implantation Bilateral Stimulation Monopolar Frequency 135 Hz Amplitude 3.5–5 V Pulse Width 120–210 μs | Rey Auditory Verbal Learning Test, Trail Making Tests-A and -B, Wechsler Adult Intelligence Scale III, Tower of London Test, HDRS-17. | FE MDD and TRD saw significant improvements over time in memory. No significant difference was observed in both groups on executive functioning, language, and processing speed. DBS was well tolerated and had no adverse effect on neuropsychological and cognitive function. | N.A. |
| Choi et al. (2015) [57] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HDRS-17 score ≥ 20; GAF score ≤ 50. | 9 | Target SCC Intraoperative Sessions: 6 min session of 3 min stimulation ON, and 3 min stimulation OFF. Number of sessions: 12 trials (one at each of the eight available contacts; four per hemisphere, plus four sham trials). Study Design Sham-controlled, Double-blind trials (one case w/single blind trial). | Acute Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 6 mA Pulse Width 90 μs | MRI with FSL analysis, Volume of Tissue Activated. | Behavioral switch was apparent to patients within the first minute of the initiation of stimulation and effects were sustained while stimulation remained on. Three common white matter bundles were affected by stimulation: (i) the uncinate fasciculus, (ii) the forceps minor, and (iii) the left cingulum bundle.Seven of nine patients with left hemispheric contact had a response to treatment at 6 months. | |
| Sun et al. (2015) [58] | NR ≥ 4 antidepressant therapies, Mean HRSD-17 score of 25 (3). | 20 | Target SCC Session: 100 min, w/15 min break EEG recording sessions/day Session 1: DBS On Session 2: DBS Random (On/Off) Session 3: DBS (Off) | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 2–7.25 mA OR 2–6 V Pulse Width 90 μs | EEG, HDRS-17. | Suppression of gamma oscillations by DBS during working memory performance and the treatment efficacy of DBS for TRD may be associated with the improved GABAergic neurotransmission, previously shown to be deficient in MDD.The present study also suggests that modifying treatment parameters to achieve suppression of gamma oscillations and increased theta-gamma coupling may lead to optimized DBS efficacy for TRD. | N.A. |
| Perez-Caballero et al. (2014) [59] | 18–70 years old with MDE; Resistant to pharmacological treatment and at most, a partial response to ECT; HAMD-17 Score ≥ 18. | 8 | Target SCG Study Design All patients received chronic DBS within 48 h after implantation. Four patients took NSAIDs for up to 30 days postoperative, four patients did not. | Implantation Quadrupolar Stimulation 135 Hz Amplitude 3.5–5 V Pulse Width 120–210 µs | HDRS-17 | At week 1 after surgery, all patients without NSAID prescription responded ** and two remitted □□□; three patients with NSAID responded **, and two remitted □□□. At week 4 after surgery, three patients without NSAID remitted □□□; no patients with NSAID responded ** | N.A. |
| Merkl et al. (2013) [60] | MDD; NR to treatments; Currently in a depressive episode as in DSM-IV Axis I disorder; HAMD-24 score of ≥ 20; HDRS-24 score ≥ 24. | 6 | Target SCG Study Design DBS on 11–19 days after electrode implantation, 24 h acute stimulation followed by sham stimulation for each of the three electrode pairs. Up to 6 months of chronic stimulation. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 2.5–10 V Pulse Width 90 µs | HAMD-24, MADRS BDI, TMT-A, TMT-B, CVLT, TAP, Boston Naming Test, Stroop Test, Word Fluency Test. | Non-significant reduction in HAMD-24, BDI, and MADRS scores for acute DBS and sham stimulation. 0/4 contact pair locations showed significant BDI and MADRS improvements. Contact pair 3/7 for Patient 4 saw a 77% reduction in HAMD-24 score and 62% reduction of MADRS score. Reduced HDRS-24, BDI, and MADRS scores at the end of chronic stimulation. Two out of six remissions □ at the end of chronic stimulation. | Mild Headache; Pain; Scalp tingling; Dizziness; Sore throat; Hardware-related; Severe NIL |
| Ramasubbu et al. (2013) [61] | Aged between 20–60 years; Diagnosed MDD; HAMD-17 score ≥ 20; NR ≥ 4 antidepressant therapies. (Enrolled patients were among the most treatment resistant). | 4 | Target SCC Study Design Double-blind DBS optimization. Open-label continuous DBS for 6 months after optimization period. Varied parameters for each patient during optimization. | Implantation Bilateral Stimulation Monopolar Frequency 0/5/20/50/130/185 Hz Amplitude 0–10.5 V Pulse Width 0/90/150/ 270/450 μs | HAMD-17, MADRS, HAM-A, CGI. | Postoperative optimization: All four patients showed maximal response at longer pulse widths; three patients experienced a 50% reduction in HAMD-17 score. Longer pulse widths were correlated to short-term improvement. Longer pulse width also induced insomnia, confusion, and drowsiness; improved by turning off stimulation. Chronic stimulation: two patients responded ** at the end of open-label DBS, with longer pulse width. Electrode targets suggested to be individualized, as opposed to standard as in movement disorders, owing to the complexity of cortical gyral anatomy | Mild Anxiety; Drowsiness; Confusion; Insomnia. |
| Torres et al. (2013) [62] | Type I bipolar depression; Poor response to ECT and pharmacotherapy. | 1 | Target SCC Study Design DBS from 15 days after implantation and follow-up for 9 months. | Implantation N.A. Stimulation Monopolar Frequency 130 Hz Amplitude 6 mA Pulse Width 91 µs | HDRS-17, BDI, MADRS, GAF, Young Mania Scale. | Scores improved across tests. Psychotic symptoms disappeared. Manic episodes reduced. | N.A. |
| Broadway et al. (2012) [63] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 12 | Target SCC Study Design DBS for up to 24 weeks. | Implantation N.A. Stimulation Monopolar Frequency 130 Hz Amplitude 6–8 mA Pulse Width 90 µs | HRSD-17, Frontal and posterior Theta cordance. | Reduced HDRS-17 scores between baseline and the end of DBS among all patients. Six patients had significantly reduced HRSD-17 scores ** at the end of DBS. Increased frontal theta cordance between baseline and week 4 in responders correlated with their decreased depressive state at later time points. | N.A. |
| Hamani et al. (2012) [64] | TRD; NR to respond to pharmacotherapy, psychotherapy, transcranial magnetic stimulation, ECT, vagus nerve stimulation. Relapsed after receiving 6 month SCC-DBS. | 1 | Target SCC Study Design Administered tranylcypromine before surgery. DBS for 6 months. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 2.5 V Pulse Width 90 µs | HAMD-17 | Before relapse: SCC-DBS reduced HAMD-17 score from 22 to 9 after 4 month DBS. After relapse: MAOI supplementation restored the therapeutic effect of DBS; HAMD-17 score lowered from 22 to 16 (after 2 weeks), to 8 (after 2 months) and to 9 (after 4 months). | N.A. |
| Holtzheimer et al. (2012) [65] | Both sexes aged 18–70 years; current MDE ≥ 12 months; NR ≥ 4 antidepressant therapies; HRSD-17 score ≥ 20; GAF score ≤ 50. | 17 | Target SCC Study Design Intraoperative testing of electrode location for 12 or 17 patients. Stimulation: (i) 4 weeks of sham stimulation, followed by (ii) 24 weeks of open label DBS for 24 weeks, followed by (iii) single-blind discontinuation for 1 week and open label stimulation for up to 2 years. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 4–8 mA Pulse Width 91 µs | HRSD-17, BDI-II, GAF. | Reduced depression and increased function. 11 patients responded ** and seven further remitted □□ after 2 year DBS. Efficacy was similar for patients with MDD and those with BP. A modest sham stimulation effect was found, likely due to a decrease in depression after the surgical intervention, but prior to entering the sham phase. | Anxiety; Worsened depression; Nausea; Headache; Infection; Suicide attempts. |
| Lozano et al. (2012) [66] | Both sexes aged 30–60 years; First MDE before 35 years; HRSD-17 score ≥ 20; GAF < 50. | 21 | Target SCG Study Design DBS for 12 months, open label. | Implantation Bilateral Stimulation N.A. Frequency 110–140 Hz Amplitude 3.5–7 mA Pulse Width 65–182 µs | HRSD-17, CGI-S. | Improved global functioning and less severe depression. 13 patients responded ***, based on HRSD-17 scores. | Gastrointestinal problems; Skin problem; Suicide; Spasms; Weight gain; Insomnia. |
| Puigdemont et al. (2012) [67] | 18–70 years old with MDE; Resistant to pharmacological treatment and at most, a partial response to ECT; HAMD-17 Score ≥ 18. | 8 | Target SCG Study Design Intraoperative feedback was provided during surgery for electrode placement. DBS began at 48 h postoperative and ended when each patient had stabilized their response for at least three consecutive visits. | Implantation Bilateral Stimulation Monopolar Frequency 135 Hz Amplitude 3.5–5 V Pulse Width 90 µs | HAMD-17, MADRS, CGI. | Seven patients responded ** and three remitted □□ after 6 month DBS. Five patients responded ** and four remitted □□ after 12 month DBS. Three out of four remitted patients after 12 month DBS had remitted after 3 month DBS. | Suicide ideation; Neck pain; Recurrence; Depression; Cephalalgia. |
| Kennedy et al. (2011) [18] | Current MDE ≥ 12 months; HRSD-17 score ≥ 20; NR ≥ 4 antidepressant therapies. | 20 | Target SCG DBS patients were monitored for 3–6 years. | Implantation Bilateral Stimulation Monopolar Frequency 124.7 Hz (average) Amplitude 4.3 V (average) Pulse Width 70.6 µs | HAMD-17 36-item Short-Form Healthy Survey Questionnaire. | 64.3% patients responded ** at the last follow-up visit. 35% patients remitted □□ at the last follow-up visit. Scores at the last visit tended towards maintenance of therapeutic scores at 3 years. | Depression; Suicidal thoughts; Suicide (All determined to be unrelated to DBS). |
| Guinjoan et al. (2010) [68] | Chronic TRD; Family history of affective disorders; Poor response to antidepressants, ECT, and psychotherapy. | 1 | Target BA25 Study Design Positioning was aided by intraoperative feedback. Bilateral DBS was conducted for 12 months. Followed by unilateral-left, then right DBS, for 6 months | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3.5–5 V Pulse Width 90 µs | HAMD-17, BDI. | Patient’s condition plateaued after 6 month bilateral DBS. Left unilateral DBS led to rapid worsening in mood. Right unilateral DBS reversed the symptoms and the patient made significant improvements over bilateral stimulation. Patient remitted at 18 months. | Orthostatic hypotension. |
| Holtzheimer and Mayberg (2010) [69] | Showed signs and symptoms of MDD; Had suicidal ideation in current MDE; Did not improve in symptoms with pharmacotherapy, psychotherapy, and ECT HAM-D score was 25 | 1 | Target SCC Study Design Positioning of electrodes was aided by intraoperative feedback 24 weeks of open-label DBS and chronic stimulation beyond the assessment | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 6 mA Pulse Width 91 µs | HAMD-17 | HAMD-17 score lowered to 9 at the end of DBS follow-up. Sustained antidepressant response up to 2 years after surgery | N.A. |
| Hamani et al. (2009) [70] | Diagnosed MDD; current MDE ≥ 12 months; HAMD-17 score >20; GAF ≤ 50; NR ≥ 4 antidepressant therapies. | 20 | Target SCG Study Design DBS began at 2 weeks after surgery and lasted for 12 months | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3–5 V Pulse Width 90 µs | HAMD-17 | 11 responded ** at the end of 6 month DBS follow-up. Electrodes in responders were positioned ventrally relative to the landmarks of the medial prefrontal lobe. | N.A. |
| Puigdemont et al. (2009) [71] | Suffered from MDD, with several MDE accompanied by psychotic symptoms; Responded poorly to pharmacotherapy and ECT; Relapse following SCG-DBS with different features; Psychotic as opposed to depressive from previous episodes. | 1 | Target SCG Study Design DBS for 4 months, then switched off because of relapse and administered ECT for 3 weeks Resumed DBS until 12 months from the beginning of DBS. | Implantation Bilateral Stimulation Monopolar Frequency 135 Hz Amplitude 3.6 V Pulse Width 90 µs | HAMD-17 | Sustained response without the need of ECT before relapse. Maintained remission in DBS after ECT until the end of follow-up. | N.A. |
| Lozano et al. (2008) [72] | Current MDE ≥ 12 months; HRSD-17 score ≥ 20; NR ≥ 4 antidepressant therapies. | 20 | Target SCG Study Design Blinded-DBS in between and after surgery, monitored for up to 1 year. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3.5–5 V Pulse Width 90 µs | HRSD-17, Beck Anxiety Inventory, BDI, CGI-S, PET scans, Neuropsychological tests. | Mean HRSD-17 score lower than baseline at all time points. 12 patients responded ** to DBS, 7 remitted □□ after 6 month DBS. 11 patients responded ** to DBS, 7 were nearly remitted or remitted □□ after 12 month DBS. Eight responses maintained from 6 month to 12 month DBS. PET Scans: decreases in orbital, medial prefrontal cortex, and insula. Increases in lateral prefrontal cortex, parietal, anterior, and posterior cingulate by 6 months; increases in metabolic activity in regions adjacent to SCG. | Seven patients without adverse effects. Wound Infection; Headache; Pain; Seizure; Worsened mood; Irritability. |
| McNeely et al. (2008) [73] | Current MDE ≥ 12 months, HRSD-17 score ≥ 20, Non-responsive (NR) ≥ 4 antidepressant therapies, | 6 | Target BA25 Continuous DBS for 12 months. | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 3–4.5 V Pulse Width 60 µs | HRSD-17 Object alternation Test Iowa gambling task Visual delayed recall memory Verbal delayed memory Verbal list learning Stroop color-word | 6 months: four responded at the end of DBS ** General Neuropsychological Performance: Manual Motor Skills: Improved for dominant and non-dominant hand by 12 months. Verbal learning: Restored impairments in two patients at the end of 12 months. No significant correlations between change in mood and neuropsychological function at 6 and 12 months. | N.A. |
| Neimat et al. (2008) [74] | Family history of severe MDD. Failed to respond to antidepressants, adjuncts, and ECT. Relapsed after ablative cingulotomy | 1 | Target BA25 Study Design Started DBS on the day after electrode implantation and lasted for 30 months | Implantation Bilateral Stimulation Monopolar Frequency 130 Hz Amplitude 4.5 V Pulse Width 60 µs | HAMD-17 | HAMD-17 score decreased from 19 before surgery to 8 at 6 months after DBS. Sustained remission until the end of DBS study (scored 7) | N.A. |
| Mayberg et al. (2005) [19] | Current MDE ≥ 12 months, HRSD-17 score ≥ 20, Non-responsive (NR) ≥ 4 antidepressant therapies. | 6 | Target BA25 Study Design 1–5 min on-off stimulation in acute DBS for 5 days postoperative. Chronic DBS for 6 months after pulse generator was implanted and optimized for 4 wks | Implantation Bilateral Stimulation Monopolar Acute: Frequency 10–130 Hz Amplitude 0.0–9.0V Pulse Width 30–250 µs Chronic: Frequency 130 Hz Amplitude 4 V Pulse Width 60 µs | HDRS-17, MADRS, CGI, Positive and Negative Affective Scale. | Acute effects: Sudden feeling of calmness Chronic effects: five patients responded ** after 2 month DBS. Response maintained in four patients at the end of 6 month DBS. Three patients achieved remission □□ or near remission at the end of 6 month DBS. | Mild Lightheadedness; Psychomotor slowing; Skin infection; Skin erosion. |
| Authors | ≤ 6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||
|---|---|---|---|---|---|---|---|---|
| Response (%) | Remission (%) | Response (%) | Remission (%) | Response (%) | Remission (%) | Response (%) | Remission (%) | |
| Sankar et al. 2020 [40] | NA | NA | NA | NA | NA | NA | NA | NA |
| Riva Posse et al. (2019) [41] | NA | NA | NA | NA | NA | NA | NA | NA |
| Eitan et al. (2018) [42] | NA | NA | NA | NA | 44.4 | NA | NA | NA |
| Merkl et al. (2018) [43] | 37.5 | 12.5 | 43 | 14.2 | 33 | 33 | 33 | NA |
| Howell et al. (2018) [44] | - | - | 33.3 | 66.7 | - | - | - | - |
| Waters et al. (2018) [45] | NA | NA | NA | NA | NA | NA | NA | NA |
| Smart et al. (2018) [46] | - | - | 78.5 | - | - | - | - | - |
| Choi et al. (2018) [47] | NA | NA | NA | NA | NA | NA | NA | NA |
| Conen et al. (2018) [48] | - | - | 28.6 | 42.9 | - | - | - | - |
| Holtzheimer et al. (2017) [15] | 22 20 (sham) | 10 7 (sham) | 28 30 (sham) | 12 7 (sham) | 54 52 (sham) | 17 20 (sham) | 48 44 (sham) | 25 12 (sham) |
| McInerney et al. (2017) [14] | - | - | 55 | - | - | - | - | - |
| Riva-Posse et al. (2018) [49] | 72.7 | 54.5 | 81.8 | 54.5 | - | - | - | - |
| Tsolaki et al. 2017 [50] | 50 | - | - | - | - | - | - | - |
| Accolla et al. (2016) [51] | - | - | - | - | 79 | 20 | - | - |
| Richieri et al. (2016) [52] | 100 (Case Study) | - | - | - | - | - | - | - |
| Hilimire et al. (2015) [53] | NA | NA | NA | NA | NA | NA | NA | NA |
| Martin-Blanco et al. (2015) [54] | NA | NA | NA | NA | NA | NA | NA | NA |
| Puigdemont et al. (2015) [55] | - | 80 | - | - | - | - | - | - |
| Serra-Blasco et al. (2015) [56] | - | - | - | - | 75 (F.E.) 87 (TRD) | - | - | - |
| Choi et al. 2015 [57] | NA | NA | NA | NA | NA | NA | NA | NA |
| Sun et al. 2015 [58] | NA | NA | NA | NA | NA | NA | NA | NA |
| Perez-Caballero et al. (2014) [59] | 50 | - | - | - | - | - | - | - |
| Merkl et al. (2013) [60] | - | - | - | 30 | - | - | - | - |
| Ramasubbu et al. (2013) [61] | 50 | - | - | - | - | - | - | - |
| Torres et al. (2013) [62] | 100 (2 Case Studies) | - | 100 (2 Case Studies) | - | 100 (2 Case Studies) | - | 100 (2 Case Studies) | - |
| Broadway et al. (2012) [63] | 50 | - | - | - | - | - | - | - |
| Hamani et al. (2012) [64] | - | 100 (Case Study) | 100 (Case Study) | - | - | - | - | - |
| Holtzheimer et al. (2012) [65] | 18 | 41 | 36 | 36 | 58 | 92 | - | - |
| Lozano et al. (2012) [66] | 57 | - | 48 | - | 62 | - | - | - |
| Puigdemont et al. (2012) [67] | 37.5 | 37.5 | 87.5 | 37.5 | 62.5 | 50 | - | - |
| Kennedy et al. (2011) [18] | - | - | 62.5 | - | 46.2 | - | 75 | - |
| Guinjoan et al. (2010) [68] | 100 (Case Study) | - | 100 (Case Study) | - | 100 (Case Study) | - | - | 100 (Case Study) |
| Holtzheimer and Mayberg (2010) [69] | 100 (Case Study) | - | 100 (Case Study) | - | 100 (Case Study) | - | 100 (Case Study) | - |
| Hamani et al. (2009) [70] | - | - | 55 | - | - | - | - | - |
| Puigdemont et al. (2009) [71] | 100 (Case Study) | - | 100 (Case Study) | - | - | - | - | - |
| Lozano et al. (2008) [72] | 35 | 10 | 60 | 35 | - | - | - | - |
| McNeely et al. (2008) [73] | 66 | NA | - | - | - | - | - | - |
| Neimat et al. (2008) [74] | 100 (Case Study) | NA | - | - | - | - | 100 (Case Study) | - |
| Mayberg et al. (2005) [19] | 66 | 50 | - | - | - | - | - | - |
| Average | 63.8% | 43.9% | 66.5% | 36.5% | 69.3% | 42.4% | 76% | 62.5% |
| Range | 18–100 | 10–100 | 28.6–100 | 12–66.7 | 33–100 | 17–92 | 33–100 | 12–100 |
| Authors | Target | Animal | Animal Models & DBS Design | Stimulation Parameters | Behavioral Tests | Outcomes |
|---|---|---|---|---|---|---|
| Jia et al. (2019) [116] | vmPFC | Sprague-Dawley rats, male. | CUS animal model. Open field test and forced swim test before DBS. | Unipolar High Frequency Frequency: 130 Hz Amplitude: 100 μA Pulse Width: 90 μs Low Frequency Frequency: 20 Hz Amplitude: 400 μA Pulse Width: 0.2 μs | Sucrose preference test | CUS rats had a lowered sucrose preference compared to control rats. |
| Open field test | No significant difference in locomotion was recorded between CUS and control groups. | |||||
| Forced swim test | Both High- and Low-Frequency Stimulation reduced immobility compared to sham rats. | |||||
| Papp et al. (2019) [117] | vmPFC | Wistar-Kyoto rats, male (DBS) Wistar rats, male [Venlafaxine(VFX)-treated] | CMS animal model. Two, 2-h DBS sessions were conducted, one on the preceding evening and the other on the following morning before each sucrose intake test and the NORT T1 session. | Frequency: 130 Hz Amplitude: 250 μA Pulse width: 90 μs | Sucrose intake test | During the first 2 wks of CMS, sucrose intake decreased >50% across groups. VFX treatment restored sucrose intake levels. |
| Novel object recognition test | Wistar Kyoto rats: DBS rescued novel object recognition test across all groups. Wistar rats: VFX rescued novel object recognition test in CMS animals administered with D2 antagonist, but not in D2-administered CMS animals. VFX also did not rescue groups administered with D3 antagonist. | |||||
| Bhaskar et al. (2018) [105] | vmPFC | Wistar rats, male. | Naïve animal model. DBS for 15 min prior to and throughout behavioral testing. | Bipolar Frequency: 100 Hz Amplitude: 200 µA Pulse Width: 100 µs | Home-cage emergence test | Enriched environment potentiated the efficacy of HFS on reduced escape latency time in the Naïve animal model. |
| Elevated plus maze | HFS with an enriched environment reduced the anxiety index and increased head dips. | |||||
| Novel object recognition test | No significant difference. | |||||
| Bregman et al. (2018) [97] | vmPFC | SERT homozygous knockout and wildtype mice, male. | Serotonin transporter (SERT) knockout model. DBS for 4 h before forced swim test and open field test. | Bilateral Monopolar Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Forced swim test | Both wild-type and knockout-DBS mice had reduced immobility time compared to sham. |
| Open field test | Knockout-DBS mice had lower locomotion counts than sham and wild-type mice. | |||||
| Lehto et al. (2018) [114] | IL | Sprague-Dawley rats, male. | Naïve animal model. All stimulation paradigms consisted of three blocks of 60 s of rest and 18 s of stimulation, ending with an additional rest period, giving a total paradigm of 4 min 54 s. | Monopolar Frequency: 20/35/70/100/130/160/200 Hz tested in randomized order. Amplitude: 1.4–1.7 mA distributed equally among the three electrode channels Pulse Width: 180-μs | N.A. | fMRI conducted to characterize changes in the brain following DBS. IL-DBS at varying stimulation parameters significantly triggered the amygdala. Orientation selective stimulation was able to recruit neuronal pathways of distinct orientations relative to the position of the electrode. |
| Papp et al. (2018) [118] | vmPFC | Wistar rats, male. | CMS animal model. Two, 2-h DBS sessions were conducted, one on the previous evening and one the next morning 15 min before each behavioral test. | Bipolar Frequency: 130 Hz, Amplitude: 250 μA, Pulse Width: 90 μs | Sucrose intake test | DBS increased sucrose intake across all treatment groups, except for imipramine-treated animals. |
| Elevated plus maze | DBS increased the anxiolytic open arm entries in all treatment groups. | |||||
| Novel object recognition test | DBS rescued the abolished novel object recognition in CMS sham-treated animals, across all treatment groups. | |||||
| Perez-Caballero et al. (2018) [115] | IL | Wistar rats, male. | Six independent sets of animals using naïve (unoperated controls) and DBS-off animals. | N.A. | Paw-pressure test | Ibuprofen, tramadol, and morphine significantly increased the paw withdrawal threshold in naïve animals relative to respective vehicle alone, demonstrating a clear analgesic effect. |
| Open field test | No analgesics altered the motor activity of rats. | |||||
| Modified forced swim test | Electrode implantation induced a significant reduction in the immobility scores of vehicle-treated animals. Ibuprofen abolished the antidepressant-like effect of electrode implantation in the modified forced swim test, increasing the DBS-off animal’s immobility. Neither morphine nor tramadol counteracted the antidepressant-like effect of DBS-off animals. | |||||
| Novelty suppressed feeding test | Electrode implantation reduced latency to feed compared to naïve animals. Ibuprofen increased latency to feed relative to VEH-treated animals. Neither morphine nor tramadol reduced the latency to feed in electrode-implanted animals. | |||||
| Torres-Sanchez et al. (2018) [110] | vmPFC | Wistar rats, male. | Naive animal model. DBS for (i) 4 h at 24 h after surgery, then (ii) 2 h at 48 h after surgery | Bipolar Monophasic Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Forced swim test | Reduced immobility time and increased climbing compared to control. |
| Volle et al. (2018) [119] | vmPFC | Sprague-Dawley rats, male. | Stimulation was delivered 1 week after surgery for either (i) a single day (acute stimulation; 8 h/day) or (ii) 12 days (chronic stimulation daily for 8 h/day) using a portable stimulator (ANS model 3510) to different groups of rats | Frequency: 130 Hz Amplitude: 200 μA Pulse Width: 90 μs | N.A. | Both treatments increase serotonin (5-HT) release, although fluoxetine resulted in a higher sustained concentration, even upon chronic treatment. Chronic DBS resulted in lowered 5HT release by Day 12. DBS reduced raphe SERT expression. DBS induced changes in 5-HT1B receptor expression, whereas fluoxetine induced changes in 5-HT1A receptors expression in the prefrontal cortex. Research highlighted different effects of both treatments on the serotonergic system. |
| Reznikov et al. (2017) [109] | IL | Sprague-Dawley rats, male. | Posttraumatic stress disorder animal model. 3-day fear conditioning DBS from 1 week after extinction recall to the end of experiment, 8 h/day, or 2 h before and 4 h after behavioral tests. | Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Extinction recall test | Higher freezing scores in DBS-weak extinction than DBS-strong extinction. |
| Open field test | No significant difference between groups. | |||||
| Novelty suppressed feeding test | Reduced latency to feeding in DBS-weak extinction, but not strong extinction. | |||||
| Elevated plus maze | No significant difference observed between groups. | |||||
| Bruchim-Samuel et al. (2016) [96] | vmPFC VTA | Flinders Sensitive Line rats, male. Sprague-Dawley rats, male. (Control) | Flinders Sensitive Line model. DBS for 15 min/day, for 10 days. | Bilateral, Monopolar vmPFC Stimulation Frequency: 20 Hz Amplitude: 400 µA Pulse Width: 200 µs VTA Stimulation (control) Frequency: 10 Hz Amplitude: 300 µA Pulse Width: 200 µs | Sweetened condensed milk intake test | No significant difference between vmPFC groups. Significant difference between VTA groups for Flinders Sensitive Line rats. |
| Novelty Exploration Test | No significant difference between vmPFC groups. Significant difference between VTA groups for Flinders sensitive line rats. | |||||
| Forced swim test | Decreased immobility for vmPFC-stimulated rats after DBS for 10 days, half relapsed at day 28. VTA-stimulated Sprague-Dawley rats had persistently reduced immobility until the end of the experiment. | |||||
| Jiménez-Sánchez et al. (2016) [33] | IL | Wistar rats, male. | Olfactory bulbectomized model animal model. DBS 1 h daily stimulation, beginning 2 days after electrode implantation before behavioral testing. | Bipolar Biphasic Frequency: 130 Hz Amplitude: 200 µA Pulse Width: 90 µs | Social interaction test | Increased duration of active contact. |
| Sucrose preference test | Increased percentage of sucrose consumption in total liquid consumption. | |||||
| Forced swim test | Reduced immobility and increased climbing but not swimming. | |||||
| Hyperemotionality test | Reduced total behavioral scores when compared to olfactory bulbectomized sham rats. | |||||
| Jiménez-Sánchez et al. (2016) [81] | IL PrL | Wistar rats, male. | Naïve animal model. DBS for 1 h daily before behavioral testing. | Bipolar Biphasic Frequency: 130 Hz Amplitude: 200 µA Pulse Width: 90 µs | Forced swim test | Reduced immobility and increased climbing in IL-DBS No significant behavioral changes in PrL-DBS. |
| Open field test | Insignificant locomotor changes in IL-DBS. | |||||
| Novelty suppressed feeding test | Decreased latency to feed in IL-DBS. | |||||
| Rummel et al. (2016) [113] | vmPFC | Flinders Sensitive Line rats, male. Congenitally learned helplessness rats, Male. | Experiment 1 Chronic intermittent DBS 1 week after surgery in Flinders sensitive line rats, 30 min each morning and extra 30-min stimulation on afternoons before the day of behavioral test. Experiment 2 Chronic continuous DBS 1 week after surgery for 16 days. Experiment 3 Chronic intermittent DBS in congenitally learned helpless rats, procedures followed that in experiment 1. | Chronic intermittent DBS Frequency: 130 Hz Amplitude: 300 µA Pulse Width: 100 µs Chronic continuous DBS Frequency: 130 Hz Amplitude: 150 µA Pulse Width: 100 µs | Sucrose consumption test | Chronic intermittent DBS increased sucrose intake in Flinders sensitive line rats but not in congenitally learned helplessness rats. Chronic continuous DBS did not affect sucrose intake in Flinders sensitive line rats and congenitally learned helplessness rats. |
| Forced swim test | Chronic intermittent DBS and chronic continuous DBS increased latency to immobility in Flinders sensitive line rats but not congenitally learned helplessness rats. | |||||
| Learned helplessness paradigm | Chronic intermittent DBS and chronic continuous DBS decreased helplessness in Flinders sensitive line rats but not cLH rats. | |||||
| Sucrose intake test | No significant difference observed. | |||||
| Novelty exploration test | No significant difference observed. | |||||
| Bambico et al. (2015) [98] | vmPFC | Fisher rats, Male. | CUS animal model CUS for ~4 weeks until anhedonia inferred by SPI scores, then performed implantation DBS for 3 weeks after implantation, 8 h per day, 7 days per week | Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Novelty-suppressed feeding test | Reduced latency to feeding in CUS-DBS animals compared to CUS-sham animals. |
| Open field test | No significant difference observed. | |||||
| Elevated plus maze test | More time in open arms in CUS-DBS animals compared to CUS-sham animals. | |||||
| Forced swim test | Reduced immobility time in CUS-DBS animals compared to CUS-sham animals. | |||||
| Edemann-Callesen et al. (2015) [94] | vmPFC; Medial forebrain bundle. | Flinders sensitive line rats, male. Sprague-Dawley rats, male. | Naïve animal model DBS was applied in an Intra-cranial self- stimulation protocol. | Bilateral, Monopolar Frequency: 20–200 Hz Amplitude: 170–560 µA Pulse Width: 100 µs | Intra-cranial self-stimulation | For Flinders Sensitive Line rats, vmPFC-DBS did not affect the reward-seeking behavior compared to medial forebrain bundle DBS. |
| Etiévant et al. (2015) [103] | IL | Sprague-Dawley rats, male. | Naïve animal model. DBS for 4 h after forced swim pre-test and 2 h before forced swim test. | Bipolar Unilateral Frequency: 130 Hz Amplitude: 150 µA Pulse Width: 60 µs | Forced swim test | Reduced immobility duration in IL-DBS compared to control. |
| Insel et al. (2015) [111] | IL | Sprague-Dawley rats, male. | Naïve animal model. DBS for 8 h per day, for 10 days. | Monopolar Bilateral Frequency: 130 Hz Amplitude: 100 μA Pulse Width: 90 μs | Spontaneous behavior recording | Coherence between ventral hippocampus and IL was reduced after 10-day DBS compared to sham in 2–4 Hz brain activity range, but was not reduced after only 1 day of treatment. Coherence was not affected by fluoxetine, indicating that IL-DBS observations were independent of the serotonergic pathways. |
| Lim et al. (2015) [102] | vmPFC | Sprague-Dawley rats, male. | Experiment 1 Naïve animal model. Experiment 2 CUS animal model. CUS for 3 weeks, each stressor lasted for 10–14 h DBS for 15 min before home-cage emergence test, before and during open field test. | Bipolar Biphasic Experiment 1 Frequency:10/100 Hz Amplitude: 100 µA Pulse Width: 100 µs Experiment 2 Frequency: 100 Hz Amplitude: 100µA Pulse Width: 100µs | Home-cage emergence test | HFS reduced escape latency time in Naïve and CUS animal model. |
| Open-field test | Insignificant effect in Naïve animals for both HFS and LFS. Increased time spent in the central zone for HFS-CUS. | |||||
| Food-intake test | HFS increased food intake in naïve animals. No significance difference observed in CUS animals. | |||||
| Sucrose-intake test | Insignificant in Naïve animals for both HFS and LFS. Increased sucrose intake in HFS-CUS. | |||||
| Forced swim test | Insignificant in Naïve for both HFS and LFS. Reduced immobility duration in HFS-CUS. | |||||
| Lim et al. (2015) [108] | PrL | Sprague-Dawley rats, male. | Naïve animal model. DBS for 15 min before and during sucrose intake test (same for forced swim test) and before sacrifice for 1 h. | Bipolar Biphasic Frequency: 100 Hz Amplitude: 100 µA Pulse Width: 100 µs | Sucrose intake test | Increased sucrose consumption. |
| Forced swim test | Reduced immobility. | |||||
| Liu et al. (2015) [99] | vmPFC | Sprague-Dawley rats, male. 4 months old and 12 months old. | Acute DBS Naïve animal model. DBS for 30 min before the behavioral tests. Chronic DBS Naïve animal model. DBS for 1 h daily including days of behavioral tests. | Bipolar Biphasic Acute DBS Frequency: 10/100 Hz Amplitude: 50/100/200/400 µA Pulse Width: 100 µs Chronic DBS Frequency: 100 Hz Amplitude: 100 µA Pulse Width: 100 µs | Novel object recognition test | Acute HFS at 200 µA produced higher novel object exploration than familiar object in short-term memory test. Chronic HFS increased novel object exploration in short- and long-term memory than familiar object, as well as the durations. |
| Morris water maze | Shorter latency to reach platform on day 1 and 2 in chronic HFS compared to sham. More time spent in the target quadrant and less in the opposite quadrant in chronic HFS compared to sham. | |||||
| Hamani et al. (2014) [100] | vmPFC | Sprague-Dawley rats, male. | Naïve animal model. DBS for 4 h after FST on day 1, 2 h before swimming on day 2 DBS 1 week after forced swim test, 4 h on day 1, 2 h on day 2, then assessed in open field test | Monopolar Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Forced swim test | Reduced immobility and increased climbing frequency between groups. |
| Open field test | No significant difference in locomotion observed. | |||||
| Laver et al. (2014) [112] | vmPFC | Sprague-Dawley rats, male. | Naïve animal model. Serotonin reuptake inhibitors/vehicle were injected i.p. 1 h and 5 h after forced swim test on day 1 and 1 h before forced swim test on day 2. DBS for 4 h on day 1 of forced swim pre-test and 2 h before forced swim test on day 2. | Monopolar Bilateral Frequency: 130 Hz Amplitude: 100 μA Pulse Width: 90 μs | Forced swim test | DBS-saline, DBS-buspirone, DBS-Risperidone, DBS-pindolol-treated animals had higher swimming and lower observed immobility frequencies. |
| Open field test | No significant difference observed | |||||
| Perez-Caballero et al. (2013) [45] | IL | Wistar rats, male. | CUS animal model. Electrode implantation after week 4 of CUS, then CUS resumed. DBS for 4 h after forced swim pre-test and 2 h before forced swim test. Some animals received pre-treatment with indomethacin or ibuprofen. | Bipolar Monophasic Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Forced swim test | Reduced immobility and increased swimming in DBS-off-IL and DBS-on-IL compared to sham- and naïve-animals. Increased immobility and reduced swimming in DBS-off-IL animals treated with NSAIDs. |
| Open field test | No significant difference. | |||||
| Rea et al. (2014) [95] | vmPFC | Flinders sensitive line rats, male. Flinders resistant line rats, male. (Control) | DBS for 30 min each morning for 2 weeks. Extra DBS for 30 min in the afternoon before the day of behavioral tests and during behavioral tests. | Monopolar Frequency: 130 Hz Amplitude: 300 µA Pulse Width: 100 µs | Forced swim test | DBS reduced immobility in both groups of rats. |
| Sucrose consumption test | DBS increased sucrose consumption in both groups of rats. | |||||
| Veerakumar et al. (2014) [104] | vmPFC | C57BL/6 mice, male. | Chronic social defeat stress animal model. DBS for 5 h/day, for 7 days. | Bipolar Unilateral Frequency: 160 Hz Amplitude: 150 µA Pulse Width: 60 µs | Social interaction test | Before DBS, defeat-susceptible mice showed lower interaction times. Defeated animals with DBS spent longer in the interaction zone than sham and similar to non-stressed animals. DBS increased the total distance traveled. |
| Hamani et al. (2012) [92] | vmPFC | Wistar rats, male. | CUS animal model. DBS for 8 h/day, for 2 weeks | Monopolar Frequency: 130 Hz Amplitude: 200 µA Pulse Width: 90 µs | Sucrose preference test | Higher preference observed in CUS-treated DBS animals when compared to CUS-sham animals. Higher sucrose consumption in CUS-treated DBS than CUS-sham alone. |
| Hamani et al. (2010) [93] | vmPFC, IL, PrL | Sprague-Dawley rats, male | Naïve animal model DBS for 4 h after FST on day 1, 2 h before swimming on day 2 | Frequency: 20 Hz/130 Hz Amplitude: 100/200/300/400 µA Pulse Width: 90 µs | Forced swim test | Parameters of 130 Hz, 90 µs, 200 µA reduced immobility the most in vmPFC-DBS, also at 100 µA and 300 µA. PrL stimulation at 130 Hz, 90 µs, 200 µA reduced immobility, but IL stimulation was insignificant. |
| Hamani et al. (2010) [91] | vmPFC | Sprague-Dawley rats, male | Naïve animal model, with serotonergic depletion, or norepinephrine lesion. DBS for Forced Swim Test Day 1: 4 h after forced swim test Day 2: 2 h before forced swim test DBS for open field test, novelty suppressed feeding test, and learned helplessness. Pre-test Day 1: 4 h Pre-test Day 2: 2 h | Monopolar Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Forced swim test | DBS reduced immobility and increased swimming counts in naïve animals. DBS in animals with ibotenic acid injection had lower immobility and higher swimming counts than control. Rats with DBS without serotonergic depletion exhibited lower immobility than DBS animals with serotonergic depletion. Rats with DBS without norepinephrine lesion had lower immobility than control, similar reduction in immobility was shown in animals with DBS and norepinephrine lesion. |
| Open field test | No significant difference. | |||||
| Novelty suppressed feeding test | DBS reduced latency to feed compared to control. | |||||
| Learned helplessness paradigm | Insignificant difference in escape latency between DBS and control. | |||||
| Animals predisposed to helplessness. DBS for 2 h before baseline assessment, 2 h before footshock at 2 days after baseline test, and DBS for 2 h before sucrose consumption test on the next day | Monopolar Frequency: 130 Hz Amplitude: 100 µA Pulse Width: 90 µs | Sucrose consumption test | DBS reduced the sucrose drinking time in animals after footshock, but this was insignificant. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairuddin, S.; Ngo, F.Y.; Lim, W.L.; Aquili, L.; Khan, N.A.; Fung, M.-L.; Chan, Y.-S.; Temel, Y.; Lim, L.W. A Decade of Progress in Deep Brain Stimulation of the Subcallosal Cingulate for the Treatment of Depression. J. Clin. Med. 2020, 9, 3260. https://doi.org/10.3390/jcm9103260
Khairuddin S, Ngo FY, Lim WL, Aquili L, Khan NA, Fung M-L, Chan Y-S, Temel Y, Lim LW. A Decade of Progress in Deep Brain Stimulation of the Subcallosal Cingulate for the Treatment of Depression. Journal of Clinical Medicine. 2020; 9(10):3260. https://doi.org/10.3390/jcm9103260
Chicago/Turabian StyleKhairuddin, Sharafuddin, Fung Yin Ngo, Wei Ling Lim, Luca Aquili, Naveed Ahmed Khan, Man-Lung Fung, Ying-Shing Chan, Yasin Temel, and Lee Wei Lim. 2020. "A Decade of Progress in Deep Brain Stimulation of the Subcallosal Cingulate for the Treatment of Depression" Journal of Clinical Medicine 9, no. 10: 3260. https://doi.org/10.3390/jcm9103260







